Abstract
Sleep-related hypermotor epilepsy (SHE), previously called nocturnal frontal lobe epilepsy (NFLE), is a focal epilepsy characterized by asymmetric tonic/dystonic posturing and/or complex hyperkinetic seizures occurring mostly during sleep. SHE fulfills the definition of rare disease with an estimated minimum prevalence of 1.8/100,000 individuals, and it represents about 10% of drug-resistant surgical cases. Although SHE and autosomal-dominant SHE (ADSHE) have been considered benign epileptic conditions for a long time, emerging data have shed light on the severity of this disorder and some peculiar features can impact negatively on the quality of life of SHE patients. In fact, seizure frequency can be very high, resulting in nocturnal sleep fragmentation with possible diurnal consequences such as excessive sleepiness and fatigue. Moreover, recent studies, adopting a systematic neuropsychological assessment, have shown deficits in memory, executive functions and visuo-spatial abilities in almost half of SHE patients. Intellectual disabilities and psychiatric disorders have also been reported in some genetic forms. SHE may also exert a negative effect on health-related quality of life, especially in domains pertaining to a patient’s role in the family, social context and patient’s illness experience.
Despite a good response to pharmacological treatment, especially with carbamazepine, 30% of SHE patients suffer from drug-resistant seizures. Finally, recent studies suggest a poor prognosis in a high percentage of SHE patients with a 20.4% cumulative probability of achieving terminal remission at 10 years from onset. For selected drug-resistant SHE patients, epilepsy surgery is the only treatment offering high probability of recovery, both for seizures and for epilepsy-related sleep alterations.
Historical background
In 1977, Pedley and Guilleminault described an unusual type of sleepwalking in six patients; all experienced episodes characterized by screaming, vocalization, complex automatisms and ambulation. The episodes ceased after phenytoin or carbamazepine treatment. Four of these patients demonstrated epileptiform abnormalities in their electroencephalograms (EEGs). On the basis of the EEGs’ abnormalities and the favorable response to therapy, authors interpreted these episodes as epileptic manifestations, despite the fact that EEG recordings of two abortive attacks failed to correlate with any paroxysmal or other abnormal electrical activity.Citation1 Some years later, further patients with complex motor attacks recurring every night during slow-wave sleep were reported; typically, the attacks consisted of twisting of the trunk and violent hyperkinetic movements of the limbs, and they were occasionally associated with tonic/dystonic posturing. Authors strongly debated the epileptic or nonepileptic origin of these episodes that were finally considered as rare motor disorders of sleep because of the complex motor clinical pattern that occurs only during sleep and the absence of EEG epileptiform abnormalities.Citation2 The term “hypnogenic paroxysmal dystonia” was used first, changed later to “nocturnal paroxysmal dystonia” (NPD).Citation3 At the same time, studies conducted in drug-resistant epileptic patients submitted to a presurgical evaluation have permitted a better understanding of the anatomo-electroclinical features of these paroxysmal manifestations, suggesting their epileptic origin.Citation4–Citation9 In particular, TharpCitation4 reported three children with seizures characterized by bizarre motor attacks recurring during wakefulness and resembling NPD episodes. One of them became seizure-free after the surgical resection of the orbital region. These data were successively confirmed by Wada et al, who demonstrated the epileptic nature and the frontal lobe origin of sleep-related seizures, similar to those classified as NPD.Citation5,Citation6 Finally, Tinuper et al recorded three patients with short-lasting motor attacks (similar to those described as NPD), showing clear-cut epileptic interictal and ictal abnormalities.Citation9 All these observations led to the coining of the term “nocturnal frontal lobe epilepsy” (NFLE). In the following years, a large series of patients were described.Citation10,Citation11 A large video-polysomnographic study of 100 consecutive NFLE cases highlighted the different intensity and durations of these sleep-related manifestations (ranging from paroxysmal arousals to nocturnal wandering) that could occur in a single patient, during a single night. The hypothesis that these episodes could represent a continuum of the same seizureCitation11,Citation12 was demonstrated by further studies using intracerebral EEG recordings techniques during the presurgical evaluation of drug-resistant sleep-related hypermotor epilepsy (SHE) patients (video-stereo-electroencephalography, SEEG).Citation13
In 2014, a Consensus Conference composed of a group of international experts in epilepsy, sleep and epidemiology was organized in order to better describe the electroclinical features of the disorder and to delineate more accurate diagnostic criteria.Citation14 During the conference, a need for a new denomination emerged, and the term “sleep-related hyper-motor epilepsy” (SHE) replaced NFLE. Indeed, the term nocturnal was judged not appropriate because seizures occur during sleep, both during the day and the night, independently of the daytime. Moreover, considering that sleep-related seizures with hyperkinetic automatisms and/or dystonic posturing have been shown to originate also from extrafrontal areas, such as temporal, insular and parietal regions,Citation15–Citation22 the term frontal was considered misleading. Finally, the term “hypermotor” was used because it was considered the best compromise able to represent both seizures with hyperkinetic automatisms or tonic–dystonic seizures. However, an update of the denomination might be required considering the last International League Against Epilepsy seizure classification.Citation23 Experts agreed that SHE should be considered a unique syndrome, independently of the etiology (genetic, lesional or both) and the brain region involved.Citation14
Clinical and diagnostic criteria for SHE
Seizures in SHE are usually brief (<2 minutes in duration), with an abrupt onset and offset and with stereotyped clinical features. Seizures are typically sleep-related (with several episodes per night) even if episodes occur rarely in wakefulness. The “hypermotor” semiology is the primary clinical pattern of the seizures, including asymmetric tonic or dystonic posturing and/or hyperkinetic automatisms. The level of awareness during and after the seizure is not a crucial clinical sign for a definitive diagnosis.
The clinical history and clinical semiology are the main criteria to establish the diagnosis as both interictal and ictal EEG may be uninformative. Three categories for the diagnosis with different levels of certainty have been identified: 1) Witnessed (possible), based on the description of the core clinical features; 2) Video Documented (clinical), based on the evaluation of a video recorded (preferably 2) hypermotor episode; and 3) Video-EEG Documented (confirmed), requiring the video-polygraphic recording of stereotyped events (one or two) and ictal or interictal epileptiform abnormalities.Citation14
Epidemiological data
Studies indicate that about 12% of epileptic patients,Citation24,Citation25 mostly with a diagnosis of focal epilepsy,Citation26,Citation27 suffered from sleep-related seizures, defined as “seizures occurring exclusively or predominantly (>90 %) during sleep”. SHE represented the diagnosis in 13% of patients referred to a tertiary center for a video-polysomnographic evaluation of nocturnal motor disordersCitation11 and in 9.4% of our population of focal drug-resistant epileptic patients (unpublished data). It is worth underlining the possible underestimation of SHE prevalence, as many patients may be misdiagnosed as parasomnias, particularly in the pediatric population.Citation28,Citation29
A prevalence study conducted in the northeast of Italy showed that SHE is a rare disease with a minimum prevalence of 1.8/100,000 individuals.Citation30,Citation31
SHE predominates in males (7:3); nonlesional forms are the most common; a family history of epilepsy is found in no more than 25% of cases; an autosomal-dominant inheritance pattern is rarely recognizable; onset of seizures peaks during childhood with the majority of cases presenting with seizures before the age of 20 years.Citation10,Citation11,Citation32–Citation34 Only 20% of drug-resistant SHE patients with hyperkinetic seizures present with this type of seizure before the age of 5 years (unpublished data). To date, the reason for this low percentage remains unknown and might reflect a later full development of behavioral neuronal networks.
Genetic background of SHE
In 1994, Sheffer et al reported a large Australian family with autosomal-dominant inherited NFLE/SHE (ADNFLE/ADSHE).Citation35 A genetic mutation of the CHRNA4 gene coding for the alpha4 subunit of the neuronal nicotinic acetylcholine receptor (nAChR)Citation36 was found. Successively, many studies described further mutations in genes (CHRNA2 and CHRNB2) coding for other nAChR subunits (alpha2 and beta2),Citation37,Citation38 the phenotype usually being indistinguishable.Citation14
More recently, other mutations besides the ones coding for the nAChR subunits have been reported in both sporadic and ADSHE cases. Combi et al found two new nucleotide variations in the corticotropin-releasing hormone gene promoter in ADSHE pedigrees and two sporadic cases.Citation39 Heron et al discovered missense mutations in the KCNT1 gene (coding for a sodium-gated potassium channel subunit) in patients with a severe phenotype, including frequent seizures, psychiatric symptoms and intellectual disabilities.Citation40
Some ADSHE families and SHE sporadic cases have been found to have mutations in DEPDC5, and NPRL2 and 3,Citation41–Citation43 all encoding proteins of the mTORC1-regulating GATOR1 complex, a key regulator of cell growth.Citation44,Citation45 The mutations were first identified in patients with familial focal epilepsy with variable foci (FFEVF)Citation46 and in epilepsy associated with a focal cortical dysplasia.Citation47,Citation48
Recently a novel missense mutation in the CABP4 gene encoding the neuronal Ca2+-binding protein 4 (CaBP4) has been found in a Chinese family, including 11 individuals diagnosed with ADSHE.Citation49
Despite a great effort to study the genetic background of SHE, a genetic cause may be recognizable in a very low percentage of sporadic cases and in less than 30% of ADSHE families, with incomplete penetrance. Moreover, to date, there are no clear-cut correlations between disease severity, genetic findings and functional effects of the known genetic mutations,Citation14 even if KCNT1 gene mutated SHE patients seem to present a more severe form.Citation40 However, further studies focused on genotype–phenotype correlations in SHE are needed.
The genetic predisposition to present seizures especially during sleep is another interesting field of research. To date, a cholinergic pathway hyperactivation and an enhanced GABA ergic function were reported in in vitro and in vivo studies, suggesting that cortical–subcortical networks involved in the mechanism of arousal can contribute in the epileptogenesis of ADSHE.Citation37 Very recently, studies performed in brain epileptogenic tissues have shown that defects in Circadian Locomotor Output Cycles Kaput (CLOCK) expression, a transcription factor that regulates the circadian rhythm and the mTOR pathway, could be responsible for the preferential occurrence of seizures during sleep.Citation50
Impact on general life and prognostic features of SHE
Although SHE and ADSHE for a long time have been considered benign epileptic conditions due to the occurrence of seizures only during sleep and the good response to treatment, many studies reported some factors that might have a negative impact on the general life of these patients.
Sleep fragmentation
Frequency of seizures in SHE patients can be very high, ranging from 1 to 20 attacks per night,Citation11 and minor motor events or paroxysmal arousals may be even more frequent. SHE patients may complain of nocturnal sleep discontinuity and consequently, excessive sleep inertia in the morning, daytime tiredness and excessive sleepiness,Citation10,Citation13,Citation33,Citation51–Citation57 impacting negatively on their quality of life. Furthermore, sleep deprivation can facilitate seizure appearance, perpetuating an erroneous loop between seizures and triggering factors.
In a case–control studyCitation58 the occurrence of sleepiness-related symptoms and subjective sleep quality were examined using questionnaires (Epworth sleepiness scale and the Bologna Questionnaire on Sleepiness-related symptoms).Citation59 SHE cases more commonly complained of “spontaneous midsleep awakenings”Citation58 and “tiredness after awakening” compared with matched controls.Citation58 Furthermore, a trend for higher values of excessive daytime somnolence was found in SHE patients, although not statistically different from the control group.Citation58
ADSHE population studies, examining the macrostructure of sleep (ie, the “classical” sleep profile), reported that patients and controls were similar.Citation10,Citation53,Citation60 However, SHE subjects with daytime complaints (ie, sleepiness, fatigue) had an increased sleep instability, as quantified by the analysis of the Cyclic Alternating Pattern (CAP).Citation56,Citation61 Moreover, seizures occurred mostly during unstable sleep,Citation56 suggesting a probable connection between sleep-related motor manifestations, sleep disruption and daytime symptoms.Citation62
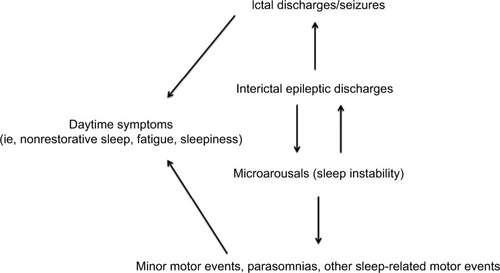
The hypothesis that sleep-related epileptic manifestations in SHE patients could favor a substantial increase of sleep instability, and vice versa, was corroborated by SEEG recordings.Citation63,Citation64 Indeed, these studies suggested that interictal epileptic discharges (IEDs) may act as an internal trigger inducing an increase in the arousal level, which in turn favors the occurrence of motor events that can be epileptic or notCitation65 (). However, from a semiological point of view, minor motor events generated by an epileptic discharge or by a simple arousal are similar,Citation63 thus a diagnosis of SHE based on the recording of only minor events is, to date, impossible. Finally, such a mutual relationship between arousal and epileptic discharges, together with genetic factors, may explain the high incidence of parasomnia and other sleep disorders in SHE patients.Citation66,Citation67
Figure 1 The vicious loop of sleep-related epileptic discharges and microarousals.
SHE and cognitive functions
Sleep deprivation exerts a negative impact on cognitive functions, including vigilance, memory retention, sensory perception and elements of executive function. Moreover, sleep deprivation may impair the functioning of cognitive systems connected to emotional networks.Citation68
From a neuropsychological point of view, the largest series of sporadic and familial cases of SHE does not report gross cognitive deficits. Psychological and cognitive deficits associated with some forms of ADSHE originally reported were considered coincidental and nonsyndromic-specific.Citation69,Citation70 More recently, some studies have put in evidence intellectual disability and psychiatric problems in some ADSHE patients carrying nAChR subunits and KCNT1 genes mutations.Citation71–Citation77 In a large cohort of sporadic and familial SHE patients, Licchetta et al (2017) reported intellectual disabilities or a borderline IQ in about 12% of cases and psychiatric disorders in 24%.Citation34 When present, the cognitive disorder in SHE seems to mainly affect memory functions as well as executive functions,Citation75 irrespective of the treatment status. A recent study conducted in a large cohort of SHE patients, through a neuropsychological standardized assessment, revealed cognitive deficits in at least half of assessed patients and worse verbal IQ scores that might reflect deficits in working memory and phonemic fluency (executive functions).Citation78 Deficits in selected executive, memory and visuospatial functions were found also in a high percentage of subjects without an apparent intellectual disability. Mutated SHE patients, compared to nonmutated ones, irrespective of the specific gene, presented a lower IQ score. Variables of clinical severity (ie, high seizure frequency, status epilepticus and bilateral convulsive seizures, poor response to antiepileptic treatment) were negatively correlated with memory and executive functions.Citation78 The finding of memory deficit may be explained by the direct negative effect of interictal and ictal epileptic activity (originating from both frontal or extrafrontal regions) and by the alterations of sleep-related encoding memory processes induced by sleep fragmentation.Citation79,Citation80
Personal and social aspects
The injury potential of sleep-related seizures seems to be lower than that during wakefulness. This is probably related to the relative safe condition of being in bed.Citation81 In SHE patients, injuries resulting from hyperkinetic seizures can be accidental.Citation82 Nevertheless, epileptic attacks in SHE may be very violent, characterized by hypermotor patterns with bimanual/bipedal activity, ballistic, rocking axial and pelvic movements and sometimes agitated ambulation. Patients can fall from the bed and get injured, and sometimes countermeasures are necessary (for example, sleeping with a mattress on the floor or putting lateral pillows on the bed). In this scenario, it is not uncommon that patients adopt avoidance behaviors in their social and sentimental life.
Ictal aggression is exceptionalCitation83 and generally not intentional. It can take the form of pushing, grasping, hitting, shoving and spitting,Citation84 and may happen if patients react to some environmental stimuli (such as the act of restraining them).Citation85
A study evaluating health-related quality of life in SHE patients compared with the general population showed that SHE constitutes a restriction on health-related quality of life, especially in domains pertaining to the patient’s role in a family and social context and the patient’s illness experience.Citation86
Seizures during wakefulness
To date, a study evaluating the risk of seizures during wake in typical SHE (100% sleep-related seizures) is lacking. Previous studies suggest that almost 30%–40% of SHE patients experience occasional seizures during wakefulnessCitation11,Citation34 that may be similar to sleep-related manifestations or only characterized by isolated subjective symptoms. SHE patients may be exposed to driving license legal restrictions, especially in case of rare focal seizures during wake.Citation87,Citation88
The problem of misdiagnosis
The differential diagnosis between SHE and parasomnias may be challenging even for experts in epileptology and sleep medicine due to possible similarities between the two sleep-related manifestations. Indeed, misdiagnosis is frequent in SHE patients because the absence of typical convulsive seizures and the presence of behavioral patterns similar to those observed in non-rapid eye movement (NREM) parasomnias and rapid eye movement (REM) behavior disorders, at least at the anamnestic evaluation. Video-EEG and video-polysomnography, considered the gold standards for a confirmed diagnosis, may be unavailable or not definitively conclusive. The high prevalence of parasomnias in SHE probands and their healthy relativesCitation11,Citation66 may create a further challenge for the differential diagnosis. Moreover, the response to antiepileptic drugs is not discriminative for the diagnosis of SHE.Citation89 A recent large cohort study reported a diagnostic delay of 12.8±10.1 years in 53.7% of SHE cases, parasomnias being the most frequent misdiagnosis (55.5%).Citation34 The problem of a correct diagnosis is an important factor that may impact over the clinical course of the disease because of inappropriate treatment and management.
The difficulty to discriminate parasomnias from SHE led experts to develop a validated scale, named Frontal Lobe Epilepsy and Parasomnias (FLEP) scale.Citation90 However, some criticism on the FLEP scale emerged, first of all its inability to distinguish nocturnal wandering from sleepwalking.Citation91 The Structured Interview for NFLE/SHE (SINFLE) represents another tool that could improve the physician’s capability to distinguish parasomnias from SHE, with high specificity but unsatisfactory sensitivity.Citation92 It is based on anamnestic information, including the presence or absence of the two major motor seizure patterns (ie, dystonic posturing or hyperkinetic automatisms) and four minor features regarding the duration of the episodes, the presence of a recognized aura, unstructured vocalization and a history of sleep-related convulsive seizures.
Prognostic features
Prognostic studies on SHE have originally included drug-resistant casesCitation33,Citation93 submitted to epilepsy surgery and showed a high percentage of patients (more than 70%) seizure-free after the intervention with a minimum follow-up of 24 months.Citation93 Only a recent reportCitation34 focused on the analysis of the long-term evolution of SHE patients (lesional and nonlesional, sporadic and familial) showing a long-term poor prognosis. Indeed, in this study, the cumulative probability of remission ranged from 20.4% at 10 years to 28.4% at 40 years from onset. The most important favorable factors affecting remission appear to be 1) the absence of any direct or indirect brain disorder (pathologic neurological examination, intellectual disability, perinatal suffering and brain abnormalities, such as focal cortical dysplasia) and 2) typical SHE (ie, 100% sleep-related seizures).Citation34
Drug resistance
Most patients show a good response to the pharmacological treatment, low doses of carbamazepine at bedtime being the first choice of therapy. However, about one-third of patients are drug-resistant.Citation11,Citation34,Citation94 Although the surgical outcome seems to be relatively good in this population, especially in patients with positive brain MRI, the presurgical evaluation and the surgical approach (including risks related to the procedures) may have a negative impact on quality of life.
Sudden unexpected death in epilepsy (SUDEP) incidence in SHE
Most of SUDEP cases occur in bed while patients are probably sleeping. A possible relationship with sleep has been found in about 60% of SUDEP cases (after considering studies including more than ten subjects who died from SUDEP).Citation95 Sleep-related settings (ie, lack of surveillance and sleeping position, especially prone position), generalized tonic–clonic seizures and autonomic changes could account for this relation, increasing SUDEP risk during sleep.Citation95
Despite the fact that occurrence of sleep-related seizures is an important risk factor for SUDEP, a retrospective study showed an incidence of SUDEP in SHE patients similar to that observed in the general epilepsy population (0.36 per 1,000 person-years).Citation96–Citation98 A low occurrence of tonic–clonic seizures in SHE may be responsible for such a lower-than-expected risk of SUDEP.Citation91 Although systematic studies are lacking, it is possible that SHE patients with an insular onset are at higher risk of SUDEP.Citation96,Citation99,Citation100 Indeed, it has been shown that seizures originating from the insula may be accompanied by important autonomic alterations.Citation95
Management strategies
Pharmacological treatment
Although controlled studies are lacking, carbamazepine seems to be the drug of choice in SHE patients.Citation2,Citation11,Citation32 Given at low doses (200–400 mg at bedtime), carbamazepine abolishes or significantly reduces seizures in about 20% and 50% of patients.Citation11 Seizures commonly recur after drug withdrawal.Citation101 Interestingly, experimental observations have found an amplified sensitivity of mutated nAChR receptors to this drug.Citation102
About one-third of SHE patients do not respond to therapyCitation10,Citation11,Citation34 and maintain a very high seizure frequency. Indeed, about 70% of these subjects had more than 25 seizures per month and these numbers may be even higher, considering that many sleep-related seizures are probably underreported.Citation11
Oxcarbazepine (at a mean dose of 30.4±11.7 mg/kg/day) has been shown to be effective in stopping seizures in a study including eight children diagnosed with SHE (ranging from 4 to 16 years of age) and followed up for a minimum of 12 months. Low doses of the drug allowed a rapid control of seizures (within 4 days from drug beginning) in six patients, whereas in two children, higher doses were required.Citation103
In a small series of SHE patients, about 90% of cases benefited from topiramate use as single or add-on therapy (from 50 to 300 mg daily at bedtime); in particular, 25% of patients became seizure-free and 62% had a reduction in seizures of at least 50% during a follow-up period ranging from 6 months to 6 years.Citation104
A study reported a good response to acetazolamide (500 mg at night) as add-on therapy to carbamazepine in three ADSHE family members.Citation105
Recent reports conducted in small series of patients, including genetic cases, suggest lacosamide 200 or 400 mg/day, as a possible add-on treatment or in monotherapy.Citation106,Citation107
Seizure frequency improved in a single patient with refractory ADSHE after nicotine transdermal patches treatment.Citation108 The favorable effect of nicotine on seizure frequency was also described in 9 of 22 patients from two European ADSHE families carrying CHRNA4 mutations.Citation109 Considering the role of the cholinergic system in arousal regulatory processes, these observations suggested a possible link between nicotine defect, alteration of arousal regulation and seizures in SHE/ADSHE patients. However, despite the reported positive effect of nicotine in reducing seizure frequency, a case–control family study, did not find a higher tendency to smoke tobacco in SHE patients and their relatives compared with the control cases.Citation110
Recently, Puligheddu et al have shown that fenofibrate, an agonist at peroxisome proliferator-activated receptor alpha (PPARa) acting as a negative modulator of nAChRs, may have beneficial effects both in mutated mouse models of SHE and pharmacoresistant SHE patients.Citation111 Interestingly, good results with fenofibrate administration have been obtained both in the mutated (CHRNA2 and 4) and nonmutated SHE patients.
Finally, it has been shown that, despite a partial reduction of nocturnal seizures, standard antiepileptic treatment is not particularly effective in reducing sleep instability compared with the pretreatment condition.Citation112
Very recently, a new therapeutic approach with quinidine was tested in a few drug-resistant epileptic patients carrying KCNT1 genetic mutations, none fitting SHE phenotype, with questionable results.Citation113,Citation114
Surgery
Epilepsy surgery provides excellent results in selected drug-resistant SHE cases both for seizures and for epilepsy-related sleep alterations (ie, frequent arousals, sleep deprivation and excessive daytime sleepiness).Citation33,Citation115
Some authors reported that more than two-thirds of patients with SHE were fully controlled after surgery (Engel’s Class Ia), and the remaining ones had a considerable decrease of both frequency and intensity of seizures (Engel’s Class II and III).Citation33,Citation93 Good surgical results are obtained in both frontal and extra-frontal SHE, although in this last group the number of operated-on patients is limited.Citation17,Citation20,Citation93,Citation99 A presurgical evaluation including SEEG recordings may be necessary, especially when brain MRI is uninformative.
An explanation for the excellent surgical results obtained in frontal and extrafrontal SHE seems to be dependent on the high incidence of cases with a histological diagnosis of type II focal cortical dysplasia (Taylor-type), a generally well-limited malformation of cortical development, known to increase the risk of sleep-related seizures independently of its location.Citation21,Citation93,Citation116 Indeed, variables statistically associated with a favorable outcome are a positive MRI, a complete removal of the epileptogenic zone and the presence of focal cortical dysplasia type II.Citation88 Patients submitted to SEEG investigation show a worse outcome as they represent more challenging cases, with an unremarkable MRI or discordant anatomo-electroclinical findings.
Other treatments
Finally, it is known that reducing factors that may promote seizures, such as sleep instability, could lead to more effective treatment strategies. Although specific studies in SHE population are lacking, disorders resulting in sleep disruption such as obstructive sleep apnea, insomnia and parasomnias may be associated with refractory focal epilepsy and have to be investigated and treated specifically.Citation117,Citation118 Hypothetically, considering that seizures in SHE are increased by sleep instability and mostly occur during N2 stage of sleep, a drug able to increase slow-wave sleep production could help in reducing seizure frequency; however, to date, there is no evidence that such a treatment may be effective.
Acknowledgments
We would like to acknowledge all of our collaborators from Niguarda Hospital (Milan) and Bellaria Hospital (Bologna).
Disclosure
The authors report no conflicts of interest in this work.
References
- PedleyTAGuilleminaultCEpisodic nocturnal wanderings responsive to anticonvulsant drug therapyAnn Neurol1977213035900905
- LugaresiECirignottaFHypnogenic paroxysmal dystonia: epileptic seizure or a new syndrome?Sleep1981421291387256073
- LugaresiECirignottaFMontagnaPNocturnal paroxysmal dystoniaJ Neurol Neurosurg Psychiatry19864943753802939199
- TharpBROrbital frontial seizures. An unique electroencephalographic and clinical syndromeEpilepsia19721356276424508104
- WadaJAPurvesSJOral and bimanual-bipedal activity as ictal manifestation of frontal lobe epilepsyEpilepsia198425668
- WadaJANocturnal recurrence of brief, intensely affective vocal and facial expression with powerful bimanual, bipedal, axial, and pelvic activity with rapid recovery as manifestations of mesial frontal lobe seizureEpilepsia198829209
- WilliamsonPDSpencerDDSpencerSSNovellyRAMattsonRHComplex partial seizures of frontal lobe originAnn Neurol19851844975044073842
- WatermanKPurvesSJKosakaBStraussEWadaJAAn epileptic syndrome caused by mesial frontal lobe seizure fociNeurology19873745775823104818
- TinuperPCerulloACirignottaFCortelliPLugaresiEMontagnaPNocturnal paroxysmal dystonia with short-lasting attacks: three cases with evidence for an epileptic frontal lobe origin of seizuresEpilepsia19903155495562401246
- OldaniAZucconiMAsseltaRAutosomal dominant nocturnal frontal lobe epilepsy. A video-polysomnographic and genetic appraisal of 40 patients and delineation of the epileptic syndromeBrain1998121Pt 22052239549500
- ProviniFPlazziGTinuperPVandiSLugaresiEMontagnaPNocturnal frontal lobe epilepsy. A clinical and polygraphic overview of 100 consecutive casesBrain1999122Pt 61017103110356056
- MontagnaPNocturnal paroxysmal dystonia and nocturnal wanderingNeurology1992427 Suppl 661671630641
- NobiliLFrancioneSMaiRNocturnal frontal lobe epilepsy: intracerebral recordings of paroxysmal motor attacks with increasing complexitySleep200326788388614655924
- TinuperPBisulliFCrossJHDefinition and diagnostic criteria of sleep-related hypermotor epilepsyNeurology201686191834184227164717
- NobiliLFrancioneSCardinaleFLo RussoGEpileptic nocturnal wanderings with a temporal lobe origin: a stereo-electroencephalographic studySleep200225666967112224845
- NobiliLCossuMMaiRSleep-related hyperkinetic seizures of temporal lobe originNeurology200462348248514872038
- MaiRSartoriIFrancioneSSleep-related hyperkinetic seizures: always a frontal onset?Neurol Sci200526Suppl 3s220s22416331400
- RyvlinPMinottiLDemarquayGNocturnal hypermotor seizures, suggesting frontal lobe epilepsy, can originate in the insulaEpilepsia200647475576516650142
- KaidoTOtsukiTNakamaHComplex behavioral automatism arising from insular cortexEpilepsy Behav20068131531916356775
- DobesbergerJOrtlerMUnterbergerISuccessful surgical treatment of insular epilepsy with nocturnal hypermotor seizuresEpilepsia200849115916218028409
- ProserpioPCossuMFrancioneSEpileptic motor behaviors during sleep: anatomo-electro-clinical featuresSleep Med201112Suppl 2S33S3822136897
- GibbsSAFigorilliMCasaceliGProserpioPNobiliLSleep Related Hypermotor Seizures with a Right Parietal OnsetJ Clin Sleep Med201511895395525902821
- FisherRSAcevedoCArzimanoglouAA practical clinical definition of epilepsyEpilepsia20145547548224730690
- ThomasRHKingWHJohnstonJASmithPEMAwake seizures after pure sleep-related epilepsy: a systematic review and implications for driving lawJournal of Neurology, Neurosurgery & Psychiatry2010812130135
- DerryCPDuncanSSleep and epilepsyEpilepsy Behav201326339440423465654
- YaqubBAWaheedGKabirajMMNocturnal epilepsies in adultsSeizure1997621451499153728
- FernándezLBSalas-PuigJPure sleep seizures: risk of seizures while awakeEpileptic Disord200791657017307714
- SchefferIAutosomal dominant frontal epilepsy misdiagnosed as sleep disorderThe Lancet19943438896515517
- WeinstockAGiglioPKerrSLDuffnerPKCohenMEHyperkinetic seizures in childrenJ Child Neurol200318851752413677576
- VignatelliLBisulliFGiovanniniGPrevalence of nocturnal frontal lobe epilepsy in the adult population of Bologna and Modena, Emilia-Romagna region, ItalySleep201538347948525406112
- VignatelliLBisulliFGiovanniniGPrevalence of Nocturnal Frontal Lobe Epilepsy in the Adult Population of Bologna and Modena, Emilia-Romagna Region, ItalySleep201538347948525406112
- SchefferIEBhatiaKPLopes-CendesIAutosomal dominant nocturnal frontal lobe epilepsy. A distinctive clinical disorderBrain1995118Pt 161737895015
- NobiliLFrancioneSMaiRSurgical treatment of drug-resistant nocturnal frontal lobe epilepsyBrain2007130Pt 256157317124189
- LicchettaLBisulliFVignatelliLSleep-related hypermotor epilepsy: Long-term outcome in a large cohortNeurology2017881707727881627
- SchefferIEBhatiaKPLopes-CendesIAutosomal dominant frontal epilepsy misdiagnosed as sleep disorderLancet199434388965155177906762
- SteinleinOKMulleyJCProppingPA missense mutation in the neuronal nicotinic acetylcholine receptor alpha 4 subunit is associated with autosomal dominant nocturnal frontal lobe epilepsyNat Genet19951122012037550350
- MariniCGuerriniRThe role of the nicotinic acetylcholine receptors in sleep-related epilepsyBiochem Pharmacol20077481308131417662253
- de FuscoMBecchettiAPatrignaniAThe nicotinic receptor beta 2 subunit is mutant in nocturnal frontal lobe epilepsyNat Genet200026327527611062464
- CombiRFerini-StrambiLMontruccoliATwo new putative susceptibility loci for ADNFLEBrain Res Bull200567425726316182932
- HeronSESmithKRBahloMMissense mutations in the sodium-gated potassium channel gene KCNT1 cause severe autosomal dominant nocturnal frontal lobe epilepsyNat Genet201244111188119023086396
- IshidaSPicardFRudolfGMutations of DEPDC5 cause autosomal dominant focal epilepsiesNat Genet201345555255523542701
- KorenkeGCEggertMThieleHNürnbergPSanderTSteinleinOKNocturnal frontal lobe epilepsy caused by a mutation in the GATOR1 complex gene NPRL3Epilepsia2016573e60e6326786403
- RicosMGHodgsonBLPippucciTMutations in the mammalian target of rapamycin pathway regulators NPRL2 and NPRL3 cause focal epilepsyAnn Neurol201679112013126505888
- Bar-PeledLChantranupongLCherniackADA Tumor suppressor complex with GAP activity for the Rag GTPases that signal amino acid sufficiency to mTORC1Science201334061361100110623723238
- IfflandPHBaybisMBarnesAELeventerRJLockhartPJCrinoPBDEPDC5 and NPRL3 modulate cell size, filopodial outgrowth, and localization of mTOR in neural progenitor cells and neuronsNeurobiol Dis201811418419329481864
- DibbensLMde VriesBDonatelloSMutations in DEPDC5 cause familial focal epilepsy with variable fociNat Genet201345554655123542697
- SimJCScerriTFanjul-FernándezMFamilial cortical dysplasia caused by mutation in the mammalian target of rapamycin regulator NPRL3Ann Neurol201679113213726285051
- SchefferIEHeronSEReganBMMutations in mammalian target of rapamycin regulator DEPDC5 cause focal epilepsy with brain malformationsAnn Neurol201475578278724585383
- ChenZHWangCZhuoMQExome sequencing identified a novel missense mutation c.464G>A (p.G155D) in Ca2+-binding protein 4 (CABP4) in a Chinese pedigree with autosomal dominant nocturnal frontal lobe epilepsyOncotarget2017845789407894729108277
- LiPFuXSmithNALoss of CLOCK Results in Dysfunction of Brain Circuits Underlying Focal EpilepsyNeuron201796238740129024662
- PeledRLaviePParoxysmal awakenings from sleep associated with excessive daytime somnolence: a form of nocturnal epilepsyNeurology198636195983941791
- MaccarioMLustmanLIParoxysmal nocturnal dystonia presenting as excessive daytime somnolenceArch Neurol19904732912942106869
- OldaniAZucconiMFerini-StrambiLBizzozeroDSmirneSAutosomal dominant nocturnal frontal lobe epilepsy: electroclinical pictureEpilepsia199637109649768822695
- OldaniAZucconiMCastronovoCFerini-StrambiLNocturnal frontal lobe epilepsy misdiagnosed as sleep apnea syndromeActa Neurol Scand199898167719696531
- SchwalenSJörgJDay time fatigue in frontal lobe epilepsy with primarily sleep-related seizures. A case reportNervenarzt19986921661709551463
- ZucconiMOldaniASmirneSFerini-StrambiLThe macrostructure and microstructure of sleep in patients with autosomal dominant nocturnal frontal lobe epilepsyJ Clin Neurophysiol2000171778610709813
- Alanis-GuevaraIPeñaECoronaTLópez-AyalaTLópez-MezaELópez-GómezMSleep disturbances, socioeconomic status, and seizure control as main predictors of quality of life in epilepsyEpilepsy Behav20057348148516098815
- VignatelliLBisulliFNaldiIExcessive daytime sleepiness and subjective sleep quality in patients with nocturnal frontal lobe epilepsy: a case-control studyEpilepsia200647Suppl 5737717239111
- RinaldiRVignatelliLD’AlessandroRValidation of symptoms related to excessive daytime sleepinessNeuroepidemiology200120424825611684901
- ZucconiMOldaniAFerini-StrambiLBizzozeroDSmirneSNocturnal Paroxysmal Arousals With Motor Behaviors During Sleep: Frontal Lobe Epilepsy or Parasomnia?Journal of Clinical Neurophysiology19971465135229458058
- ParrinoLFerriRBruniOTerzanoMGCyclic alternating pattern (CAP): the marker of sleep instabilitySleep Med Rev201216274521616693
- ParrinoLHalaszPTassinariCATerzanoMGCAP, epilepsy and motor events during sleep: the unifying role of arousalSleep Med Rev200610426728516809057
- TerzaghiMSartoriIMaiRSleep-related minor motor events in nocturnal frontal lobe epilepsyEpilepsia200748233534117295628
- TerzaghiMSartoriIMaiRCoupling of minor motor events and epileptiform discharges with arousal fluctuations in NFLEEpilepsia200849467067618028403
- GibbsSAProserpioPTerzaghiMSleep-related epileptic behaviors and non-REM-related parasomnias: Insights from stereo-EEGSleep Med Rev20162542026164370
- BisulliFVignatelliLNaldiIIncreased frequency of arousal parasomnias in families with nocturnal frontal lobe epilepsy: a common mechanism?Epilepsia20105191852186020477848
- NobiliLNocturnal frontal lobe epilepsy and non-rapid eye movement sleep parasomnias: differences and similaritiesSleep Med Rev200711425125417628315
- KillgoreWDEffects of sleep deprivation on cognitionProg Brain Res201018510512921075236
- MclellanAPhillipsHARitteyCPhenotypic comparison of two Scottish families with mutations in different genes causing autosomal dominant nocturnal frontal lobe epilepsyEpilepsia200344461361712681012
- KhatamiRNeumannMSchulzHKölmelHWA family with autosomal dominant nocturnal frontal lobe epilepsy and mental retardationJ Neurol1998245128098109840354
- ChoYWMotamediGKLaufenbergIA Korean kindred with autosomal dominant nocturnal frontal lobe epilepsy and mental retardationArch Neurol200360111625163214623738
- ChoYWYiSDLimJGKimDKMotamediGKAutosomal dominant nocturnal frontal lobe epilepsy and mild memory impairment associated with CHRNB2 mutation I312M in the neuronal nicotinic acetylcholine receptorEpilepsy Behav200813236136518534914
- ItoMKobayashiKFujiiTElectroclinical picture of autosomal dominant nocturnal frontal lobe epilepsy in a Japanese familyEpilepsia2000411525810643924
- MagnussonAStordalEBrodtkorbESteinleinOSchizophrenia, psychotic illness and other psychiatric symptoms in families with autosomal dominant nocturnal frontal lobe epilepsy caused by different mutationsPsychiatr Genet2003132919512782965
- WoodAGSalingMMFediMNeuropsychological function in patients with a single gene mutation associated with autosomal dominant nocturnal frontal lobe epilepsyEpilepsy Behav201017453153520189461
- HeronSESmithKRBahloMMissense mutations in the sodium-gated potassium channel gene KCNT1 cause severe autosomal dominant nocturnal frontal lobe epilepsyNat Genet201244111188119023086396
- PicardFPegnaAJArntsbergVNeuropsychological disturbances in frontal lobe epilepsy due to mutated nicotinic receptorsEpilepsy Behav200914235435919059498
- LicchettaLPodaR VProfile of neuropsychological impairment in Sleep-related Hypermotor EpilepsySleep Med20184881529843024
- CentenoMThompsonPJKoeppMJHelmstaedterCDuncanJSMemory in frontal lobe epilepsyEpilepsy Res2010912–312313220800996
- KreutzmannJCHavekesRAbelTMeerloPSleep deprivation and hippocampal vulnerability: changes in neuronal plasticity, neurogenesis and cognitive functionNeuroscience201530917319025937398
- WirrellECEpilepsy-related injuriesEpilepsia200647Suppl 17986
- SchenckCHMilnerDMHurwitzTDBundlieSRMahowaldMWA polysomnographic and clinical report on sleep-related injury in 100 adult patientsAm J Psychiatry19891469116611732764174
- TassinariCATassiLCalandra-BuonauraGBiting behavior, aggression, and seizuresEpilepsia200546565466315857430
- SiclariFKhatamiRUrbaniokFViolence in sleepBrain2010133Pt 123494350921126993
- Delgado-EscuetaAVMattsonRHKingLSpecial report. The nature of aggression during epileptic seizuresN Engl J Med1981305127117167266614
- LicchettaLVignatelliLBisulliFHealth-related quality of life in patients with nocturnal focal epilepsyBoll Lega It Epil2006133/134299300
- ThomasRHKingWHJohnstonJASmithPEAwake seizures after pure sleep-related epilepsy: a systematic review and implications for driving lawJ Neurol Neurosurg Psychiatry201081213013520145025
- BeghiESanderJWEpilepsy and drivingBMJ20053317508606116002855
- TinuperPBisulliFFrom nocturnal frontal lobe epilepsy to Sleep-Related Hypermotor Epilepsy: A 35-year diagnostic challengeSeizure201744879228027860
- DerryCPDaveyMJohnsMDistinguishing sleep disorders from seizures: diagnosing bumps in the nightArch of Neurol20066370570916682539
- ManniRTerzaghiMRepettoAThe FLEP scale in diagnosing nocturnal frontal lobe epilepsy, NREM and REM parasomnias: data from a tertiary sleep and epilepsy unitEpilepsia20084991581158518410366
- BisulliFVignatelliLNaldiIDiagnostic accuracy of a structured interview for nocturnal frontal lobe epilepsy (SINFLE): a proposal for developing diagnostic criteriaSleep Med2012131818722137114
- LosurdoAProserpioPCardinaleFDrug-resistant focal sleep related epilepsy: results and predictors of surgical outcomeEpilepsy Res2014108595396224679947
- HirschESellalFMatonBRumbachLMarescauxCNocturnal paroxysmal dystonia: a clinical form of focal epilepsyNeurophysiol Clin19942432072178090154
- NobiliLProserpioPRubboliGMontanoNDidatoGTassinariCASudden unexpected death in epilepsy (SUDEP) and sleepSleep Med Rev201115423724620951616
- MostacciBBisulliFVignatelliLIncidence of sudden unexpected death in nocturnal frontal lobe epilepsy: a cohort studySleep Med201516223223625600783
- MostacciBBisulliFVignatelliLIncidence of sudden unexpected death in epilepsy in sleep-related hypermotor epilepsy, formerly named nocturnal frontal lobe epilepsySleep Med2017299827915205
- LambertsRJThijsRDLaffanALanganYSanderJWSudden unexpected death in epilepsy: people with nocturnal seizures may be at highest riskEpilepsia201253225325722192074
- ProserpioPCossuMFrancioneSInsular-opercular seizures manifesting with sleep-related paroxysmal motor behaviors: a stereo-EEG studyEpilepsia201152101781179121883183
- RyvlinPAvoid falling into the depths of the insular trapEpileptic Disord20068Suppl 2S375617012071
- ProviniFPlazziGLugaresiEFrom nocturnal paroxysmal dystonia to nocturnal frontal lobe epilepsyClin Neurophysiol2000111Suppl 2S2S810996549
- PicardFBertrandSSteinleinOKBertrandDMutated nicotinic receptors responsible for autosomal dominant nocturnal frontal lobe epilepsy are more sensitive to carbamazepineEpilepsia19994091198120910487182
- RajuGPSarcoDPPoduriARivielloJJBerginAMTakeokaMOxcarbazepine in children with nocturnal frontal-lobe epilepsyPediatr Neurol200737534534917950420
- OldaniAManconiMZucconiMMartinelliCFerini-StrambiLTopiramate treatment for nocturnal frontal lobe epilepsySeizure200615864965216973383
- VaradkarSDuncanJSCrossJHAcetazolamide and autosomal dominant nocturnal frontal lobe epilepsyEpilepsia200344798698712823586
- LiguoriCRomigiAPlacidiFSarpaMGMercuriNBIzziFEffective treatment of nocturnal frontal lobe epilepsy with lacosamide: a report of two casesSleep Med20162312112226922622
- SamarasekeraSRBerkovicSFSchefferIEA case series of lacosamide as adjunctive therapy in refractory sleep-related hypermotor epilepsy (previously nocturnal frontal lobe epilepsy)J Sleep Res2018e12669e1266929479765
- WilloughbyJOPopeKJEatonVNicotine as an antiepileptic agent in ADNFLE: an N-of-one studyEpilepsia20034491238124012919397
- BrodtkorbEPicardFTobacco habits modulate autosomal dominant nocturnal frontal lobe epilepsyEpilepsy Behav20069351552016931165
- NaldiIBisulliFVignatelliLTobacco habits in nocturnal frontal lobe epilepsyEpilepsy Behav201326111411723246147
- PulighedduMMelisMPillollaGRationale for an adjunctive therapy with fenofibrate in pharmacoresistant nocturnal frontal lobe epilepsyEpilepsia201758101762177028766701
- de PaolisFColizziEMilioliGEffects of antiepileptic treatment on sleep and seizures in nocturnal frontal lobe epilepsySleep Med201314759760423746822
- MikatiMAJiangYHCarboniMQuinidine in the treatment of KCNT1-positive epilepsiesAnn Neurol201578699599926369628
- AbdelnourEGallentineWMcdonaldMSachdevMJiangYHMikatiMADoes age affect response to quinidine in patients with KCNT1 mutations? Report of three new cases and review of the literatureSeizure2018551329291456
- NobiliLSartoriITerzaghiMRelationship of epileptic discharges to arousal instability and periodic leg movements in a case of nocturnal frontal lobe epilepsy: a stereo-EEG studySleep200629570170416774161
- NobiliLCardinaleFMagliolaUTaylor’s focal cortical dysplasia increases the risk of sleep-related epilepsyEpilepsia200950122599260419519797
- ManniRTerzaghiMComorbidity between epilepsy and sleep disordersEpilepsy Res201090317117720570109
- MalowBAFoldvary-SchaeferNVaughnBVTreating obstructive sleep apnea in adults with epilepsy: a randomized pilot trialNeurology200871857257718711110
