Abstract
Obstructive sleep apnea (OSA) is increasingly being recognized as a major health burden with strong focus on the associated cardiovascular risk. Studies from the last two decades have provided strong evidence for a causal role of OSA in the development of systemic hypertension. The acute physiological changes that occur during apnea promote nocturnal hypertension and may lead to the development of sustained daytime hypertension via the pathways of sympathetic activation, inflammation, oxidative stress, and endothelial dysfunction. This review will focus on the acute hemodynamic disturbances and associated intermittent hypoxia that characterize OSA and the potential pathophysiological mechanisms responsible for the development of hypertension in OSA. In addition the epidemiology of OSA and hypertension, as well as the role of treatment of OSA, in improving blood pressure control will be examined.
Introduction
Obstructive sleep apnea (OSA) is characterized by recurrent periods of complete or partial collapse of the upper airway during sleep (apneas and hypopneas), causing sleep fragmentation and frequent awakenings which often result in excessive daytime sleepiness. In more severe forms of the disease, periods of obstructed breathing result in profound intermittent hypoxia (IH) with underlying bursts in sympathetic nerve activity (SNA) and dramatic increases in heart rate (HR) and blood pressure (BP). The severity of OSA is determined by the apnea hypopnea index (AHI)Citation1 which is a measure of the number of periods of obstructed breathing per hour of sleep. The prevalence of OSA (AHI > 5 events/hour) with associated daytime sleepiness has been estimated at between 2% and 7% in diverse middle aged adult populations,Citation2 whilst the prevalence of OSA (AHI > 1 event/hour) in children is between 1%–3%.Citation3 Adults with OSA are typically centrally obese, and although this obesity is strongly causally linked to the condition, there is an increased prevalence of cardiovascular morbidity and mortality amongst OSA sufferers above what would be expected from obesity alone. In addition to OSA, patients often present with one or more comorbidities including dyslipidemia, glucose intolerance, and hypertension. In the past two decades, research has focused on establishing whether OSA increases cardiovascular risk, either via the exacerbation of these risk factors or by other mechanisms. Amongst these, the strongest evidence that OSA lies on the causal pathway to cardiovascular disease is via the promotion of hypertension (). It is therefore likely that the development of cardiovascular complications, including coronary artery disease and stroke, in these patients is at least partially driven by the underlying OSA.
Figure 1 Schematic diagram showing the pathophysiological mechanisms linking obstructive sleep apnea with the development of hypertension.
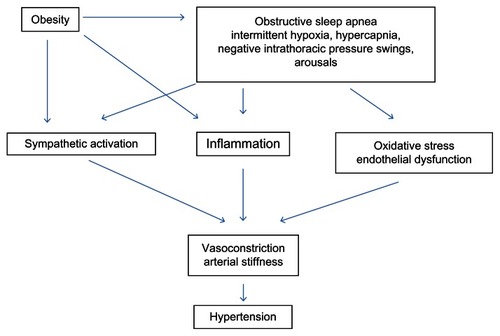
In this review we will present an overview of how the acute hemodynamic disturbances and associated IH that characterize OSA during sleep may promote not only nocturnal hypertension, but also sustained daytime hypertension. We will summarize the epidemiological evidence linking OSA to hypertension and the potential pathophysiological mechanisms responsible. We will also critically evaluate the role of OSA treatment in improving BP control. Finally, we will outline how future research may potentially increase our understanding of the OSA-hypertension link and how management of both conditions may reduce risk of adverse outcomes.
Normal blood pressure and cardiovascular risk
In healthy individuals, sleep is associated with a 10%–15% reduction in systolic and diastolic BP compared to wakefulness.Citation4 Referred to as “BP dipping”, this reduction coincides with the sympathetic withdrawal and subsequent parasympathetic predominance that occurs when going from wake to non-rapid eye movement (NREM) sleep. Indeed all measures of cardiovascular activity show diurnal variations in activity with levels higher during the day and reducing during sleep, due to the interacting effects of the sleep–wake and circadian cycles. Compared with wakefulness, NREM sleep is associated with lower BP, HR, cardiac output, and systemic vascular resistance. Rapid eye movement (REM) sleep on the other hand is punctuated by transient surges in SNA, HR, and BP. However, as REM constitutes only approximately 20% of total sleep, the net effect on cardiovascular measures is still a reduction from wake levels.
Sleep-related BP dipping is considered important for cardiovascular health, whilst absent or diminished nocturnal dipping of BP has been shown to be a strong independent predictor of cardiovascular risk. In the Ohasama studyCitation5 of 1464 individuals, it was shown that nighttime, as well as daytime BP measured by 24 hour ambulatory blood pressure monitoring (ABPM), were linearly related with stroke risk. In addition, in the Anglo-Scandinavian Cardiac Outcomes Trial,Citation6 increased nighttime systolic BP (SBP) was associated with an increased risk of cardiovascular events. Other population studies have confirmed the cardiovascular benefit of the normal sleep-related decrease in BP.Citation7,Citation8
Importantly, it has also been shown that ABPM is superior to clinic measurement in predicting cardiovascular mortality. The Dublin Outcome StudyCitation9 measured both clinic and ABPM in 5292 individuals and demonstrated that nighttime BP was overall the best predictor of cardiovascular risk, with a 10 mmHg increase in mean nighttime SBP being associated with a 21% increase in cardiovascular mortality. The measurement of nighttime BP may be particularly important in people who are taking antihypertensive medication because in these individuals, daytime SBP loses its predictive value for fatal and nonfatal cardiovascular events. In contrast, nighttime SBP predicts mortality and nonfatal events regardless of treatment status.Citation10 In the context of this review, ABPM likely provides the best prognostic value in OSA patients because they typically have raised nocturnal BP and many are treated with antihypertensive medication.
Hemodynamic effects of OSA
The acute hemodynamic effects of OSA have been well described in the literature. The effects of upper airway occlusion and subsequent hypoxia, hypercapnia, large negative intrathoracic pressure swings, and arousal from sleep, all lead to acute increases in peripheral vasoconstriction, resulting in increased BP.
During upper airway occlusion, both HR and BP initially decrease and then progressively increase. Peripheral SNA also increases, most likely caused by the synergistic influence of hypoxia and hypercapnia. Central hypercapnic chemoreceptor stimulation is a potent stimulus for ventilation and SNA.Citation11,Citation12 Hypoxic stimulation of peripheral chemoreceptors also increases ventilation and causes systemic vasoconstriction, with both acute and chronic increases in SNA.Citation13 Combined hypercapnic and hypoxic stimulation produces a synergistic increase in SNA that is more than additive.Citation12 Upon resumption of ventilation at airway reopening, and often coincident with arousal from sleep, there is a large surge in HR (tachycardia)Citation14 superimposed upon hypoxia-induced peripheral vasoconstriction, resulting in a large increase in BP. Resumption of ventilation also marks the abrupt decline in SNA and this is most likely due to inhibition via stimulation of pulmonary afferents.
Cardiovascular analyses during and post obstructive events in adults have reported acute increases in BP and HR in excess of 20 mmHg and 15 beats per minute (bpm), respectively. Mean BP following an obstructive apnea has been shown to increase by 32 mmHg during NREM and 42 mmHg during REM sleep.Citation15 Similarly, two studies both reported BP surges > 20 mmHg.Citation16,Citation17 Increases in HR of 15 bpm overall have been reported,Citation18 whilst increases of 18 bpm in NREM and 30 bpm in REM have also been reported.Citation19 Whilst these reports in adults describe larger HR and BP responses in REM sleep as compared with NREM, these respiratory events are usually longer and associated with more profound oxygen desaturation. In contrast, although the magnitudes of cardiovascular changes are similar in children, the response is larger in NREM even when event duration and oxygen desaturation are controlled for.Citation20 This sleep state difference is most likely due to the increased ventilatory response to hypoxia leading to a larger inspiratory effort upon resumption of breathing. The lack of an independent effect of sleep state in adults may be due to a reduction in ventilatory and cardiac chronotropic responses to hypoxia from childhood to adulthood.Citation21
A further contributor to the hemodynamic changes with apnea is arousal from sleep which often coincides with apnea termination. The arousal state itself involves an increase in cardiac sympathetic activation and a withdrawal of parasympathetic activity.Citation22 It is well documented in adults that even spontaneous arousal causes significant cardiovascular changes that are beyond functional requirements. Arousal has also been found to be a strong determinant of cardiovascular changes at the termination of obstructive events in adultsCitation23 and children,Citation20 with the change in cardiovascular activity postevent being proportional to the duration of arousal. Thus, while cardiovascular activity increases upon resumption of ventilation, arousal also exerts a further influence on the magnitude of the perturbation.
Mechanisms promoting hypertension in OSA
Sympathetic activation
The most noteworthy mechanism by which OSA may increase BP is through the acute and chronic increases in sympathetic activation associated with the condition. This effect is not limited to the acute apnea but manifests as sustained increases in sympathetic activation lasting through the day. Muscle SNA (MSNA) measured directly by microneurography has been shown to be elevated during sleep and remains elevated through the day compared with controls, long after the direct hemodynamic effects of apnea have subsided.Citation24 Plasma catecholamine levels have also been shown to be elevated in awake patients with OSA compared with controls.Citation25 Additionally, numerous studies have reported obesity independent elevations in both nocturnalCitation26 and 24 hour urinary catecholaminesCitation27–Citation30 in patients with OSA.Citation31 It is this heightened sympathetic activity that has been postulated as a mechanism for systemic hypertension in OSA by peripheral vascular remodeling and increased vascular resistance. Elevations in sympathetic activity in OSA appear to be strongly linked to chemoreflex activation driven by IH. Hypoxic stimulation of the carotid body leads to a reflex increase sympathetic activity, ventilation, HR, and BP. Notably, chronic IH has been shown to lead to long-lasting elevations in sympathetic activity by long-term facilitation.Citation32
Animal and human studies have explored the role of IH on sympathoactivation and subsequent BP elevation. Rats exposed to IH (8 hours/day over 35 days) showed a striking increase in mean BP of 13.7 mmHg when compared with controls.Citation33 Subsequent studies found that not only did the IH lead to a significant increase in BP in rats, but surgical denervation of peripheral chemoreceptors prevented the increase.Citation34 Furthermore, adrenal demedullation and chemical denervation of the peripheral sympathetic nervous system also prevented the increase in BP. Human studies utilizing IH have been able to show increases in sympathetic activity and BP over a shorter time period. Mean BP increased by 4 mmHg in ten males exposed to IH for 6 hours/day for 4 days.Citation35 Similarly, in twelve healthy subjects, 2 weeks of IH exposure increased SBP and diastolic BP (DBP) (by 8/5 mmHg) and MSNA.Citation36 The role of sleep arousals in the development of hypertension was examined in dogs by simulating obstructive apneas during sleep.Citation37 When comparing repetitively induced apneas with arousal from sleep with auditory induced arousals without respiratory disturbance, nocturnal mean BP increased during both protocols, but only the apneas resulted in an increase in daytime BP (~16 mmHg). This study suggests that the association between OSA and hypertension is specific to the apnea-arousal cycle and cannot be explained by increased arousals alone.
In addition to the role of hypoxia, permanent dysregulation of the cardiovascular control system may lead to sympathetic overactivity. The arterial baroreceptors are mechanoreceptors mainly located in the carotid sinuses and aortic arch. They respond to changes in carotid or aortic stretch elicited by rises or falls in arterial pressure. The repetitive cardiovascular surges previously described may have an important role in the impairment of baroreflex control seen in OSA patients.Citation38–Citation40
Whilst IH increases chemoreflex function, data is emerging showing that it also decreases baroreflex function. This imbalance between the chemoreflex and baroreflex contributes to the sympathetic activation by IH and may be mediated by the generation of reactive oxygen species (see section on “Oxidative stress”).Citation41
Activation of the renin-angiotensin-aldosterone system
Activation of the renin-angiotensin-aldosterone system (RAAS) is another potential mechanism for the development of hypertension in OSA. The RAAS is a complex hormone system that regulates BP. Briefly, angiotensin II is a potent vasoconstrictor which also stimulates the secretion of the hormone aldosterone which alters sodium handling, resulting in fluid retention which also increases BP. One study reported increased angiotensin II and aldosterone levels in 24 patients with OSA when compared to 18 control subjects,Citation42 whilst a second study demonstrated that the increase in BP from IH is abolished by blockade of the angiotensin II receptors.Citation43 This suggests a role for the RAAS in the pathophysiology of hypertension associated with IH in OSA; however, more studies are required.
Inflammation
The repetitive oxygen desaturation, and more importantly resaturation, that characterizes IH in OSA may also contribute to the development of inflammation. Systemic inflammation is strongly linked to the pathogenesis of atherosclerosis and hypertension; however, its role in OSA is confounded by the presence of obesity, a chronic inflammatory state. Circulating inflammatory markers such as tumor necrosis factor-alpha (TNF-α), C-reactive protein (CRP), and interleukin-6 (IL6) have been reported to be elevated in OSA, although some studies have yielded conflicting results possibly due to the confounding effects of obesity. One study found elevated CRP and IL6 levels in OSA patients when compared with controls, however, the patients with OSA were more obese.Citation44 Subsequent studies have shown increased levels of CRP in patients with OSA that were independent of body mass index (BMI).Citation45–Citation47 Conversely, two studies found that obesity per se, rather than OSA, is a better predictor of CRP.Citation48,Citation49 Furthermore, a large community study failed to detect an independent association between CRP and OSA after adjustment for BMI,Citation50 suggesting that the OSA-CRP relationship may be primarily driven by obesity. However, the Icelandic Sleep Apnea Cohort (n = 454)Citation51 recently found that OSA severity is an independent predictor of IL 6 and CRP levels but interacts with obesity such that this association is found only in obese OSA patients.
The evidence for increased levels of the pro-inflammatory cytokine TNF-α, however, is far more convincing. Several case-control studies have demonstrated elevated circulating TNF-α in patients with OSA when compared to controls, independent of obesity.Citation52,Citation53 Importantly, the oxygen desaturation index has been shown to be independently associated with TNF-α levels in OSA, supporting the key role of IH as a mediator of the inflammatory response.Citation54
Oxidative stress
IH in OSA may also contribute to the development of hypertension by increasing oxidative stress. Oxidative stress results in the production of reactive oxygen species and reduces circulating nitric oxide (NO), a key endothelial derived molecule that mediates control of vascular tone. Impaired NO release from endothelial cells leads to vasoconstriction and is regarded as an initiator and promoter of cardiovascular disease in patients with OSA. In this context, several studies have documented increased markers of oxidative stress in OSA patients when compared to controls, including superoxide levels in blood neutrophilsCitation55 and 8-isoprostane levels in blood and exhaled breath condensate.Citation56,Citation57 One study found that exhaled 8-isoprostane levels correlated positively with the AHI,Citation56 whilst another study also found lower nitrate and nitrite levels (reflecting lower overall NO production) in the OSA group.Citation57
Endothelial dysfunction
Endothelial dysfunction is thought to play a fundamental role in the development of atherosclerosis and hypertension, and often precedes cardiovascular disease. Several studies have reported endothelial dysfunction by impaired endothelium-dependent vasodilation in patients with OSA independent of obesity.Citation58–Citation60 This is likely mediated by a reduction in nitric oxide,Citation61 possibly due to increased oxidative stress. Furthermore, there have been at least two randomized trials with continuous positive airway pressure (CPAP) demonstrating improved endothelial function with treatment of OSA.Citation62,Citation63
Epidemiology of OSA and hypertension
The increased prevalence of hypertension in OSA populations, and of OSA in hypertension populations, has been a driving force for establishing the direction of causality.
Prevalence of OSA in hypertension
In studies which have examined the prevalence of OSA amongst hypertensive populations, 20%–40% of individuals have been subsequently diagnosed with comorbid OSACitation64–Citation66 with levels as high as 71% amongst drug resistant hypertensives.Citation67
Prevalence of hypertension in OSA
There are also multiple epidemiological studies in both clinic and community dwelling populations examining the prevalence and incidence of hypertension in OSA. In most, hypertension is identified from a static measure of office BP exceeding 140/90 mmHg and/or the prescription of BP lowering medication. A vast majority are cross-sectional studies, some of which include > 1500 subjects. An advantage of these larger studies is their adequate power to explore confounding effects from traditional risk factors including obesity, gender, age, and smoking. In a study involving 2677 adults who were referred to a sleep clinic, after adjusting for age, BMI, and gender, the odds of hypertension increased by 1% for every unit (event/hour) increase in the AHI, with the prevalence levels for hypertension being 22.8%, 36.5%, 46%, and 53.6% in subjects with no, mild, moderate, and severe OSA, respectively.Citation68 Similarly, in a studyCitation69 of 1741 community dwelling subjects with suspected OSA who subsequently underwent polysomnography, both mild and moderate to severe OSA was significantly associated with the presence of hypertension. The age and BMI adjusted odds ratio (OR) for hypertension in mild OSA was 2.29, whilst that for moderate or severe OSA was 6.85. Interestingly, when the analysis was stratified by age and gender, this association was predominantly present in younger (<50 years old) males.Citation69
There are also some large prospective studies that were initially designed to examine the prevalence of OSA in the community which have also examined the OSA-hypertension association. The most notable of these community based studies (which are less prone to the pitfalls of selection bias) are the Wisconsin Sleep Cohort Study (WSCS) and the Sleep Heart Health Study (SHHS).Citation70,Citation72 In the WSCS, the cross-sectional analysis of 1060 participants aged 30–60 years revealed that BP increased linearly with increasing AHI independent of age, gender, and BMI.Citation70 In addition, the BMI adjusted OR for having hypertension (SBP ≥ 140 mmHg, and/or DBP ≥ 90 mmHg, or use of BP medication) was 1.21, 1.75, and 3.07 for AHI cutoffs set at 5, 15, and 30 events/hour, respectively. The increase in risk of hypertension in this community sample was 4% for every unit increase in AHI. An important add-on component to this study has been the inclusion of ABPM in 147 subjects.Citation71 The data revealed that individuals with OSA had a 9/5 mmHg and 9/4 mmHg greater daytime and nighttime BP, respectively, than a nonOSA group. Furthermore, after controlling for obesity, age, and gender, there was a dose-response relationship between OSA severity and hypertension with ORs ranging from 2 for milder OSA (AHI = 5 events/hour) to 5 for more severe OSA (AHI = 25 events/hour).
In an older (>40 years) and ethnically more diverse group from the SHHS involving 6132 subjects, the BMI, smoking, and alcohol intake adjusted OR for hypertension comparing severe OSA to no OSA was lower, but still significant, at 1.37.Citation72 Significant associations were still present in analyses stratified by gender, age, ethnicity, and BMI. Furthermore, in a subanalysisCitation72 of 3670 subjects not taking antihypertensive medication, AHI was positively associated with BP in a dose dependent manner. It is important, however, to highlight that the level of risk is somewhat weakened in older individuals to the point where a subsequent analysis revealedCitation73 no association with hypertension for any AHI severity in subjects aged > 60 years. In contrast, significant associations were present for all levels of OSA in subjects aged between 40–59 years.Citation73 Although there are no clear reasons for the lack of association between OSA and hypertension in the elderly, survival bias or perhaps the age-related reduction in the cardiovascular response to arousal from sleepCitation74 may play a role.
Although a meta-analysis of pediatric studies published before 2007Citation75 found no evidence that OSA in childhood is associated with elevated BP, as BP elevation seen in association with childhood OSA rarely surpasses the 95th percentile, more recent large studies have shown an association. ABPM in 306 children from the general community showed, independent of obesity, increasing BP levels with increasing OSA severity, and children with moderate to severe disease (AHI > 5 events/hour) were at significantly higher risk for nocturnal systolic (OR 3.9) and diastolic (OR 3.3) hypertension.Citation76 A second ABPM study also reported higher nocturnal and diurnal BP in children with OSA (>5 events/hour).Citation77 In addition, in 140 children who had ABPM every 15 minutes, 24 hour BP and HR were significantly increased in children with OSA when compared with healthy controls.Citation78 Similar findings occurred in a population sample of 700 elementary school children where there was a dose-response relationship between AHI and SBP after adjusting for multiple confounders including age, gender, and BMI.Citation79 However, as only one quarter of the sample had OSA and most of these were mild in severity, the strength of the association is unclear. Interestingly, snoring was significantly associated with BP, independent of OSA. However, this finding is in contrast to a later study showing that parentally reported snoring was not independently associated with BP, carotid artery intima media thickness, or measures of arterial stiffness.Citation80
Incident hypertension in OSA
Collectively, the cross-sectional studies strongly support an association between OSA and hypertension; however, the adjusted ORs for hypertension seem to vary considerably between studies. This variation may be due to differences in a number of factors between studies including baseline age and BMI, as well as ethnically homogenous versus diverse populations. As with all cross-sectional studies however, they are unable to demonstrate causality since the temporal development of OSA and hypertension in relation to one another are unknown. To try and answer this question, there have been four longitudinal cohort studies which have allowed examination of incident and/or persistence of hypertension at follow-up. In the WSCS, 709 participants were followed up to 4 years after baseline.Citation81 After adjusting for baseline hypertension status, age, gender, alcohol, and smoking, relative to an AHI of 0 events/hour at baseline, the ORs for the presence of hypertension at follow-up were 1.42 with an AHI of 0.1–4.9 events/hour, 2.03 with an AHI of 5.0–14.9 events/hour, and 2.89 with an AHI of >15.0 events/hour. Similar findings occurred in the more recent Zaragoza Sleep Cohort Study, a prospective study of 1889 subjects without hypertension who were referred for polysomnographic investigation of OSA.Citation82 The investigators assessed the occurrence of new onset hypertension in subjects without OSA and in subjects who remained untreated for their OSA. Compared to controls, all untreated groups had significantly increased hazard ratios for developing new onset hypertension. Of particular importance, there has also been a longitudinal analysisCitation83 over 7.2 years of the WSCS subsample that underwent ABPM (n = 328). The odds for incident systolic nondipping were 3.1 and 4.4 for mild (AHI = 5–15 events/hour) and moderate to severe (AHI ≥ 15 events/hour) OSA, respectively.Citation83
In contrast, incident hypertension in OSA is not supported by either of the two other community cohort studies.Citation84,Citation85 The SHHS followed up 2470 subjects for 5 years who were free of hypertension at baseline and found that the risk for incident hypertension significantly increased with increasing baseline AHI.Citation84 However, in the models which adjusted for baseline BMI, the risk was markedly attenuated and became nonsignificant. In addition, the Vitoria Cohort StudyCitation85 in Spain assessed hypertensive status after 7.5 years in 1180 subjects aged 30–70 years who were free from hypertension at baseline. At baseline, all participants underwent a home-based respiratory study and then approximately half also underwent polysomnography to validate the home study. The age, gender, BMI, alcohol, caffeine, and tobacco consumption adjusted OR for incident hypertension according to respiratory disturbance index was not statistically significant leading the authors to conclude that a causal relationship between OSA and hypertension in the middle aged general population may not exist.Citation85 Finally, we are not aware of any longitudinal cohort studies in children where objectively defined measures of OSA at baseline predict incident hypertension.
Overall, cross-sectional studies show clear associations between OSA severity and hypertension in adults but the data in children is less clear. However, not all longitudinal studies in adults support a causal relationship. Further cohort studies which begin at ages where OSA and hypertension are both absent but continue during the evolution of either condition are needed to more clearly discern the natural history of the two disease processes.
Treatment of OSA and treatment of hypertension in OSA patients
Effects of OSA treatment on blood pressure
There are multiple observational studies suggesting that treatment of OSA with CPAP lowers BP. However, it has only been in the past decade that these effects have been more rigorously tested using randomized control trial (RCT) designs. To date, there have been at least 19 RCTs with over 1600 patients examining the effects of OSA treatment on BP.Citation86 In these studies, the control involved sham (subtherapeutic) devices, placebo pills, or no treatment. Most, but not all, studies demonstrate a BP lowering effect; however, the responses are widely variable. This variability in response has been attributed to the heterogeneity of enrolled patients in relation to age, OSA severity, sleepiness, and hypertensive status (including coexisting pharmacological antihypertensive treatment), as well as variability in treatment compliance and duration. In general, for studies with CPAP, the greatest antihypertensive effects occurred when nightly compliance was >5 hours and patients had preexisting hypertension and severe OSA. The effects are particularly marked when trials measured 24 hour ABPM where CPAP treatment was associated with reductions in both systolic and diastolic pressures during wakefulness and sleep.Citation86
In an attempt to more clearly define the response to treatment, three meta-analyses were conducted, all in 2007.Citation87–Citation89 Two analyses,Citation87,Citation88 suggested an overall small (1.5–2.5 mmHg) reduction in systolic and diastolic BP, whilst the third analysis suggested no significant reduction in any pressures.Citation89 Since these analyses, there have been a number of additional trialsCitation90–Citation92 including two large studies from Spain targeting predominantly severe OSA patients, and a third study from India targeting OSA patients with a high prevalence of metabolic syndrome. The first Spanish study, using ABPM, targeted hypertensive patients who had predominantly severe OSA and found a small (<3 mmHg) BP reduction after 3 months.Citation91 The second Spanish study, using office BP targeted nonsleepy patients, also with predominantly severe OSA, found a reduction in diastolic BP that only became evident after 12 months of treatment.Citation92 The Indian study was a 3-month CPAP-Sham CPAP crossover study where BP during CPAP was 3.9/2.5 mmHg lower than on Sham CPAP.Citation90
The overall average change in BP across all trials can be summarized as of a modest magnitude. Nevertheless, even these modest changes are likely to significantly reduce the occurrence of cardiovascular events.Citation93
More recently, there have been two studies that are the first to explore the association between CPAP therapy for OSA and incident hypertension.Citation82,Citation94 The (previously mentioned) Zaragoza Sleep Cohort Study is a prospective observational study which found significantly lower risk of incident hypertension after 12.2 years of follow-up amongst compliant CPAP users compared to nonusers.Citation82 However, it is important to consider that the observational nature of this study makes it prone to selection bias, including the possibility that CPAP users were healthier and more compliant with medication compared to the nonCPAP group. In contrast, the second study, also from Spain, followed 723 nonsleepy OSA patients randomized to CPAP or no intervention for 4 years.Citation94 The authors found a nonsignificant reduction in incident hypertension in the CPAP group when compared to the nonCPAP group. However, in a post hoc analysis, compliant CPAP use (>4 hours/night) was associated with reduction in incident hypertension. This highlights the importance of good treatment compliance in modulating the antihypertensive effect.
Pharmacological treatments effects on blood pressure
There are surprisingly few studies examining the impact of BP lowering medication in OSA patients with hypertension. Only one small RCT with 20 patients examined the five major antihypertensive drug classes.Citation95 The study used a balanced incomplete block randomization design with each patient receiving two drugs, each for 6 weeks, in a randomized order with a 3 week washout. This resulted in each agent being tested in eight patients. This study found similar reductions in daytime BP (~10 mmHg) across all classes. Although the small sample size could have limited the ability to detect between class differences in BP lowering, beta-blocker and diuretic therapy did reduce nighttime BP more than the other agents. Unfortunately, this poses a problem because both beta-blockers and diuretics have been associated with increased risk of new onset diabetes,Citation96,Citation97 with beta-blocker therapy being associated with a greater incidence of cardiovascular morbidity and mortality.Citation98 There is, however, some encouraging new data suggesting that antihypertensive treatment with a mineralocorticoid receptor antagonist in OSA patients with resistant hypertension substantially improves both BP and OSA severity.Citation99 The latter effect may be mediated through a reduction in upper airway edema, and if confirmed in larger randomized trials, these agents may provide a promising treatment for hypertension in this group.
Summary and future directions
There is an abundance of evidence implicating OSA as an important secondary cause of hypertension. The mechanisms likely involve sustained, increased sympathetic activation associated with intermittent hypoxia. In the long-term, BP elevation, together with other less well understood processes, is likely to explain the increase in cardiovascular related morbidity, events, and death that are seen in this group. While there is good evidence that effective treatment of more severe OSA in patients with hypertension improves BP, future research should be directed at more clearly identifying factors that determine individual antihypertensive responses with OSA treatment. In addition, further studies are required to explore the effect of long-term treatment of OSA on BP. Whilst OSA-induced hypertension could lead to further vascular remodeling or structural cardiac changes, it also remains to be demonstrated whether BP reduction decreases OSA-mediated cardiovascular risk. Given the consistent relationship between hypertension and risk of cardiovascular disease (such as stroke and heart failure), it is important to adopt strategies which have a maximal BP lowering effect. In populations with OSA and hypertension, the best strategy likely involves combining OSA treatment with antihypertensive medication. This combination is likely to be more effective in lowering both nocturnal and daytime BP than either treatment alone. The subsequent reduction in cardiovascular risk may be substantial.
Disclosure
The authors report no conflicts of interest in this work.
References
- EpsteinLJKristoDStrolloPJJrAdult Obstructive Sleep Apnea Task Force of the American Academy of Sleep MedicineClinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adultsJ Clin Sleep Med200915:5326327619960649
- PunjabiNMCaffoBSGoodwinJLSleep-disordered breathing and mortality: a prospective cohort studyPLoS Med200968e100013219688045
- EbertCSDrakeAFThe impact of sleep-disordered breathing on cognition and behavior in children: a review and meta-synthesis of the literatureOtolaryngol Head Neck Surg2004131681482615577774
- TrinderJKleimanJCarringtonMAutonomic activity during human sleep as a function of time and sleep stageJ Sleep Res200110425326411903855
- OhkuboTHozawaANagaiKPrediction of stroke by ambulatory blood pressure monitoring versus screening blood pressure measurements in a general population: the Ohasama studyJ Hypertens200018784785410930181
- DolanEStantonAVThomSAmbulatory blood pressure monitoring predicts cardiovascular events in treated hypertensive patients – an Anglo-Scandinavian cardiac outcomes trial substudyJ Hypertens200927487688519516185
- KarioKPickeringTGMatsuoTHoshideSSchwartzJEShimadaKStroke prognosis and abnormal nocturnal blood pressure falls in older hypertensivesHypertension200138485285711641298
- Ben-DovIZKarkJDBen-IshayDMeklerJBen-ArieLBursztynMPredictors of all-cause mortality in clinical ambulatory monitoring: unique aspects of blood pressure during sleepHypertension20074961235124117389258
- DolanEStantonAThijsLSuperiority of ambulatory over clinic blood pressure measurement in predicting mortality: the Dublin outcome studyHypertension200546115616115939805
- BoggiaJLiYThijsLInternational Database on Ambulatory blood pressure monitoring in relation to Cardiovascular Outcomes (IDACO) Investigators. Prognostic accuracy of day versus night ambulatory blood pressure: a cohort studyLancet200737095941219122917920917
- Berthon-JonesMSullivanCEVentilation and arousal responses to hypercapnia in normal sleeping humansJ Appl Physiol198457159676432751
- SomersVKMarkALZavalaDCAbboudFMContrasting effects of hypoxia and hypercapnia on ventilation and sympathetic activity in humansJ Appl Physiol1989675210121062513316
- LeuenbergerUJacobESweerLWaravdekarNZwillichCSinowayLSurges of muscle sympathetic nerve activity during obstructive apnea are linked to hypoxemiaJ Appl Physiol19957925815887592221
- GarpestadEKatayamaHParkerJAStroke volume and cardiac output decrease at termination of obstructive apneasJ Appl Physiol1992735174317481474046
- OkabeSHidaWKikuchiYRole of hypoxia on increased blood pressure in patients with obstructive sleep apnoeaThorax199550128347886644
- AliNJDaviesRJFleethamJAStradlingJRThe acute effects of continuous positive airway pressure and oxygen administration on blood pressure during obstructive sleep apneaChest19921016152615321600769
- DaviesRJCrosbyJVardi-VisyKClarkeMStradlingJRNon-invasive beat to beat arterial blood pressure during non-REM sleep in obstructive sleep apnoea and snoringThorax19944943353398202903
- JelicSBartelsMNMateikaJHNgaiPDeMeersmanREBasnerRCArterial stiffness increases during obstructive sleep apneasSleep200225885085512489890
- StoohsRGuilleminaultCCardiovascular changes associated with obstructive sleep apnea syndromeJ Appl Physiol19927225835891559936
- O’DriscollDMFosterAMNgMLAcute cardiovascular changes with obstructive events in children with sleep disordered breathingSleep200932101265127119848356
- MarcusCLGlombWBBasinskiDJDavidsonSLKeensTGDevelopmental pattern of hypercapnic and hypoxic ventilatory responses from childhood to adulthoodJ Appl Physiol19947613143208175523
- HornerRLBrooksDKozarLFTseSPhillipsonEAImmediate effects of arousal from sleep on cardiac autonomic outflow in the absence of breathing in dogsJ Appl Physiol19957911511627559214
- YoonIYJeongDUDegree of arousal is most correlated with blood pressure reactivity during sleep in obstructive sleep apneaJ Korean Med Sci200116670771111748349
- SomersVKDykenMEClaryMPAbboudFMSympathetic neural mechanisms in obstructive sleep apneaJ Clin Invest1995964189719047560081
- CarlsonJTHednerJElamMEjnellHSellgrenJWallinBGAugmented resting sympathetic activity in awake patients with obstructive sleep apneaChest19931036176317688404098
- O’DriscollDMHorneRSDaveyMJIncreased sympathetic activity in children with obstructive sleep apnea: cardiovascular implicationsSleep Med201112548348821521626
- FletcherECMillerJSchaafJWFletcherJGUrinary catecholamines before and after tracheostomy in patients with obstructive sleep apnea and hypertensionSleep198710135443563246
- Garcia-RioFRacioneroMAPinoJMSleep apnea and hypertensionChest200011751417142510807831
- MarroneORiccobonoLSalvaggioAMirabellaABonannoABonsignoreMRCatecholamines and blood pressure in obstructive sleep apnea syndromeChest199310337227278449058
- SolinPKayeDMLittlePJBerginPRichardsonMNaughtonMTImpact of sleep apnea on sympathetic nervous system activity in heart failureChest200312341119112612684302
- LamJCYanCSLaiAYDeterminants of daytime blood pressure in relation to obstructive sleep apnea in menLung2009187529129819653037
- BabcockMABadrMSLong-term facilitation of ventilation in humans during NREM sleepSleep199821770971611286347
- FletcherECLesskeJQianWMillerCC3rdUngerTRepetitive, episodic hypoxia causes diurnal elevation of blood pressure in ratsHypertension1992196 Pt 15555611592450
- LesskeJFletcherECBaoGUngerTHypertension caused by chronic intermittent hypoxia – influence of chemoreceptors and sympathetic nervous systemJ Hypertens19971512 Pt 2159316039488210
- FosterGEBrugniauxJVPialouxVCardiovascular and cerebrovascular responses to acute hypoxia following exposure to intermittent hypoxia in healthy humansJ Physiol2009587Pt 133287329919417094
- TamisierRPepinJLRemyJ14 nights of intermittent hypoxia elevate daytime blood pressure and sympathetic activity in healthy humansEur Respir J201137111912820525723
- BrooksDHornerRLKozarLFRender-TeixeiraCLPhillipsonEAObstructive sleep apnea as a cause of systemic hypertension. Evidence from a canine modelJ Clin Invest19979911061099011563
- ParatiGDi RienzoMBonsignoreMRAutonomic cardiac regulation in obstructive sleep apnea syndrome: evidence from spontaneous baroreflex analysis during sleepJ Hypertens19971512 Pt 2162116269488213
- BonsignoreMRParatiGInsalacoGContinuous positive airway pressure treatment improves baroreflex control of heart rate during sleep in severe obstructive sleep apnea syndromeAm J Respir Crit Care Med2002166327928612153958
- LombardiCParatiGCortelliPDaytime sleepiness and neural cardiac modulation in sleep-related breathing disordersJ Sleep Res200817326327018503513
- PrabhakarNRKumarGKPengYJSympatho-adrenal activation by chronic intermittent hypoxiaJ Appl Physiol201211381304131022723632
- MollerDSLindPStrungeBPedersenEBAbnormal vasoactive hormones and 24-hour blood pressure in obstructive sleep apneaAm J Hypertens200316427428012670743
- FosterGEHanlyPJAhmedSBBeaudinAEPialouxVPoulinMJIntermittent hypoxia increases arterial blood pressure in humans through a Renin-Angiotensin system-dependent mechanismHypertension201056336937720625082
- YokoeTMinoguchiKMatsuoHElevated levels of C-reactive protein and interleukin-6 in patients with obstructive sleep apnea syndrome are decreased by nasal continuous positive airway pressureCirculation200310781129113412615790
- HayashiMFujimotoKUrushibataKTakamizawaAKinoshitaOKuboKHypoxia-sensitive molecules may modulate the development of atherosclerosis in sleep apnoea syndromeRespirology2006111243116423198
- CanMAcikgozSMunganGSerum cardiovascular risk factors in obstructive sleep apneaChest2006129223323716478836
- KokturkOCiftciTUMollarecepECiftciBElevated C-reactive protein levels and increased cardiovascular risk in patients with obstructive sleep apnea syndromeInt Heart J200546580180916272771
- GuilleminaultCKirisogluCOhayonMMC-reactive protein and sleep-disordered breathingSleep20042781507151115683141
- RyanSNolanGMHanniganECunninghamSTaylorCMcNicholasWTCardiovascular risk markers in obstructive sleep apnoea syndrome and correlation with obesityThorax200762650951417251313
- TaheriSAustinDLinLNietoFJYoungTMignotECorrelates of serum C-reactive protein (CRP) – no association with sleep duration or sleep disordered breathingSleep200730899199617702268
- ArnardottirESMaislinGSchwabRJThe interaction of obstructive sleep apnea and obesity on the inflammatory markers C-reactive protein and interleukin-6: the Icelandic Sleep Apnea CohortSleep201235792193222754038
- CiftciTUKokturkOBukanNBilgihanAThe relationship between serum cytokine levels with obesity and obstructive sleep apnea syndromeCytokine2004282879115381186
- MinoguchiKTazakiTYokoeTElevated production of tumor necrosis factor-alpha by monocytes in patients with obstructive sleep apnea syndromeChest200412651473147915539715
- RyanSTaylorCTMcNicholasWTPredictors of elevated nuclear factor-kappaB-dependent genes in obstructive sleep apnea syndromeAm J Respir Crit Care Med2006174782483016840748
- SchulzRMahmoudiSHattarKEnhanced release of superoxide from polymorphonuclear neutrophils in obstructive sleep apnea. Impact of continuous positive airway pressure therapyAm J Respir Crit Care Med20001622 Pt 156657010934088
- CarpagnanoGEKharitonovSARestaOFoschino-BarbaroMPGramiccioniEBarnesPJ8-Isoprostane, a marker of oxidative stress, is increased in exhaled breath condensate of patients with obstructive sleep apnea after night and is reduced by continuous positive airway pressure therapyChest200312441386139214555570
- Alonso-FernandezAGarcia-RioFAriasMAEffects of CPAP on oxidative stress and nitrate efficiency in sleep apnoea: a randomised trialThorax200964758158619074930
- KatoMRoberts-ThomsonPPhillipsBGImpairment of endothelium-dependent vasodilation of resistance vessels in patients with obstructive sleep apneaCirculation2000102212607261011085964
- NamtvedtSKHisdalJRandbyAImpaired endothelial function in persons with obstructive sleep apnoea: impact of obesityHeart2012991303423048165
- KohlerMCraigSNicollDLeesonPDaviesRJStradlingJREndothelial function and arterial stiffness in minimally symptomatic obstructive sleep apneaAm J Respir Crit Care Med2008178998498818658111
- IpMSLamBChanLYCirculating nitric oxide is suppressed in obstructive sleep apnea and is reversed by nasal continuous positive airway pressureAm J Respir Crit Care Med200016262166217111112132
- IpMSTseHFLamBTsangKWLamWKEndothelial function in obstructive sleep apnea and response to treatmentAm J Respir Crit Care Med2004169334835314551167
- CrossMDMillsNLAl-AbriMContinuous positive airway pressure improves vascular function in obstructive sleep apnoea/hypopnoea syndrome: a randomised controlled trialThorax200863757858318390635
- FletcherECDeBehnkeRDLovoiMSGorinABUndiagnosed sleep apnea in patients with essential hypertensionAnn Intern Med198510321901954014900
- LaviePBen-YosefRRubinAEPrevalence of sleep apnea syndrome among patients with essential hypertensionAm Heart J198410823733766464973
- WorsnopCJNaughtonMTBarterCEMorganTOAndersonAIPierceRJThe prevalence of obstructive sleep apnea in hypertensivesAm J Respir Crit Care Med199815711111159445287
- GoncalvesSCMartinezDGusMObstructive sleep apnea and resistant hypertension: a case-control studyChest200713261858186218079220
- LaviePHererPHoffsteinVObstructive sleep apnoea syndrome as a risk factor for hypertension: population studyBMJ2000320723347948210678860
- BixlerEOVgontzasANLinHMAssociation of hypertension and sleep-disordered breathingArch Intern Med2000160152289229510927725
- YoungTPeppardPPaltaMPopulation-based study of sleep-disordered breathing as a risk factor for hypertensionArch Intern Med199715715174617529250236
- HlaKMYoungTBBidwellTPaltaMSkatrudJBDempseyJSleep apnea and hypertension. A population-based studyAnn Intern Med199412053823888304655
- NietoFJYoungTBLindBKAssociation of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health StudyJAMA2000283141829183610770144
- HaasDCFosterGLNietoFJAge-dependent associations between sleep-disordered breathing and hypertension: importance of discriminating between systolic/diastolic hypertension and isolated systolic hypertension in the Sleep Heart Health StudyCirculation2005111561462115699282
- GoffEAO’DriscollDMSimondsAKTrinderJMorrellMJThe cardiovascular response to arousal from sleep decreases with age in healthy adultsSleep20083171009101718652096
- ZintzarasEKaditisAGSleep-disordered breathing and blood pressure in children: a meta-analysisArch Pediatr Adolesc Med2007161217217817283303
- LiAMAuCTSungRYAmbulatory blood pressure in children with obstructive sleep apnoea: a community based studyThorax200863980380918388205
- LeungLCNgDKLauMWTwenty-four-hour ambulatory BP in snoring children with obstructive sleep apnea syndromeChest200613041009101717035432
- AminRSomersVKMcConnellKActivity-adjusted 24-hour ambulatory blood pressure and cardiac remodeling in children with sleep disordered breathingHypertension2008511849118071053
- BixlerEOVgontzasANLinHMBlood pressure associated with sleep-disordered breathing in a population sample of childrenHypertension200852584184618838624
- MarshallNSAyerJGToelleBGSnoring is not associated with adverse effects on blood pressure, arterial structure or function in 8-year-old children: the Childhood Asthma Prevention Study (CAPS)J Paediatr Child Health201147851852321535282
- PeppardPEYoungTPaltaMSkatrudJProspective study of the association between sleep-disordered breathing and hypertensionN Engl J Med2000342191378138410805822
- MarinJMAgustiAVillarIAssociation between treated and untreated obstructive sleep apnea and risk of hypertensionJAMA2012307202169217622618924
- HlaKMYoungTFinnLPeppardPESzklo-CoxeMStubbsMLongitudinal association of sleep-disordered breathing and nondipping of nocturnal blood pressure in the Wisconsin Sleep Cohort StudySleep200831679580018548823
- O’ConnorGTCaffoBNewmanABProspective study of sleep-disordered breathing and hypertension: the Sleep Heart Health StudyAm J Respir Crit Care Med2009179121159116419264976
- Cano-PumaregaIDuran-CantollaJAizpuruFObstructive sleep apnea and systemic hypertension: longitudinal study in the general population: the Vitoria Sleep CohortAm J Respir Crit Care Med2011184111299130421868499
- ParatiGLombardiCHednerJEuropean Respiratory Society, EU COST ACTION B26 membersPosition paper on the management of patients with obstructive sleep apnea and hypertension: joint recommendations by the European Society of Hypertension, by the European Respiratory Society and by the members of European COST (COoperation in Scientific and Technological research) ACTION B26 on obstructive sleep apneaJ Hypertens201230463364622406463
- BazzanoLAKhanZReynoldsKHeJEffect of nocturnal nasal continuous positive airway pressure on blood pressure in obstructive sleep apneaHypertension200750241742317548722
- HaentjensPVan MeerhaegheAMoscarielloAThe impact of continuous positive airway pressure on blood pressure in patients with obstructive sleep apnea syndrome: evidence from a meta-analysis of placebo-controlled randomized trialsArch Intern Med2007167875776417452537
- AlajmiMMulgrewATFoxJImpact of continuous positive airway pressure therapy on blood pressure in patients with obstructive sleep apnea hypopnea: a meta-analysis of randomized controlled trialsLung20071852677217393240
- SharmaSKAgrawalSDamodaranDCPAP for the metabolic syndrome in patients with obstructive sleep apneaN Engl J Med2011365242277228622168642
- Duran-CantollaJAizpuruFMontserratJMSpanish Sleep and Breathing GroupContinuous positive airway pressure as treatment for systemic hypertension in people with obstructive sleep apnoea: randomised controlled trialBMJ2010341c599121106625
- BarbeFDuran-CantollaJCapoteFSpanish Sleep and Breathing GroupLong-term effect of continuous positive airway pressure in hypertensive patients with sleep apneaAm J Respir Crit Care Med2010181771872620007932
- TurnbullFEffects of different blood-pressure-lowering regimens on major cardiovascular events: results of prospectively-designed overviews of randomised trialsLancet200336293951527153514615107
- BarbeFDuran-CantollaJSanchez-de-la-TorreMSpanish Sleep and Breathing NetworkEffect of continuous positive airway pressure on the incidence of hypertension and cardiovascular events in nonsleepy patients with obstructive sleep apnea: a randomized controlled trialJAMA2012307202161216822618923
- KraicziHHednerJPekerYGroteLComparison of atenolol, amlodipine, enalapril, hydrochlorothiazide, and losartan for antihypertensive treatment in patients with obstructive sleep apneaAm J Respir Crit Care Med200016151423142810806134
- ZillichAJGargJBasuSBakrisGLCarterBLThiazide diuretics, potassium, and the development of diabetes: a quantitative reviewHypertension200648221922416801488
- BangaloreSParkarSGrossmanEMesserliFHA meta-analysis of 94,492 patients with hypertension treated with beta blockers to determine the risk of new-onset diabetes mellitusAm J Cardiol200710081254126217920367
- LindholmLHCarlbergBSamuelssonOShould β blockers remain first choice in the treatment of primary hypertension? A meta-analysisLancet200536694961545155316257341
- GaddamKPimentaEThomasSJSpironolactone reduces severity of obstructive sleep apnoea in patients with resistant hypertension: a preliminary reportJ Hum Hypertens201024853253720016520