Abstract
Exogenous melatonin can be used to treat sleep disturbance in adults, children, and adolescents. While its short-term use is considered safe, there are some concerns that long-term use might delay children’s sexual maturation, possibly by disrupting the decline in nocturnal melatonin levels that occur at the onset of puberty. This narrative review aimed to summarize some of the current knowledge about the potential effects of exogenous melatonin on puberty. We found no clinical studies that experimentally tested the effects of melatonin on pubertal timing in children, but we reviewed the small number of observational studies. We also drew on animal data to try to answer our question. The photoperiod and melatonin-mediated seasonal transitions in sexual activity and breeding in some mammals across the seasons have been used as a model of sexual development in mammals, including humans. The switch from non-sexual activity (in the non-breeding period) to sexual activity (in the breeding period) has been likened to the onset of puberty as there are similarities between the two. We conclude that to investigate an association between melatonin and pubertal timing, it will be important to conduct long-term randomized controlled trials of latency age children and also examine the cellular and systems-level interactions between melatonin and kisspeptin, a recently identified neuropeptide with a locus of action at the gonadotropin releasing hormone neurons that is important in contributing to the timing of puberty onset.
Overview and purpose
In recent years, there has been an increase in the use of melatonin for the treatment of sleep disturbances with positive results. Human studies have shown that short-term use of melatonin is safe, even in high doses, with only mild adverse effects such as dizziness, headache, nausea, and sleepiness.Citation1 Carefully timed exogenous melatonin effectively reduces sleep latency while restoring chronobiological alignment of sleep with the internal biological clockCitation2,Citation3 without negative alteration of sleep architecture and other side effects observed with most hypnotics. However, melatonin use with adolescents and children is still off-label and concerns have been raised that its long-term use may affect sexual maturation.Citation4 The present review first offers a brief synopsis of the nature and properties of melatonin, the prevalence of sleep disorders in children and adolescents, the current understanding of mechanisms of onset of puberty, and a brief review of kisspeptin’s effects on the gonadotropin releasing hormone (GnRH) neurons as a potential model of puberty. This is followed by a review of some of the existing animal and human studies on melatonin and the onset of puberty. We conclude with a comment about non-pharmacological interventions for sleep disturbance and future direction of research.
Properties and effects of melatonin
The neurohormone, melatonin (N-acetyl-5-methoxytrypta-mine), is produced in the pineal gland of mammals. It first came to attention in 1958 when Lerner, a dermatologist, found that melatonin could cause the lightning of frog skin.Citation5 Subsequently in 1960, he and his colleagues defined melatonin’s chemical structure.Citation6 Since then, melatonin has been found to affect a wide range of physiological processes such as; circadian rhythms,Citation7 sleep-wake cycles,Citation8 sexual maturation,Citation9 aging,Citation10 and exerts neuroendocrine,Citation11 cardiovascular,Citation12 and oncostatic effects.Citation13 Many laboratory studies conducted under well-controlled conditions have shown that the administration of exogenous melatonin acutely affects sleep and thermoregulation in humans, and that the soporific effect of melatonin is mediated by an increase in distal heat loss through increased skin temperature over the course of the sleep cycle.Citation14–Citation16 In healthy volunteers, a single melatonin dose of 0.3 mg or 1.0 mg orally has been reported to have significant soporific effects.Citation17 Although exogenous melatonin is often referred to as a hypnotic, it has been proposed that soporific is a more accurate term than hypnotic when referring to the sleepiness-inducing characteristics of melatonin. Exogenous melatonin is also considered as a chronobiotic due to its ability to shift the timing of sleep and circadian rhythms.Citation3,Citation18
The photoneuroendocrine system
In mammals, photoperiodic (or relative length of light and dark periods) information is received at the level of the retina and is transmitted through a multi-synaptic neural pathway to the pineal gland to modulate melatonin secretion.Citation19 More specifically, the endogenous production and release of melatonin are suppressed by exposure to bright light. Pineal melatonin secretion is also regulated by the main biological clock in the suprachiasmatic nucleus of the hypothalamus.Citation20 In humans, melatonin release under dim light conditions usually starts in the evening, with some averages ranging between 19:30 and 21:30 in adults, and between 19:00 and 21:00 in children from 6–12 years of age.Citation21 The duration of melatonin secretion varies according to day length and provides an endocrine representation of the photoperiod.Citation22 When administered in the daytime, exogenous melatonin has an elimination half-life of 35–45 minutes.Citation23
Prevalence of sleep disturbances in children and adolescents, and melatonin use
It is estimated that 15%–25% of children and adolescents in the general population have sleep disturbances.Citation24,Citation25 A higher prevalence of sleep disturbances is seen in children and adolescents with medical and psychiatric conditions. For example, the estimated rates of sleep disturbances range from 30%–53% in individuals with autism spectrum disorder,Citation26 and up to 70% in those with attention deficit hyperactivity disorder.Citation27 In individuals with neurodevelopmental disorder, the prevalence of sleep disturbance is estimated to be 13%–86%.Citation28 With respect to anxiety disorders in children and adolescents, 80%–90% report at least one sleep-related problem.Citation29,Citation30 In major depression, the prevalence of sleep disturbances is estimated to be 72.7%.Citation31 In line with the considerable changes affecting the biological clock during this developmental period,Citation32,Citation33 sleep disturbances occurring in late childhood and adolescence often take the form of delayed sleep phase.Citation34,Citation35 If untreated, such sleep disturbances can negatively impact children, adolescents, and their families with respect to physical and mental health, social, academic, and cognitive functioning.Citation36–Citation38
Off-label, over-the-counter exogenous melatonin use in children and youth has grown tremendously in recent years. For example, Hartz et alCitation39 studied recurrent pediatric melatonin use across Norway. From 2004–2012, the prevalence of people using melatonin between the ages of 4 and 17 increased from 3.4–11.0 per 1,000 in boys and 1.5–7.7 per 1,000 in girls. Twenty-nine percent of boys and 23% of girls were recurrent melatonin users, with highest level of recurrent use in young children 4–8 years of age. Most of the children or youth had a diagnosis of a psychiatric or neurologic disorder.Citation39
Melatonin and sexual maturation
In a cross-sectional study, Waldhauser et alCitation40 examined nocturnal serum melatonin levels in 367 individuals ranging from 3 days to 90 years of age. They noted that the highest nighttime serum melatonin levels were found in very young individuals (1–3 years), with mean levels dropping progressively by 80% throughout childhood until adolescence and young adulthood (15–20 years). This is consistent with an earlier study in which they described a 75% drop in nocturnal serum melatonin levels when comparing children aged 1–5 years to young adults.Citation41 There are some indications that the drop in nocturnal melatonin levels during adolescence parallels sexual maturation processes.Citation42 Accordingly, the decline in melatonin levels has been found to relate to the progression of Tanner stages.Citation43 These observations, together with animal studies showing that exogenous melatonin can suppress GnRH secretion,Citation44 contributed to the emergence of concerns about the possibility that exogenous melatonin supplementation may affect children’s sexual maturation.Citation4
In brief, given how common the use of melatonin has become in the treatment of sleep disturbances in children and adolescents, it is imperative to review the evidence from clinical and basic science studies to answer the question of whether melatonin could affect pubertal timing in adolescence.
Physiology of puberty
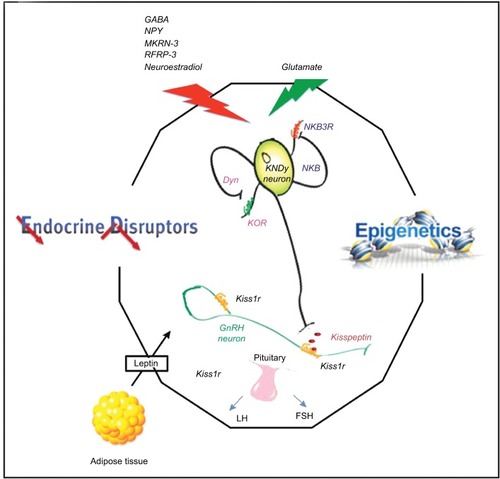
Puberty, the process by which a child becomes sexually mature and capable of sexual reproduction, is associated with a growth spurt and the development of secondary sexual characteristics. This is the product of a complex series of neuroendocrine steps in which the primary mechanisms are still unclear.Citation45 The start of puberty is marked by a sustained increase in the pulsatile release of GnRHCitation46 by a small population of GnRH neurons which extend axons from the preoptic area and the infundibular nucleus of the hypothalamus.Citation47 The hypothalamus forms part of the hypothalamic-pituitary-gonadal axis which controls the onset and progression of puberty. This axis is active in the embryonic and early postnatal stages of life and is subsequently restrained during childhood only to be reactivated during the initiation of puberty.Citation48 The exact mechanisms which trigger the onset of puberty are unknown. However, it is believed that the augmentation of activating factors leading to GnRH secretion, together with the suppression of inhibitory factors of GnRH secretion result in the initiation of puberty. These activating and inhibitory factors involve neurotransmitters and neuropeptides which originate in the hypothalamus in addition to peripheral or gonadal signals. In humans, the timing of puberty is highly variable due to several factors such as: gender; epigenetic and genetic factors; intrauterine conditions; nutrition; light-dark cycles; climatic conditions; hormones (eg, leptin, ghrelin, IGF-1, sex steroid); and exposure to endocrine-disrupting chemicals in the environment.Citation49 , taken from Livadas and Chrosusos,Citation47 illustrates the interaction of hypothalamic factors and peripheral signals for puberty onset.
Figure 1 Interaction of hypothalamic factors and peripheral signals on puberty onset.
Kisspeptin signaling, effects on GnRH neurons, and sensitivity to melatonin
As reported by Liu and Herbison,Citation50 the cancer suppressor gene, KISS1, was discovered in 1996 in Hershey, Philadelphia and named KISS1, after the Hershey’s Kisses chocolate.Citation51
It encoded for a group of four neuropeptides, called kisspeptins. Their receptor was found to be the orphan G protein-coupled receptor GPR54 also known as KISS1R. Of the four kisspeptins, kisspeptin 10 has been used the most in research.Citation51
In 2005, kisspeptin was discovered to be the most potent activator of GnRH neuronal excitabilityCitation52 and the direct effects of kisspeptin are believed to be critical for both the electrophysiological and reproductive actions of kisspeptin.Citation53–Citation55 In rodents (hamsters and rats) and sheep, most GnRH neurons (greater than 90%) express the receptor, Kiss1r. Kiss1r is the non-human ortholog of the human KISS1R. It is believed that the mammalian reproductive system is driven by pulsatile release of GnRH and that kisspeptin’s effects on reproduction are mediated through GnRH neurons. It is noted however that in humans not all GnRH neurons receive kisspeptin neuron input, suggesting that additional molecules regulate the process of human puberty.Citation47
Animal studies have shown that melatonin supplementation reduces kisspeptin under conditions of prolonged photope-riod,Citation58 while endogenous melatonin suppression (following pinealectomy) abolishes the effects of reduced photoperiod on kisspeptin expression.Citation59 In addition, a recent study demonstrated that exogenous melatonin first induces a reduction in kisspeptin gene expression in the acute phase, but that longer effects lead to an increase in kisspeptin gene expressionCitation60 which could then actively stimulate the hypothalamic-pituitary-gonadal axis. Overall, this suggests that the effects of melatonin on the reproduction system may be modulated by kisspeptin and that these effects may differ considerably between the acute and longer-term phases of melatonin supplementation.
We therefore conducted a non-systematic review of the literature of studies assessing the effects of melatonin on puberty and the potential modulating role of kisspeptin.
Literature search: strategy and selection of studies
Medline and PubMed were searched for papers published in English up to 2018 using the search terms “melatonin”, “puberty”, “puberty onset”, “hypothalamic-pituitary-gonadal axis”, “kisspeptin”, “children”, and “adolescents”. The relevant articles were read and the reference lists were searched and those identified as relevant were also read. We first report on animal studies, followed by human studies.
Animal studies
Exogenous melatonin and puberty
There are several reasons for including animal studies in this narrative review. The first rests in the fact that experimental studies that would directly test the hypothesis that exogenous administration of melatonin might delay children’s sexual maturation by assigning children to long-term melatonin treatment or placebo, do not currently exist and would be unethical. Second, the answer to the question may be informed by considering models of seasonal breeding in some mammals. For example, the two most commonly employed mammalian experimental models used to explain the endogenous rhythms governing reproduction involve hamsters (Syrian and Siberian) and sheep.Citation61–Citation64 It has previously been suggested the transition from the non-breeding (anestrous) season to the breeding season in these animals could represent a mechanism similar to puberty.Citation65 Numerous similarities can be drawn between puberty and the transition to anestrous season. For example, in both pre-pubertal and seasonally anestrous ewes, preovulatory luteinizing hormone (LH) surges, which cause ovulation, do not occur. Also, in both cases, the neural wiring and innate ability to produce an LH surge are present,Citation66–Citation68 but appear to be suppressed. Prior to puberty, LH secretion is pulsatile, as is the case during the anestrous season, but the frequency of LH pulses is markedly reduced.Citation69 While these similarities are interesting, it is noted that the precise neuroendocrine systems which govern puberty and reproductive functions are not fully understood.Citation28,Citation45,Citation49
In the next sections, we provide a few examples of the effects of exogenous melatonin and pinealectomy on pubertal onset in animals. Subsequently, we present some investigations on the relationship between the photoperiod and changes in kisspeptin expression between breeding and non-breeding systems. also summarizes examples of the effects of exogenous melatonin administration on the timing of puberty in lambs, gilts, rats, and male Siberian hamsters.
Table 1 Examples of the effects of exogenous melatonin administration on the timing of puberty in lambs, gilts, rats, and male Siberian hamsters
In the male Djungarian hamster, which usually starts puberty 25 days after birth,Citation70 a 10-day treatment with short days or melatonin during the pre-pubertal period (day 15 to day 25) arrested the onset of puberty.Citation71 While one study failed to see any significant effect of melatonin-filled implants on the timing of puberty in gilts,Citation72 another study showed that prepubertal ewe lambs treated with melatonin implants had puberty onset delayed by 4 weeks compared to controls.Citation73 Conversely, another study showed that oral administration of melatonin to Suffolk prepubertal ewe lambs, from 10 weeks of age onward, advanced the onset of puberty by 3 weeks compared to control lambs maintained under the same natural photoperiod.Citation74 This may be in line with another study reporting that pinealectomy delayed the onset of puberty by 12 weeks in ewe lambs.Citation75 To summarize, there are some indications that exogenous melatonin can alter the timing of puberty in seasonally breeding mammals, but findings to date seem to be inconsistent.
Kisspeptin, melatonin, and puberty
Investigators considered whether photoperiod could be a primary stimulus for changes in kisspeptin expression between breeding and nonbreeding seasons. It has been demonstrated that, in Soay ewes, the photoperiodic changes in kisspeptin levels were related to changes in the pattern of melatonin secretion.Citation76 The Soay ewe is a short-day breeder, breeding during Autumn and Winter when days are short and nights are long (ie, entailing longer periods of melatonin secretion). Similarly, Simonneaux et al,Citation58 also found that kisspeptin expression was influenced by the photoperiod in male Syrian hamsters, which are long-day breeders. During the long-day period (Spring and Summer) the duration of melatonin secretion is shorter compared to short-day periods (Autumn and Winter). Kisspeptin cells are, however, not known to express melatonin receptors,Citation77 suggesting that there could be intermediary processes (yet to be elucidated) between the photoperiod, melatonin, and kisspeptin expression. Simonneaux, Ancel, Poirel, and GauerCitation78 proposed that RFRP-3, a member of the RRamide peptide group, known to inhibit GnRH, could play such an intermediary role. , from Simmonneaux et al,Citation78 illustrates photo-inhibitory melatonergic message in the male Syrian hamster.
Figure 2 Working model indicating how the photo-inhibitory melatonergic message in short day conditions is integrated in the hypothalamus to further regulate the gonadotropic axis in the male Syrian hamster.
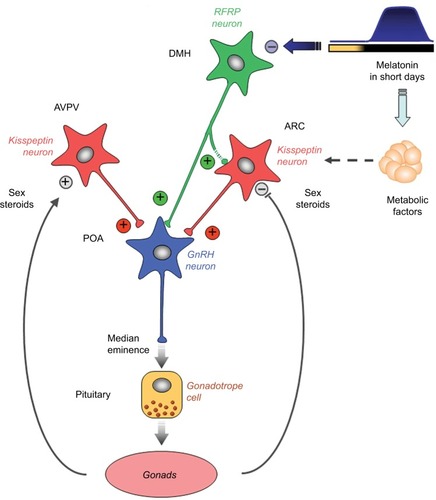
Another postulated pathway for melatonin’s inhibitory effect in the period of reproductive quiescence in hamsters during short days (Autumn and Winter) is that melatonin may alter and reinforce the negative feedback effect of sex steroids on kisspeptin expression.Citation59,Citation79
On the other hand, there is evidence that even in the seasonally breeding mammals the hypothalamic-pituitary-gonadal axis may escape the driving influence of light and melatonin on the timing of fertility and reproduction at least under experimental conditions. It is known that after 10 weeks of exposure to short days (Autumn and Winter and longer periods of melatonin exposure) some hamsters become sexually inactive and this was associated with a decrease in Kiss1 expression in the hypothalamus (arcuate nucleus). If the short-day exposure continues over 20–30 weeks, the hamsters are known to become refractory to the inhibitory effects of short day lengths and become sexually active spontaneously – implying an escape from the effect of a long period of exposure to melatonin. The usual cycle becomes reset only after prolonged exposure to long-day conditions (Spring and Summer). It was found that the sexual reactivation was associated with an increase and Kiss1 expression similar to findings in the Spring and Summer.Citation59,Citation80
In summary, there is evidence that in some seasonal breeders photoperiodic and melatonin level changes are associated with changes in kisspeptin signaling. However, some studies failed to show any significant association, or suggested that there could be temporary dissociations.
Human studies: pubertal timing in pediatric melatonin use
Only three human studies have tracked pubertal timing along the course of exogenous melatonin use, and all examined pubertal timing as a secondary outcome while the primary focus was on melatonin effectiveness or dosing. Two studies involved the Meldos Trial, which was a randomized controlled trial investigating optimal melatonin dosing for intractable insomnia in children and youth.Citation81
The first report encompasses data on 59 of the 69 6–12-year-old participants who had completed the Meldos Trial at least a year before.Citation81 The children, having used melatonin for 6 months or longer, were asked to fill out questionnaires including self-report Tanner staging with pictures, mother’s age of menarche, and boys’ ages of first ejaculation for them and for their fathers. The ages across Tanner stage were compared to Dutch population data. Only 19 of the participants reached possible pubertal age during this study, and only 62% of the boys and 91% of the girls answered the puberty questions. In this limited sample, there was no evidence of pubertal timing being different from the population norms.
This group was again followed up 9–12 years later, comparing the 33 participants who completed the follow-up phase to publicly available population samples on perceived pubertal timing (early, normal, late).Citation82 In this subsequent study, 31.3% of the participants reported a late perceived pubertal timing, compared to the population sample control rate of 17%.
Carr et alCitation83 conducted a longitudinal study of melatonin treatment in 44 children with neurodevelopmental disorders and treatment-resistant circadian rhythm sleep disorders. The baseline study was a placebo-controlled double-blind crossover trial of sustained-release melatonin. The follow-up study consisted of a structured caregiver telephone interview every 3 months for up to 3.8 years. The median age of puberty was 11.5 years, with a range of 12–15 years. Precocious puberty was present in five children with severe neurodevelopmental disability, most of whom experienced it prior to the melatonin treatment. In the remaining children, pubertal timing was considered within normal limits, with a mean age of 13.4 years ±1.4 years.
In summary, there are only few studies that have examined pubertal timing in children and youth given melatonin on a long-term basis. Drawing any conclusions from the three that exist is difficult because they have very small samples, incomplete follow-up, and poor measures of pubertal timing.
It is also useful to consider indirect human studies. Magee et alCitation84 suggested the possibility of a role of exposure to light in human puberty, as they noted that blind girls tended to have earlier menarche. However, these reports were not subsequently confirmed.Citation49 In a similar vein, other studies suggested that menarche occurred more frequently in the Winter than in the Summer,Citation85–Citation87 suggesting that light would have an inhibiting effect on the onset of puberty. This is however contrary to the finding that the long Winter months in the Arctic may be associated with reduced pituitary-gonadal function and low conception rates.Citation88
As to the finding that the level of melatonin falls in the lead-up to puberty in humans,Citation89–Citation91 it was observed that the decrease in melatonin secretion occurs usually after the onset of puberty, between Tanner stages II and III.Citation90
Discussion
In this narrative review, we highlighted that there is some evidence that exogenous melatonin may alter pubertal timing in some animals, that photoperiodic and melatonin level changes may be associated with changes in kisspeptin signaling in some seasonal breeders, and that one of the longitudinal studies in humans suggested that long-term melatonin use might be linked to a delay in puberty onset; however, the determination of puberty delay was subjective (perceived), and not objective. These findings are all based on very limited research and the findings are not consistent across studies. Hence, drawing any strong conclusions at this stage is difficult.
To the extent that the transition from the sexually inactive to sexually active phase could be an animal model for the onset of puberty and light, darkness and melatonin are important in this transition, it could be argued that perhaps melatonin plays an important role in the onset of puberty in seasonally breeding mammals. Whether or not melatonin could play an important role in the pubertal onset in humans is still an open question.
Since humans are not seasonal breeders like hamsters and sheep, an argument could also be made that the human hypothalamic-pituitary-gonadal axis may have, during the process of evolution, escaped the driving influence of light and melatonin on the timing of fertility and reproduction.
Future directions
We suggest three future areas of research to advance our knowledge about the effects of long-term use of exogenous melatonin on pubertal timing in children and adolescents. First, established methodologies of longitudinal studies of pubertal timingCitation81,Citation82,Citation92 should be applied to observational studies in children who are spontaneously electing to take melatonin. It would be ideal to start with children who are as young as possible and follow them annually, measuring the somatic manifestations of puberty (eg, age of menarche, Tanner stage development, age at first ejaculation). Because the normal age range of puberty in girls begins at 8 years of age and at 9 in boys, studies should recruit girls and boys at young ages, ideally 5 or 6 years of age. Second, it will be important to re-examine and get a better understanding of the reasons for the inconsistent findings in studies involving exogenous melatonin administration and pubertal onset in animals. Third, further investigations are needed to clarify the potential role of cellular and systems-level interactions between melatonin and kisspeptin, a neuropeptide which acts on GnRN neurons and is considered important in the timing of puberty onset. Such studies can be done at a basic science level.
Conclusion
Our review suggests that the role of melatonin in sexual maturation and the timing of puberty is understudied in humans. The three human studies that have examined the question have done so as an ancillary research question in small samples of children and youth, some of whom had neurodevelopmental disorders. This limits the generalizability to the general population and is insufficient evidence to draw conclusions for patients with mental health and neurological disorders. Further experimental studies on the impact of melatonin on puberty, notably in non-seasonal mammals, and advances in the research about the intermediary processes between melatonin and kisspeptin activation, could ultimately inform us about the potential influence of exogenous melatonin on puberty.
We would be remiss not to mention that, aside from melatonin intake, non-pharmacological interventions can be effective for the treatment of sleep disturbances in children and adolescents. They have been found to be more beneficial than placebo, alternative treatments, and frequently used medications in children, adolescents, and adults.Citation93 Non-pharmacological approaches range from sleep hygiene and psychoeducation, cognitive behavioral therapy for insomnia, physical exercise,Citation94 and mindfulness-based meditation,Citation95 to relaxation-based therapies.Citation96
Author contributions
AB, SG, SA, RR, KP, and PT contributed to the rationale and design of the paper. The manuscript was written by AB. AB, SG, SA, RR, KP, PT, and JDK conducted searches of pertinent literature. All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Acknowledgments
Financial support was provided by funds from the Psychiatry Associates at the Children’s Hospital of Eastern Ontario and from the Institute of Mental Health Research University of Ottawa.
Disclosure
The authors report no conflicts of interest in this work.
References
- AndersenLPHGögenurIRosenbergJReiterRJThe safety of melatonin in humansClin Drug Investig2016363169175
- van GeijlswijkIMvan der HeijdenKBEgbertsACKorziliusHPSmitsMGDose finding of melatonin for chronic idiopathic childhood sleep onset insomnia: an RCTPsychopharmacology2010212337939120668840
- CardinaliDPFurioAMReyesMPBruscoLIThe use of chronobiotics in the resynchronization of the sleep-wake cycleCancer Causes Control200617460160916596316
- KennawayDJPotential safety issues in the use of the hormone melatonin in paediatricsJ Paediatr Child Health201551658458925643981
- LernerABCaseJDTakahashiYLeeTHMoriWIsolation of melatonin, the pineal gland factor that lightens melanocytesJ Am Chem Soc1958
- LernerABCaseJDTakahashiYIsolation of melatonin and 5-methoxyindole-3-acetic acid from bovine pineal glandsJ Biol Chem19602351992199714415935
- GilletteMUTischkauSASuprachiasmatic nucleus: the brain’s circadian clockRecent Prog Horm Res1999543358 discussion 58–5910548871
- ReiterRJPineal melatonin: cell biology of its synthesis and of its physiological interactionsEndocr Rev19911221511801649044
- Díaz LópezBDíaz RodríguezEUrquijoCFernández ÁlvarezCMelatonin influences on the neuroendocrine-reproductive axisAnn N Y Acad Sci2005105733736416399905
- PierpaoliWRegelsonWPineal control of aging: effect of melatonin and pineal grafting on aging miceProc Natl Acad Sci USA19949127877918290600
- CardinaliDPPévetPBasic aspects of melatonin actionSleep Med Rev19982317519015310500
- ReiterRJTanDXKorkmazAThe circadian melatonin rhythm and its modulation: Possible impact on hypertensionJ Hypertens Suppl2009276S17S2019633446
- KorkmazATamuraHManchesterLCOgdenGBTanDXReiterRJCombination of melatonin and a peroxisome proliferator-activated receptor-gamma agonist induces apoptosis in a breast cancer cell lineJ Pineal Res200946111511618798787
- CajochenCKrauchiKWirz-JusticeARole of melatonin in the regulation of human circadian rhythms and sleepJ Neuroendocrinol200315443243712622846
- KrauchiKCajochenCWirz-JusticeAA relationship between heat loss and sleepiness: effects of postural change and melatonin administrationJ Appl Physiol19978311341399216955
- DawsonDvan den HeuvelCJIntegrating the actions of melatonin on human physiologyAnn Med1998301951029556095
- AttenburrowMEJCowenPJSharpleyALLow dose melatonin improves sleep in healthy middle-aged subjectsPsychopharmacology199612621791818856838
- Wirz-JusticeAArmstrongSMMelatonin: nature’s soporific?J Sleep Res1996521371418795816
- HastingsMHMaywoodESReddyABTwo decades of circadian timeJ Neuroendocrinol200820681281918601704
- SimonneauxVRibelaygaCGeneration of the melatonin endocrine message in mammals: a review of the complex regulation of melatonin synthesis by norepinephrine, peptides, and other pineal transmittersPharmacol Rev200355232539512773631
- van GeijlswijkIMKorziliusHPSmitsMGThe use of exogenous melatonin in delayed sleep phase disorder: a meta-analysisSleep201033121605161421120122
- TosiniGFukuharaCPhotic and circadian regulation of retinal melatonin in mammalsJ Neuroendocrinol200315436436912622835
- FourtillanJBBrissonAMGobinPIngrandIDecourtJPGiraultJBioavailability of melatonin in humans after day-time administration of D(7) melatoninBiopharm Drug Dispos2000211152211038434
- ShamseerLVohraSComplementary, Holistic, and Integrative Medicine: Melatonin Definition and Description Available from: www.cfsan.fda.gov/Accessed November 12, 2018
- OwensJClassification and epidemiology of childhood sleep disordersPrim Care200835353354618710669
- SivertsenBPosserudMBGillbergCLundervoldAJHysingMSleep problems in children with autism spectrum problems: a longitudinal population-based studyAutism201216213915021478225
- CorteseSFaraoneSVKonofalELecendreuxMSleep in children with attention-deficit/hyperactivity disorder: meta-analysis of subjective and objective studiesJ Am Acad Child Adolesc Psychiatry200948989490819625983
- SajithSGClarkeDMelatonin and sleep disorders associated with intellectual disability: a clinical reviewJ Intellect Disabil Res200751 Pt 121317181598
- ChaseRMPincusDBSleep-related problems in children and adolescents with anxiety disordersBehav Sleep Med20119422423622003976
- AlfanoCAGinsburgGSKingeryJNSleep-related problems among children and adolescents with anxiety disordersJ Am Acad Child Adolesc Psychiatry200746222423217242626
- LiuXBuysseDJGentzlerALInsomnia and hypersomnia associated with depressive phenomenology and comorbidity in childhood depressionSleep2007301839017310868
- CarpenterJRobillardRTherapyIHVariations in the Sleep–Wake Cycle from Childhood to Adulthood: Chronobiological PerspectivesCiteseer2015 Available from: http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.838.9127&rep=rep1&type=pdfAccessed November 22, 2018
- TonettiLAdanADi MiliaLRandlerCNataleVMeasures of circadian preference in childhood and adolescence: A reviewEur Psychiatry201530557658225726892
- CrowleySJAceboCCarskadonMASleep, circadian rhythms, and delayed phase in adolescenceSleep Med20078660261217383934
- ThorpyMJKormanESpielmanAJGlovinskyPBDelayed sleep phase syndrome in adolescentsJ Adolesc Heal Care1988912227
- FalloneGOwensJADeaneJSleepiness in children and adolescents: clinical implicationsSleep Med Rev20026428730612531133
- BrandSKirovRSleep and its importance in adolescence and in common adolescent somatic and psychiatric conditionsInt J Gen Med2011442521731894
- SadehACognitive-behavioral treatment for childhood sleep disordersClin Psychol Rev200525561262815979219
- HartzIHandalMTverdalASkurtveitSPaediatric off-label use of melatonin-A register linkage study between the norwegian prescription database and patient registerBasic Clin Pharmacol Toxicol2015117426727325892306
- WaldhauserFWeiszenbacherGTatzerEAlterations in nocturnal serum melatonin levels in humans with growth and agingJ Clin Endocrinol Metab19886636486523350912
- WaldhauserFWaldhauserMLiebermanHRDengMHLynchHJWurtmanRJBioavailability of oral melatonin in humansNeuroendocrinology19843943073136493445
- WaldhauserFWeiszenbacherGFrischHZeitlhuberUWaldhauserMWurtmanRJFall in nocturnal serum melatonin during prepuberty and pubescenceLancet1984183743623656141425
- CrowleySJAceboCCarskadonMAHuman puberty: salivary melatonin profiles in constant conditionsDev Psychobiol201254446847321953482
- RoyDBelshamDDMelatonin receptor activation regulates GnRH gene expression and secretion in GT1-7 GnRH neurons. Signal transduction mechanismsJ Biol Chem2002277125125811684691
- TerasawaEFernandezDLNeurobiological mechanisms of the onset of puberty in primatesEndocr Rev200122111115111159818
- BelchetzPEPlantTMNakaiYKeoghEJKnobilEHypophysial responses to continuous and intermittent delivery of hypopthalamic gonadotropin-releasing hormoneScience19782024368631633100883
- LivadasSChrousosGPControl of the onset of pubertyCurr Opin Pediatr201628455155827386974
- WenninkJMDelemarre-van de WaalHASchoemakerRSchoemakerHSchoemakerJLuiteinizing hormone and follicle stimulatiing hormone scretion patterns in girls throughout puberty measures using highly sensitive immunoradiometric assaysClin Endocrinol1990333333344
- ParentASTeilmannGJuulASkakkebaekNEToppariJBourguignonJPThe timing of normal puberty and the age limits of sexual precocity: variations around the world, secular trends, and changes after migrationEndocr Rev200324566869314570750
- LiuXHerbisonAEKisspeptin regulation of neuronal activity throughout the central nervous systemEndocrinol Metab2016312193205
- LeeJHMieleMEHicksDJKiSS-1, a novel human malignant melanoma metastasis-suppressor geneJ Natl Cancer Inst19968823173117378944003
- HanSKGottschMLLeeKJActivation of gonadotropin-releasing hormone neurons by kisspeptin as a neuroendocrine switch for the onset of pubertyJ Neurosci20052549113491135616339030
- KirilovMClarksonJLiuXDependence of fertility on kisspeptin-Gpr54 signaling at the GnRH neuronNat Commun201341249224051579
- NovairaHJSonkoMLHoffmanGDisrupted kisspeptin signaling in GnRH neurons leads to hypogonadotrophic hypogonadismMol Endocrinol201428222523824422632
- LeónSBarrosoAVázquezMJDirect Actions of Kisspeptins on GnRH Neurons Permit Attainment of Fertility but are Insufficient to Fully Preserve Gonadotropic Axis ActivitySci Rep201661920626755241
- MessagerSChatzidakiEEMaDKisspeptin directly stimulates gonadotropin-releasing hormone release via G protein-coupled receptor 54Proc Natl Acad Sci USA200510251761176615665093
- IrwigMSFraleyGSSmithJTKisspeptin activation of gonadotropin releasing hormone neurons and regulation of KiSS-1 mRNA in the male ratNeuroendocrinology200480426427215665556
- SimonneauxVAnselLRevelFGKlosenPPévetPMikkelsenJDKisspeptin and the seasonal control of reproduction in hamstersPeptides200930114615318619505
- RevelFGSaboureauMMasson-PévetMPévetPMikkelsenJDSimonneauxVKisspeptin mediates the photoperiodic control of reproduction in hamstersCurr Biol200616171730173516950111
- GingerichSWangXLeePKThe generation of an array of clonal, immortalized cell models from the rat hypothalamus: analysis of melatonin effects on kisspeptin and gonadotropin-inhibitory hormone neuronsNeuroscience200916241134114019463905
- LarkinJEJonesJZuckerITemperature dependence of gonadal regression in Syrian hamsters exposed to short day lengthsAm J Physiol Regul Integr Comp Physiol20022823R744R75211832395
- LehmanMNCoolenLMGoodmanRLViguieCBillingsHJKarschFJSeasonal plasticity in the brain: the use of large animal models for neuroanatomical researchReprod Suppl20025914916512698979
- PaulMJZuckerISchwartzWJTracking the seasons: the internal calendars of vertebratesPhilos Trans R Soc B Biol Sci20083631490341361
- GoodmanRLJansenHTBillingsHJCoolenLMLehmanMNNeural systems mediating seasonal breeding in the eweJ Neuroendocrinol201022767468120456601
- FosterDLPuberty in the female sheepKnobilENeillJDPhysiology of ReproductionRaven Press1988New York
- FosterDLKarschFJDevelopment of the mechanism regulating the preovulatory surge of luteinizing hormone in sheepEndocrinology1975975120512091183409
- TranCTEdeyTNFindlayJKPituitary response of prepuberal lambs to oestradiol-17 betaAust J Biol Sci1979324–5463120739
- ClarkeIJGonadotrophin-releasing hormone secretion (GnRH) in anoestrous ewes and the induction of GnRH surges by oestrogenJ Endocrinol198811733553603292694
- FosterDLLemonsJAJaffeRBNiswenderGDSequential patterns of circulating luteinizing hormone and follicle-stimulating hormone in female sheep from early postnatal life through the first estrous cyclesEndocrinology19759749859941238249
- YellonSMGoldmanBDPhotoperiod control of reproductive development in the male Djungarian hamster (Phodopus sungorus)Endocrinology198411426646706418534
- BuchananKLYellonSMDelayed puberty in the male Djungarian hamster: effect of short photoperiod or melatonin treatment on the GnRH neuronal systemNeuroendocrinology1991542961021766555
- KennawayDJHughesPEvan WettereWHMelatonin implants do not alter estrogen feedback or advance puberty in giltsAnim Reprod Sci2015156132225618532
- KennawayDJPeekJCGilmoreTARoylesPPituitary response to LHRH, LH pulsatility and plasma melatonin and prolactin changes in ewe lambs treated with melatonin implants to delay pubertyJ Reprod Fertil19867811371483531504
- RecabarrenSELobosAHenriquezJPeneipilCPariloJEffect of daily melatonin treatment on the profile of luteinizing hormone secretion in prepubertal ewesAgrociencia19981430315
- KennawayDJGilmoreTADunstanEAPinealectomy delays puberty in ewe lambsJ Reprod Fertil19857411191254040570
- WagnerGCJohnstonJDClarkeIJLincolnGAHazleriggDGRedefining the limits of day length responsiveness in a seasonal mammalEndocrinology20081491323917901234
- YellonSMFosterDLMelatonin rhythms time photoperiod-induced puberty in the female lambEndocrinology1986119144493720672
- SimonneauxVAncelCPoirelVJGauerFKisspeptins and RFRP-3 act in concert to synchronize rodent reproduction with seasonsFront Neurosci201372223550229
- MalpauxBMigaudMTricoireHChemineauPBiology of mammalian photoperiodism and the critical role of the pineal gland and melatoninJ Biol Rhythms200116433634711506379
- GoldmanBDMammalian photoperiodic system: formal properties and neuroendocrine mechanisms of photoperiodic time measurementJ Biol Rhythms200116428330111506375
- van GeijlswijkIMMolRHEgbertsTCSmitsMGEvaluation of sleep, puberty and mental health in children with long-term melatonin treatment for chronic idiopathic childhood sleep onset insomniaPsychopharmacology2011216111112021340475
- ZwartTSmitsMEgbertsTRademakerCvan GeijlswijkILong-Term Melatonin Therapy for Adolescents and Young Adults with Chronic Sleep Onset Insomnia and Late Melatonin Onset: Evaluation of Sleep Quality, Chronotype, and Lifestyle Factors Compared to Age-Related Randomly Selected Population CohortsHealthcare2018612328711504
- CarrRWasdellMBHamiltonDLong-term effectiveness outcome of melatonin therapy in children with treatment-resistant circadian rhythm sleep disordersJ Pineal Res200743435135917910603
- MageeKBasinskaJQuarringtonBStancerHCBlindness and menarcheLife Sci1970917125435855
- BojlénKBentzonMWSeasonal variation in the occurrence of menarcheDan Med Bull19742151611684605089
- AlbrightDLVodaAMSmolenskyMHHsiBPDeckerMSeasonal characteristics of and age at menarcheChronobiol Int1990732512582268887
- CohenPMonth at menarche: a re-evaluation of the seasonal hypothesisAnn Hum Biol19932021982028447664
- RojanskyNBrzezinskiASchenkerJGSeasonality in human reproduction: an updateHum Reprod1992767357451323571
- SilmanRELeoneRMHooperRJPreeceMAMelatonin, the pineal gland and human pubertyNature19792825736301303503201
- SaltiRGalluzziFBindiGNocturnal melatonin patterns in childrenJ Clin Endocrinol Metab20008562137214410852442
- CavalloARitschelWAPharmacokinetics of melatonin in human sexual maturationJ Clin Endocrinol Metab1996815188218868626852
- MarceauKRamNHoutsRMGrimmKJSusmanEJIndividual differences in boys’ and girls’ timing and tempo of puberty: modeling development with nonlinear growth modelsDev Psychol20114751389140921639623
- TaylorDJRoaneBMTreatment of insomnia in adults and children: a practice-friendly review of researchJ Clin Psychol201066111137114720890946
- KalakNGerberMKirovRDaily morning running for 3 weeks improved sleep and psychological functioning in healthy adolescents compared with controlsJ Adolesc Health201251661562223174473
- CreswellJDIrwinMRBurklundLJMindfulness-Based Stress Reduction training reduces loneliness and pro-inflammatory gene expression in older adults: a small randomized controlled trialBrain Behav Immun20122671095110122820409
- MorinCMBootzinRRBuysseDJEdingerJDEspieCALichsteinKLPsychological and behavioral treatment of insomnia:update of the recent evidence (1998–2004)Sleep200629111398141417162986
- KennawayDJLight, neurotransmitters and the suprachiasmatic nucleus control of pineal melatonin production in the ratNeurosignals199764–6247254
