Abstract
Objectives
Detecting sleep latency during the Multiple Sleep Latency Test (MSLT) using electroencephalogram (scalp-EEG) is time-consuming. The aim of this study was to evaluate the efficacy of a novel in-ear sensor (in-ear EEG) to detect the sleep latency, compared to scalp-EEG, during MSLT in healthy adults, with and without sleep restriction.
Methods
We recruited 25 healthy adults (28.5±5.3 years) who participated in two MSLTs with simultaneous recording of scalp and in-ear EEG. Each test followed a randomly assigned sleep restriction (≤5 hours sleep) or usual night sleep (≥7 hours sleep). Reaction time and Stroop test were used to assess the functional impact of the sleep restriction. The EEGs were scored blind to the mode of measurement and study conditions, using American Academy of Sleep Medicine 2012 criteria. The Agreement between the scalp and in-ear EEG was assessed using Bland-Altman analysis.
Results
Technically acceptable data were obtained from 23 adults during 69 out of 92 naps in the sleep restriction condition and 25 adults during 85 out of 100 naps in the usual night sleep. Meaningful sleep restrictions were confirmed by an increase in the reaction time (mean ± SD: 238±30 ms vs 228±27 ms; P=0.045). In the sleep restriction condition, the in-ear EEG exhibited a sensitivity of 0.93 and specificity of 0.80 for detecting sleep latency, with a substantial agreement (κ=0.71), whereas after the usual night’s sleep, the in-ear EEG exhibited a sensitivity of 0.91 and specificity of 0.89, again with a substantial agreement (κ=0.79).
Conclusion
The in-ear sensor was able to detect reduced sleep latency following sleep restriction, which was sufficient to impair both the reaction time and cognitive function. Substantial agreement was observed between the scalp and in-ear EEG when measuring sleep latency. This new in-ear EEG technology is shown to have a significant value as a convenient measure for sleep latency.
Introduction
Excessive daytime sleepiness (EDS) is a common problem affecting ~12% of the general population.Citation1–Citation3 The causes of EDS include both primary sleep disorders and secondary medical conditions that disrupt sleep, eg, those causing pain. In addition, poor sleep habits, whether chosen by the patient or imposed by occupational circumstances, can cause EDS. The consequences of EDS also vary widely, ranging from underperformance at school in the young population, to fatal road traffic accidents (RTAs) in adults. A recent Cochrane Review suggested that EDS is associated with school absenteeism and poor academic performance.Citation4 Among drivers, EDS is thought to be a contributing factor in 5%–7% of all RTAs.Citation5,Citation6 For example, in the USA only, RTAs due to sleep or drowsiness while driving was reported to result in 8,952 deaths and 220,000 serious injuries between 2004 and 2013.Citation7 Similar underperformance and accidents issues arise among other occupations too. Reis et alCitation8 have shown that the prevalence of EDS among pilots was around 60%Citation8 and Cotrim et alCitation9 found that EDS was highly prevalent among train drivers, peaking at 72% during night shifts.
Currently, evaluation of EDS in real-world situations is limited to subjective measures of sleepiness, with objective tests, such as the Multiple Sleep Latency Test (MSLT) and the Maintenance of Wakefulness Test (MWT), mainly being undertaken in a sleep laboratory.Citation10–Citation12 In both these tests, the electroencephalography (EEG) recorded from the scalp (scalp-EEG) is used to detect sleep latency, a standard measure of sleepiness. They are the standard diagnostic tests for patients with hypersomnia of central origins such as narcolepsy. However, neither the MSLT nor the MWT has achieved universal acceptance because they require patients to stay in the laboratory for the whole day; this renders the investigation inconvenient, relatively costly and not representative of real-world situations. A common subjective measure of sleepiness is the Epworth Sleepiness Scale (ESS)Citation13 which has been used extensively in many countries.Citation14–Citation26 However, ESS is known to be poorly correlated with objective measures of sleepinessCitation27 and functional outcomes.Citation28 Therefore, the development of a robust, non-invasive, wearable EEG-based method for detecting sleep latency could be of significant value to facilitate the measurement and understanding of EDS.
Our group have previously reported a wearable sensor that measures EEG from the external auditory meatus (in-ear EEG).Citation29,Citation30 In preliminary studies, we have shown that the in-ear EEG can be used to detect slow wave sleep during short sleep studies (<1 hour) in four healthy adults.Citation31 The aim of the present study was to evaluate the efficacy of the in-ear EEG to detect sleep latency during MSLT in healthy adults. We have tested the hypothesis that the in-ear EEG was not inferior to the scalp-EEG to detect sleep latency during MSLT. For robustness, we have used the intervention of voluntary sleep restriction and confirmed that this was a stimulus of appropriate magnitude by measuring reaction time and cognitive impairment associated with our sleep restriction intervention.
Methods
Participants
We recruited 25 (8 female) healthy adults, who were identified using advertisements posted on campus at Imperial College London, UK; the majority of participants were students or employees. Participants were excluded from the study if they reported any sleep disorders or neurological conditions, the use of sleep medications, reported shift working, a long-haul flight in the past 2 weeks, a hearing impairment or ear infection. Participants gave written informed consent and the study was approved by Imperial College London Research Ethics Committee (ICREC_12_1_1).
Screening
Communication with potential participants was conducted initially through emails and then by face-to-face interviews, in order to ensure that the inclusion and exclusion criteria were met and to familiarize the participants with the in-ear EEG sensor. If participants met the criteria and agreed to participate, they were assigned dates for the study (two day-time laboratory visits). The order of the studies (sleep restriction and usual night sleep) was randomized.
Protocol
A prospective, randomized crossover design was used, where the participants were asked to come to the Imperial College signal processing EEG sleep laboratory either after a single night of sleep restriction (≤5 hours) or usual night sleep (≥7 hours). Adherence to the study protocol on the night before the MSLT was confirmed by asking participants to log-in to a webpage every hour from 18:00 until the bed time and again when they first woke-up. Participants were asked to refrain from having caffeine or any stimulants on the day of the study.
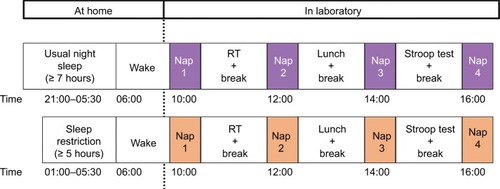
The MSLT protocol is shown in . Participants arrived at the lab at 09:30 and the first nap (nap 1) started at 10:00; naps then occurred every 2 hours and the fourth nap (nap 4) was finished by 17:00.Citation32,Citation33 The setup time was ~30 minutes for attachment of both the scalp and in-ear EEG. The impedance was checked before each nap to maintain the quality of the signals. The scalp EEG electrodes were firmly attached throughout the study. The in-ear EEG sensor was also attached throughout the study unless the participant requested to remove it in between naps. If the in-ear sensor was removed in between naps, the sensor was then reinserted before each nap and impedance was checked again. The scalp-EEG electrodes were placed at the C3 and C4 positions according to the 10–20 montage, with the ground electrode placed on the forehead. The electrooculogram (right/left EOG) and electromyogram (chin EMG) were not recorded.
Figure 1 The MSLT protocol.
Abbreviation: MSLT, Multiple Sleep Latency Test; RT, reaction time.
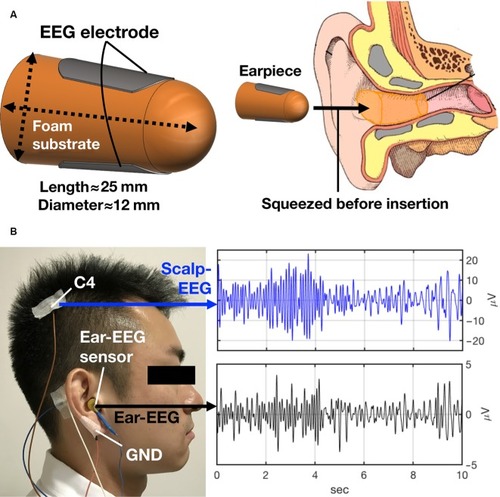
Full details of the in-ear EEG sensor are given in our previous publication.Citation30,Citation31 In brief, the in-ear EEG sensor is of ~25 mm length and 12 mm diameter. The two EEG elec trodes are embedded onto the sensor diametrically opposite one another (). The flexible viscoelastic sensor was inserted into the external auditory meatus by squeezing it to fit into the ear canal. The left or right ear was used for placement, according to participants’ preference. The reference and ground electrodes for the in-ear EEG were placed on the helix and earlobe, respectively, using gold cup electrodes (Bio-medical, Clinton Township, MI, USA). An example of a participant wearing scalp and in-ear EEG and corresponding signals is shown in . Prior to the application of gold cup electrodes, skin was prepared using abrasive gel (Nuprep, Bio-medical). Earwax was removed from the ear using ear cotton buds, and Signa Gel was applied on the in-ear EEG sensor before inserting it into the ear (Parker Laboratories, Inc, Fairfield, NJ, USA). Scalp and in-ear EEG were recorded simultaneously using g-USBamp, a 24-bit biosignal amplifier (g.tec medical engineering GmbH, Schiedlberg, Austria). Data were acquired with a sampling rate of 1.2 kS/s. The impedance was checked before the start of the recording for both the in-ear and scalp EEG, and we aimed to keep these below 10 kΩ. We did not amplify the signals. A fourth-order Butterworth bandpass filter with passband 3–14 Hz was applied to both scalp and in-ear EEG.
Figure 2 The in-ear EEG sensor.
Abbreviations: EEG, electroencephalography; GND, ground.
Once the EEG electrodes were placed, participants were asked to sleep on a comfortable bed in a quiet and dark room following standardized instructions.Citation32,Citation33 Each nap was of 20 minutes duration, and if the participant slept they were allowed to continue for the duration of the nap.
Measurement of functional outcomes of sleep restriction
After nap 1, participants performed a Multiple Unprepared Reaction Time using the Oxford Sleep Resistance Test (Stowood Scientific, Oxford, UK).Citation34 After nap 3, participants performed a Stroop test.Citation35 Results were recorded in the form of the number of mistakes and the duration taken to complete the task.
Data analysis
Data were analyzed blinded to the order allocation (sleep restriction or usual night sleep) and the mode of measurement (scalp-EEG or in-ear EEG). The EEG scoring was performed by a single scorer according to the American Academy of Sleep Medicine criteria, 2012 version 2.0.Citation36 Sleep latency was defined as the start of the first epoch scored at any stage other than stage wake. This included the attenuation of the alpha rhythm (8–13 Hz) of more than 50% of an epoch and replaced by a low-amplitude, mixed-frequency activity (4–7 Hz). For participants who did not generate alpha activity, sleep latency was defined as first appearance of sleep spindle and/or K-complex. Epochs were labelled as “unable to analyze” if >50% of the EEG signal (scalp or in-ear) showed artifact that prevented the scorer from clearly distinguishing the stage or if the electrodes did not record data due to technical problems. Naps were then removed from the analysis if over 50% of the nap could not be scored. When the scoring was completed, the data sets were un-blinded, to enable statistical analysis.
Sample size and statistical analysis
The sample size was calculated using the null hypothesis that the in-ear EEG was not inferior to the scalp-EEG, with a non-inferiority margin of 3 minutes and population variance of 4 minutes. The margin of 3 minutes was selected, as sleep latency has previously been reported to be reduced by 3 minutes following sleep restriction.Citation37 For the 5% significance level and 80% power we estimated that 25 participants were required for this study.
The primary outcome of this study was the sleep latency measured from the in-ear EEG compared to the scalp-EEG, with and without sleep restriction. Data were evaluated by the mean and SD or number and percent (%) for categorical variables. The sleep latency measured from scalp and in-ear EEG was compared using either a paired t-test or Wilcoxon-signed rank sum test, as appropriate to the distribution of the data. Any nap that did not have a “pair” (either scalp or in-ear EEG) was deleted from the comparison (pair-wise deletion). Given the non-inferiority nature of this study, a CI for the mean difference was constructed using the non-inferiority margin of 3 minutes, in order to assess whether indeed in-ear EEG was not inferior to the scalp-EEG. To evaluate the sensitivity and specificity of in-ear EEG against the scalp-EEG, the sleep latency score was categorized into “positive” and “negative” for both devices and that categorization was used to assess the sensitivity and specificity. Agreement between scalp and in-ear EEG in detecting sleep latency was performed using the Bland-Altman analyses.Citation38 The limits of agreement were defined as mean±1.96 SD. All statistical tests other than the non-inferiority test were two-sided and the statistical significance was set at P<0.05. The IBM SPSS 23.0 software and Excel 2013 were used for statistical analysis, whereas GraphPad Prism 7 and Matlab 2016b (MathWorks, Inc) were used for generating the figures.
Results
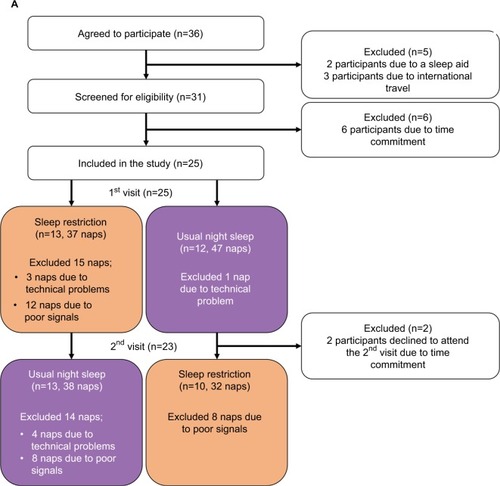
We screened 36 healthy adults of which 25 (8 female) were included in the study; details of recruitment are shown in the consort diagram (). The mean age of the participants was 28.5±5.3 years. By design, since we sought healthy adults, the mean ESS score prior to the study was normal (6±3).
Figure 3 Consort flowchart.
Comparison of in-ear EEG vs scalp-EEG
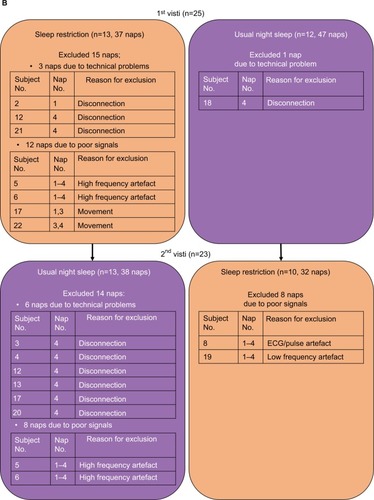
Technically acceptable data were obtained from 23 adults during 69/92 naps in the sleep restriction condition and 25 adults during 85/100 naps in the usual night sleep. Naps were excluded from the analysis either because signals could not be scored (28 naps) or due to technical problems (10 naps). Specific details regarding which naps were excluded are given in . Unscoreable signals included: 1) high frequency artifacts (16 naps, all of which came from the same participants, n=2 [4 naps×2 conditions×2 participants=16 naps]); 2) electrocardiogram (ECG)/pulse artifacts (4 naps); 3) low-frequency artifacts consistent with respiratory frequency (four naps); 4) movement (4 naps). The majority of naps excluded due to technical problems were from nap 4 in the usual night sleep condition. The group mean comparison between scalp-EEG and in-ear EEG in detecting sleep latency is shown in .
Table 1 Comparison between in-ear-EEG and scalp-EEG in detecting sleep latencyTable Footnoteb
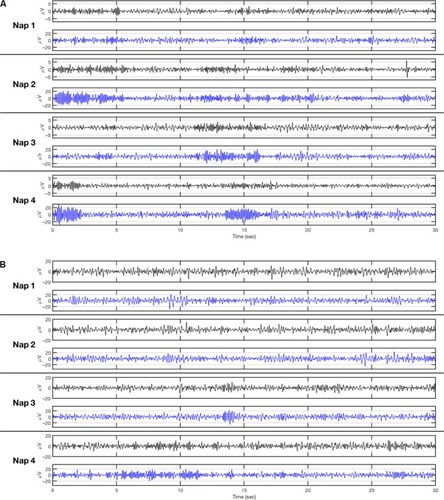
The majority of our participants (22 out of 25) wore the in-ear sensor throughout the MSLT, including hours between naps, and only one participant commented negatively about the comfort of the in-ear EEG sensor. Individual examples of scalp and in-ear EEG during four naps for a participant who kept the in-ear sensor throughout the test, and a participant who removed the in-ear sensor between naps, are shown in , respectively.
Figure 4 Examples of signals from scalp-EEG and in-ear EEG.
Abbreviation: EEG, electroencephalogram.
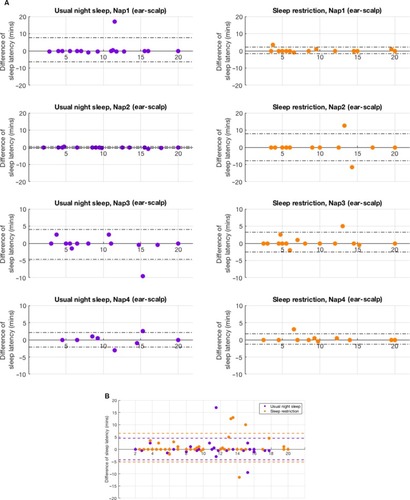
The difference between scalp and in-ear EEG in detecting sleep latency was <3 minutes in 84% (58 out of 69) following sleep restriction and 94% (80 out of 85) of participants after usual night sleep. In the sleep restriction condition, the in-ear EEG exhibited a sensitivity of 0.93 and specificity of 0.80 for detecting sleep latency, with a substantial agreement (κ=0.71), while after the usual night sleep, the in-ear EEG exhibited a sensitivity of 0.91 and specificity of 0.89, again with a substantial agreement (κ=0.79), compared to scalp-EEG. Bland-Altman analysis () revealed good agreement between the two methods (in-ear EEG minus scalp-EEG) for all naps combined ().
Figure 5 Bland-Altman agreement for scalp-EEG vs in-ear EEG.
Abbreviation: EEG, electroencephalogram.
Changes in sleep latency, reaction time and cognitive function following sleep restriction
The mean sleep latency decreased significantly after sleep restriction compared to after usual night sleep in scalp-EEG (10.4±4.8 minutes, 12.4±5.2 minutes; P=0.014) and in-ear EEG (10.9±5.1 minutes, 12.7±4.9 minutes; P=0.019).
Participants fell asleep in 83% (57 out of 69) of naps after sleep restriction and in 64% (55 out of 85) of naps after usual night sleep. Comparison of individual naps showed that nap 3 and nap 4 exhibited greater differences between sleep restriction and usual night sleep (P=0.004, P=0.005) compared with nap 1 and 2 (P=0.115, P=0.792).
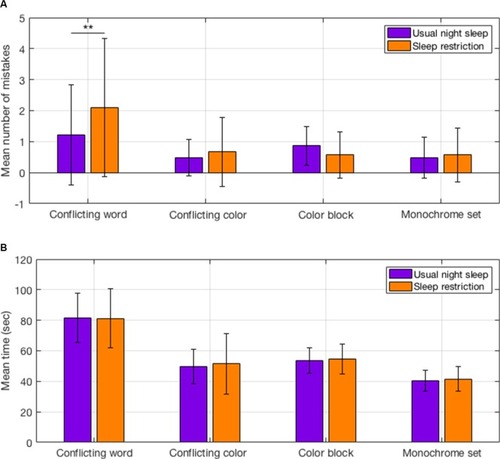
There was a modest but statistically significant increase in reaction time after sleep restriction compared with usual night sleep (238.4±30.4 ms, 228.5±27.1 ms; P=0.045). When completing the Stroop test, the number of mistakes in the conflicting word task increased significantly after sleep restriction compared to after usual night sleep (2±2, 1±1, P=0.003). No significant increases were observed in the conflicting color, color block, or monochrome tasks (). The duration taken to complete each task increased for the conflicting color, color block, and monochrome tasks after sleep restriction, but this was not statistically significant ().
Figure 6 Stroop test after sleep restriction and after usual night sleep.
Discussion
The main finding of this study was that the in-ear EEG sensor was able to detect the sleep latency with substantial agreement, compared to the scalp-EEG. As a consequence, the in-ear EEG sensor was also able to detect the shortened sleep latency, induced by sleep restriction, which was associated with measurable functional impairment, as indicated by the reduced reaction time, and cognitive function.
Critique of the method
The participants in this study were all healthy adults. The performance of the in-ear EEG in patients with sleep disorders is yet to be examined, therefore at the moment we cannot comment on the applicability of the current data, compared to patients with sleep disorders. Likewise, discussion applies to older people with more fragmented and less well-defined sleep architecture.
Another limitation of our study was that an overnight polysomnography was not carried out prior to the MSLT, defined by the standard protocol.Citation32 Therefore, the sleep restriction was not objectively documented. It could have been that the participants slept in between logging into the website during the sleep restriction and/or may have stayed awake during the usual night sleep duration. However, the reaction time and cognitive ability measured by Stroop test were significantly impaired following the sleep restriction, suggesting that our participants adhered to the protocol. Previous studies, utilizing acute sleep restriction ≤5 hours, have found a decreased sleep latency of 2 minutes.Citation39 The sleep restriction in our study also produced a reduction in sleep latency of 2 minutes. In addition, reaction time was increased, and cognitive ability was impaired.Citation40 Our finding that the number of errors on the conflicting word task increased following sleep restriction is also consistent with previous studies.Citation41 In our study, Stroop test was performed after nap 3, which enabled us to test the maximum impact of the decline in cognitive abilities as this seems to deteriorate as cumulative time awake increased.Citation42 Previous studies also showed that the time of the day (morning vs afternoon) impacted the cognitive abilities after sleep restrictions due to the circadian dip.Citation43
The current study extends our previous findings that the in-ear sensor can detect non-REM (N3) stage,Citation31 but it does not extend to REM sleep, indeed we are not claiming the applicability of the sensor in the evaluation or diagnosis of specific sleep stages beyond sleep latency or sleep disorders.
The in-ear EEG
In this study, the in-ear sensor was made from viscoelastic material, to enable expansion to fit tightly in the ear canal.Citation30 The size of the in-ear sensor was also the same for all participants, even though the size of the ear canal may be different in some participants; so it could be that the in-ear EEG sensor did not fit properly with the ear canal. Therefore, naps were removed from the analysis due to poor signal quality from the in-ear EEG; specifically ECG artifacts, high frequency artifacts, low-frequency artifacts, or movements artifacts may observed as a consequence of the conduction problem between the skin and the in-ear sensor. A potential development could be to make the in-ear EEG sensor available in different sizes, and this could increase the retention of the in-ear sensor. This may be important when studying females in whom the average ear diameter is smaller (8.5±0.7 mm) compared to males (9.7±1.1 mm), and when studying different ethnicities, as European people typically have a larger ear canal diameter compared to those of Asian descent.Citation44
In our study, the in-ear EEG analysis was carried out offline; capturing and analyzing data in real time requires an automated algorithm. The development of such algorithms has proved elusive for polysomnography, although this might be easier for the in-ear EEG since the interference of the EEG recorded from the ear canal is minimal.Citation45–Citation47
Recording the EEG from within the ear has been investigated by different research groups.Citation48,Citation49 However, there are distinct differences between our in-ear EEG device and other devices. Nguyen et alCitation48 have used an off-the-shelf foam-based earplug that can be inserted in both ears simultaneously. One of the ear pieces has two passive electrodes, and the reference and ground electrodes are placed on the other ear piece. The two earpieces are connected with a wire cable. The device that was used by Mikkelsen et alCitation49 is a custom-made hard-shell in-ear EEG. The ear piece has six electrodes in total, all embedded in the same earpiece and referenced to a passive electrode placed next to the scalp Cz electrode. Therefore, our device is different in several ways. It is made from a viscoelastic earplug based on “one-size-fits-all,” and used only in one ear (left or right). Additionally, the reference and ground electrodes of our in-ear EEG device were placed on the helix and earlobe, respectively, using gold cup electrodes.
Significance of the findings
The in-ear EEG sensor has at least two characteristics that make it convenient to use. First, the memory foam mounting enables the sensor to be fitted comfortably and safely inside the ear after squeezing it from all sides. It also allows the sensor to expand evenly so it remains in place. Secondly, the sensor incorporates a conductive cloth cover, which is flexible and soft to make the sensor comfortable to wear. While the present data confirm that the in-ear EEG can identify sleep latency accurately enough to detect the short sleep latency elicited by sleep restriction in healthy adults, further development is needed to understand how this can become a useful tool to capture sleepiness in the real world.Citation50
In our study, the majority of our participants wore the in-ear EEG sensor throughout the MSLT, including the hours between naps. The quality of the EEG signals between a participant who wore the sensor throughout the MSLT and a participant who remove the sensor were almost the same (). These figures demonstrate that the transition from alpha activity to theta activity in scalp-EEG is very similar to the in-ear EEG. Therefore the quality of the signals does not change over time and this suggests that the in-ear EEG sensor is applicable for long-term recordings, such as an overnight recording. The investigation of the applicability of the in-ear EEG overnight is currently underway.
A logical development of our study could be to develop a real-world MSLT; however, unless functionally significant data could be obtained from less than four naps the procedure would still be time-consuming for the participant, even if less so for the investigator. Sunwoo et alCitation51 have shown that compared to four naps, single nap from MSLT can detect reasonably well individuals with sleep latency of less than 8 minutes.
Another potential use for our sensor might be domiciliary measurement of sleep latency. Although not easily done for practical reasons it could be that, eg, sleep latency measured at home on several consecutive nights might lead to a better understanding of idiopathic hypersomnia, which is currently typically monitored by actigraphy.Citation52 A further potential development for the in-ear EEG sensor would be in real-time detection of sleep, eg, a system to detect sleepiness during driving. This would entail development of an automated scoring algorithm. Nakamura et alCitation53 have developed an algorithm to classify sleep stages with 90% agreement, compared to conventional scoring. In this context, a strength of the in-ear EEG is that it allows “free movement” and is not visibly obtrusive; it may be possible to modify its use in environments where polysomnography is not feasible.
Finally, although not tested in this study, our findings suggest that in-ear EEG could also be used to simplify MSLT/MWT. This could be useful for some high-risk occupations where remaining alert is a requirement. For example, military pilots with hypersomnia in the USA are advised, by the USA Air Force, to be tested for alertness using MWT.Citation54 Similarly, Australia recommended MWT to assess the fitness for driving in patients with sleep disorders.Citation55 One could imagine a scenario where a modified simple MSLT/MWT was used to screen employees in high-risk occupations before starting their shifts.
Conclusion
Our findings have shown that sleep latency can be detected by the in-ear EEG, during MSLT with and without sleep restriction, with substantial agreement compared to the gold standard scalp-EEG. The majority of participants in our study found the in-ear EEG sensor comfortable and were able to keep it in situ all day. Also, our data showed that the sleep restriction was sufficient to shorten the sleep latency, with a decline on cognitive ability elicited by sleep restriction. We believe that the in-ear EEG will provide new opportunities in clinical and occupational medicine to monitor real world sleep.
Acknowledgments
The authors wish to thank all of the participants who contributed to this study.
Disclosure
The authors report no conflicts of interest in this work.
References
- JaussentIBouyerJAncelinMLExcessive sleepiness is predictive of cognitive decline in the elderlySleep20123591201120722942498
- PallesenSNordhusIHOmvikSSivertsenBTellGSBjorvatnBPrevalence and risk factors of subjective sleepiness in the general adult populationSleep200730561962417552377
- KimHYoungTSubjective daytime sleepiness: dimensions and correlates in the general populationSleep200528562563416171277
- MarxRTanner-SmithEEDavisonCMLater school start times for supporting the education, health, and well-being of high school studentsCochrane Database Syst Rev20177CD00946728670711
- McnicholasWTRodensteinDSleep apnoea and driving risk: the need for regulationEur Respir Rev20152413860260626621974
- TefftBCPrevalence of motor vehicle crashes involving drowsy drivers, United States, 1999–2008Accid Anal Prev20124518018622269499
- BurksSVAndersonJEBombykMNonadherence with employer-mandated sleep apnea treatment and increased risk of serious truck crashesSleep201639596797527070139
- ReisCMestreCCanhãoHGradwellDPaivaTSleep complaints and fatigue of airline pilotsSleep Sci201692737727656269
- CotrimTCarvalhaisJNetoCTelesJNoriegaPRebeloFDeterminants of sleepiness at work among railway control workersAppl Ergon20175829330027633225
- RichardsonGSCarskadonMAFlaggWvan den HoedJDementWCMitlerMMExcessive daytime sleepiness in man: multiple sleep latency measurement in narcoleptic and control subjectsElectroencephalogr Clin Neurophysiol197845562162781764
- JohnsMWSensitivity and specificity of the multiple sleep latency test (MSLT), the maintenance of wakefulness test and the Epworth Sleepiness Scale: failure of the MSLT as a gold standardJ Sleep Res20009151110733683
- MitlerMMGujavartyKSBrowmanCPMaintenance of wakefulness test: a polysomnographic technique for evaluation treatment efficacy in patients with excessive somnolenceElectroencephalogr Clin Neurophysiol19825366586616177511
- JohnsMWA new method for measuring daytime sleepiness: the Epworth Sleepiness ScaleSleep19911465405451798888
- ChenNHJohnsMWLiHYValidation of a Chinese version of the Epworth sleepiness scaleQual Life Res200211881782112482165
- IzciBArdicSFiratHSahinAAltinorsMKaracanIReliability and validity studies of the Turkish version of the Epworth Sleepiness ScaleSleep Breath200812216116817922157
- BlochKESchochODZhangJNRussiEWGerman version of the Epworth Sleepiness ScaleRespiration199966544044710516541
- TsaraVSerasliEAmfilochiouAConstantinidisTChristakiPGreek version of the Epworth Sleepiness ScaleSleep Breath200482919515211393
- BertolaziANFagondesSCHoffLSPedroVDMenna BarretoSSJohnsMWPortuguese-language version of the Epworth sleepiness scale: validation for use in BrazilJ Bras Pneumol200935987788319820814
- BeiskeKKKjelsbergFNRuudEAStavemKReliability and validity of a Norwegian version of the Epworth sleepiness scaleSleep Breath2009131657218560916
- Rosales-MayorERey de CastroJHuayanayLZagacetaKValidation and modification of the Epworth Sleepiness Scale in Peruvian populationSleep Breath2012161596921279696
- VignatelliLPlazziGItalian version of the Epworth Sleepiness Scale: external validityNeurol Sci200323629530012624716
- BanhiranWAssanasenPNopmaneejumruslersCMetheetrairutCEpworth sleepiness scale in obstructive sleep disordered breathing: the reliability and validity of the Thai versionSleep Breath201115357157720835769
- KopitovicITrajanovicNProdicSThe Serbian version of the Epworth Sleepiness ScaleSleep Breath201115477578021053085
- ChoYWLeeJHSonHKLeeSHShinCJohnsMWThe reliability and validity of the Korean version of the Epworth sleepiness scaleSleep Breath201115337738420358406
- PecoticRDodigIPValicMIvkovicNDogasZThe evaluation of the Croatian version of the Epworth sleepiness scale and STOP questionnaire as screening tools for obstructive sleep apnea syndromeSleep Breath201216379380221874368
- AhmedAEFataniAAl-HarbiAValidation of the Arabic version of the Epworth Sleepiness ScaleJ Epidemiol Glob Health20144429730225455647
- ChervinRDAldrichMSThe Epworth Sleepiness Scale may not reflect objective measures of sleepiness or sleep apneaNeurology19995211251259921859
- WallerKLMortensenELAvlundKSubjective sleep quality and daytime sleepiness in late midlife and their association with age-related changes in cognitionSleep Med20161716517326188599
- LooneyDKidmosePParkCThe in-the-ear recording concept: user-centered and wearable brain monitoringIEEE Pulse201236324223247157
- GoverdovskyVLooneyDKidmosePMandicDPIn-ear EEG from viscoelastic generic earpieces: robust and unobtrusive 24/7 monitoringIEEE Sens J2016161271277
- LooneyDGoverdovskyVRosenzweigIMorrellMJMandicDPWearable in-ear encephalography sensor for monitoring sleep. Preliminary observations from Nap studiesAnn Am Thorac Soc201613122229223327684316
- CarskadonMADementWCMitlerMMRothTWestbrookPRKeenanSGuidelines for the Multiple Sleep Latency Test (MSLT): a standard measure of sleepinessSleep1986945195243809866
- ArandDBonnetMHurwitzTMitlerMRosaRSangalRBThe clinical use of the MSLT and MWTSleep200528112314415700728
- BennettLSStradlingJRDaviesRJA behavioural test to assess daytime sleepiness in obstructive sleep apnoeaJ Sleep Res1997621421459377534
- StroopJRStudies of interference in serial verbal reactionsJ Exp Psychol1935186643662
- BerryRBBrooksRGamaldoCEHardingSMMarcusCVaughnBThe AASM Manual for the Scoring of Sleep and Associated Events Rules, Terminology and Technical SpecificationsDarienAmerican Academy of Sleep Medicine2012
- PejovicSBastaMVgontzasANEffects of recovery sleep after one work week of mild sleep restriction on interleukin-6 and cortisol secretion and daytime sleepiness and performanceAm J Physiol Endocrinol Metab20133057E890E89623941878
- BlandJMAltmanDGStatistical methods for assessing agreement between two methods of clinical measurementLancet1986184763073102868172
- BelenkyGWesenstenNJThorneDRPatterns of performance degradation and restoration during sleep restriction and subsequent recovery: a sleep dose-response studyJ Sleep Res200312111212603781
- LimJDingesDFSleep deprivation and vigilant attentionAnn N Y Acad Sci2008112930532218591490
- MacleodCMHalf a century of research on the Stroop effect: an integrative reviewPsychol Bull199110921632032034749
- DoranSMVan DongenHPDingesDFSustained attention performance during sleep deprivation: evidence of state instabilityArch Ital Biol2001139325326711330205
- MolliconeDJVan DongenHPRogersNLBanksSDingesDFTime of day effects on neurobehavioral performance during chronic sleep restrictionAviat Space Environ Med201081873574420681233
- StaabWJSjursenWPrevesDA one-size disposable hearing aid is introducedHear J200053436
- KappelSLLooneyDMandicDPKidmosePPhysiological artifacts in scalp EEG and ear-EEGBiomed Eng Online201716110328800744
- TonoyanYLooneyDMandicDPVan HulleMMDiscriminating multiple emotional states from EEG using a data-adaptive, multi-scale information-theoretic approachInt J Neural Syst2016262165000526829885
- NakamuraTGoverdovskyVMorrellMJMandicDPAutomatic sleep monitoring using ear-EEGIEEE J Transl Eng Health Med2017518
- NguyenAAlqurashiRRaghebiZBanaei-KashaniFHalbowerACVuTLIBS: a lightweight and inexpensive in-ear sensing system for automatic whole-night sleep stage monitoringGetMobile: Mobile Comput Commun20172133134
- MikkelsenKBVilladsenDBOttoMKidmosePAutomatic sleep staging using ear-EEGBiomed Eng Online201716111128927417
- GoverdovskyVvon RosenbergWNakamuraTHearables: multimodal physiological in-ear sensingSci Rep201771694828761162
- SunwooBYJacksonNMaislinGGurubhagavatulaIGeorgeCFPackAIReliability of a single objective measure in assessing sleepinessSleep201235114915822215929
- SateiaMJInternational classification of sleep disorders, third editionChest201414651387139425367475
- NakamuraTAdjeiTAlqurashiYLooneyDMorrellMJMandicDPComplexity science for sleep stage classification from EEGProceedings of IEEE International Joint Conference on Neural Networks (IJCNN)438743942017
- GrossmanABarenboimEAzariaBShererYGoldsteinLThe maintenance of wakefulness test as a predictor of alertness in aircrew members with idiopathic hypersomniaAviat Space Environ Med200475328128315018299
- LloydSAssessing fitness to drive for commercial and private vehicle drivers (medical standards for licensing and clinical management guidelines)—a resource for health professionals in AustraliaOccup Med2012626472473
