Abstract
The intestine is the largest bacterial ecosystem and immune response organ of the human body. The microbiota regulates the metabolic and immune functions of the host through their metabolites. Short-chain fatty acids (SCFAs) are part of the metabolites of the gut microbiota (GM), providing energy to intestinal epithelial cells and regulating the immune system. A decrease in SCFA-producing bacteria, imbalanced effector T-helper cells (Th cells), and increasing corresponding inflammatory cytokine were found in both animal models and clinical patients with obstructive sleep apnea (OSA) and hypertension (HTN). Intervention with probiotics, prebiotics, or postbiotics in animal models simulating OSA-associated HTN restored blood pressure to normal, which allows the hypothesis that GM are involved in the pathophysiology of OSA-induced HTN patients through their metabolites’ SCFAs; however, the exact regulatory mechanism is not completely clear. This review describes the potential mechanisms of SCFAs, a major metabolite of the GM, in the pathology of OSA-induced HTN, from the perspective of immune system regulation in the available studies.
Introduction
Gut microbiota (GM) dysbiosis caused by obstructive sleep apnea (OSA) and hypertension (HTN) has received increasing attention. OSA and HTN are both common chronic diseases in middle-aged and elderly people; they share many common pathogenic factors, including age, gender, race, obesity, etc., so, they often coexist.Citation1 It is worth mentioning that obesity is the main risk factor for OSA. Morbid obesity is common in OSA patients,Citation2 and is also an important factor leading to HTN and HTN-related target organ damage.Citation3 However, excluding the common pathogenic factors still showed that OSA is an independent risk factor for HTN; about 35% of general OSA patients have primary HTN,Citation4 and the severity of HTN is positively correlated with the apnea-hypopnea index (AHI).Citation5,Citation6 Additionally, OSA-related comorbidities mediate intermittent hypoxia or chronic inflammation depending on the severity of OSA such as cardiovascular events, ora-nasal disorders, or neurocognition.Citation7–Citation9 In an animal model of chronic intermittent hypoxia (CIH)-induced OSA, a gavaged with cecal contents from OSA-induced HTN rats into normotensive OSA rats resulted in a significant increase in blood pressure after 7–14 days, suggesting a causal relationship between GM dysbiosis and HTN.Citation10
Gut commensal bacteria, through their metabolites such as short-chain fatty acids (SCFAs), trimethylamine-N-oxide (TMAO), and primary or secondary bile acids, communicated with the host through metabolism and immune host homeostasis.Citation11–Citation13 Among these, SCFAs are the main metabolite produced by the fermentation of indigestible dietary fibre in the colon by numerous bacteria. Acetate, propionate, and butyrate account for more than 95% of SCFAs (the proportion structure in the intestine is 3:1:1).Citation13 Butyrate, a nutrient in the colon epithelium, is mainly absorbed in the colon epithelium and promotes the close connection between the intestinal epithelium. Propionate and butyrate are mainly metabolized by liver cells after passing through the portal vein, and only a small amount of SCFAs enters the systemic circulation.Citation14 SCFA-producing bacteria mainly include Anaerobic bacteria, Bifidobacterium, Lactobacillus, Streptococcus, and fungi.Citation15 SCFAs are involved in the host physiopathological processes through regulation of the immune system,Citation16 adipogenesis,Citation17 oxidative stressCitation18 and sensitivity to insulin,Citation19 and its receptors are widely present in the intestinal epithelium, adipose, pancreatic islets, T cells, sympathetic nerves, and so on. It directly or indirectly regulates immunity through the host metabolism as a hormonal signaling molecule through specific receptors.Citation15,Citation20 SCFAs produced by intestinal bacteria play an important role in maintaining blood pressure stability and immune system homeostasis at normal physiological concentrations, moreover, propionate also protects from hypertensive end-organ damage.Citation21
Changes in GM have been observed in many diseases, including inflammatory bowel disease (IBD),Citation22 type 2 diabetes,Citation23 obesity,Citation24 psychiatric disorders,Citation25 asthma,Citation26 and cardiovascular disease.Citation27 In recent years, Changes of GM dysbiosis with its metabolites have been observed in patients with HTN and OSA.Citation28,Citation29 However, the relevant regulatory mechanisms are not yet cleared. In this review, we will discuss the GM dysbiosis and the changes of SCFAs associated with OSA, which result in an immune imbalance in OSA-associated HTN patients, and elaborate on the potential mechanisms of SCFAs via the immune pathway in OSA-associated HTN patients.
Immunoregulatory Effect of SCFAs on Host
The intestinal tract is the largest micro ecosystem and the largest immune organ in the human body, its four main phylum are Firmicutes, Bacteroidetes, Actinobacteria, and Proteobacteria. The Firmicutes to Bacteroidetes (F/B) ratio is commonly used as a biomarker to assess pathological status. An increased F/B ratio is widely considered a sign of GM imbalance.Citation30,Citation31
Gut commensal bacteria communicate with the host physiology and immune system through their metabolites. In recent years, the understanding of host immune regulation by SCFAs have gradually emphasized; they can influence the expression of intrinsic immune cells and cytokines in a microenvironment through their trophic effects on the intestinal mucosaCitation32 and maintain host homeostasis by regulating the differentiation of T lymphocytes for adaptive immune response according to the needs of the host.Citation33 Long-term CIH and sleep fragmentation (SF) are the main hallmarks of OSA, leading to inappropriate sympathetic and renin-angiotensin-sin-aldosterone system (RASS) activation and insufficiency of blood supply to systemic organs. In OSA patients, intermittent hypoxia leads to intestinal mucosal ischemia-reperfusion injury and forms an anaerobic environment and insufficient oxygen supply to intestinal mucosa, leading to a change in gut bacteria structure and abundance, an increase in the F/B ratio, and decrease in SCFA-producing bacteria and anti-inflammation-associated bacteria, influencing intestinal barrier integrity.Citation29,Citation34,Citation35 This aggravates intestinal mucosal nutrition disorder and leads to intestinal microenvironment disruption and intestinal epithelial tissue damage, resulting in GM dysbiosis and even local IBD, which is particularly obvious in severe OSA patients.Citation36,Citation37 Plasma intestinal fatty acid binding protein (I-FABP) is a small molecule protein released into circulation when intestinal wall membrane integrity is lost and is a highly sensitive marker of ischemic intestinal mucosa.Citation38 Plasma I-FABP levels in OSA patients were significantly higher than those in healthy controls.Citation39 The Plasma D-lactate acid (D-LA) level reflected intestinal mucosal permeability and damage degree in OSA patients and was positively correlated with AHI.Citation40
SCFAs participate in the adaptive immune response by acting on intracellular specific G-protein-coupled receptors (GPR)Citation41 and histone deacetylase (HDAC),Citation33 affecting vasodilation, atherosclerotic progression, and blood pressure regulation. The main GPRs of SCFAs include Gpr41 (also known as free fatty acid receptor 3, FFAR3), Gpr43 (FFAR2), Gpr109a (niacin receptor, also known as HCAR2), and the G protein-coupled olfactory receptor 78 (Olfr78).
Local Immune Regulation by SCFAs in the Intestine
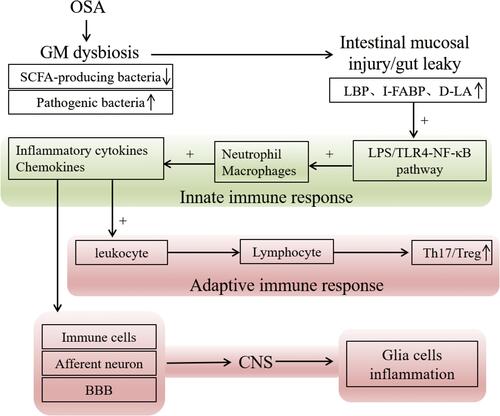
SCFAs, mainly acetate, provides nutrients for intestinal epithelial cells (IECs). It promotes the differentiation of intestinal epithelial goblet cells and mucus secretion,Citation42 which is conducive to increasing the tight junction of IECs and improving the immune defence of IECsCitation43,Citation44 to inhibit bacteria and their metabolites, such as lipopolysaccharide (LPS), a cell wall component of Gram-negative bacteria, from the intestine to the systemic circulation.Citation45 The LPS-binding protein (LBP) serves as a surrogate marker of LPS from the gut, examined for underlying low-grade endotoxemia, children and adults with OSA all exhibited increased LBP levels, which were strongly positively correlated with insulin resistance and dyslipidemia.Citation46,Citation47 When SCFAs levels decrease, the intestinal mucosal barrier function is weakened, pathogen-associated molecular patterns (PAMPs) from bacteria, viruses and fungi activate Toll-like receptors (TLRs) signaling through the disrupted intestinal mucosa,Citation48 and LPS moves into the body’s circulation through TLR4 stimulation of dendritic cells (DCs),Citation49 activating nuclear factor kappa B (NF-κB), mobilizing antimicrobial defenses, recruiting neutrophils and macrophages, and leading to the production of proinflammatory cytokines and chemokines and chemokines.Citation36 Chemokines migrate leukocytes to the lesion site and activate it; monocytes differentiate into macrophages at the lesion site and become foam cells after swallowing lipids, which are the main pathological factor of inflammatory mediators for insulin resistance and atherosclerosis.Citation50 The intestinal microenvironment of inflammatory cytokines and immune dysregulation induces T cell differentiation direction, protects the organism from harmful microorganisms through an adaptive immune response.Citation51 Proinflammatory cytokines reach the CNS via afferent neurons, immune cells, and the blood-brain barrier (BBB),Citation52 activating microglia and releasing proinflammatory cytokines to initiate defense responses; however, excessive inflammatory responses cause damage to neurons leading to potential neurocognitive dysfunction. Studies have shown that SCFAs counteract the LPS-induced inflammatory response.Citation53 Interestingly, under normal physiological conditions, SCFAs promote the growth and development of microglia in the brain, increasing cellular immunity and the immune defense of the brain.Citation54 It is thus clear that SCFAs have immunomodulatory capacity not only in the intestinal and peripheral circulation but also in the nervous system ().
Figure 1 OSA causes immune disorders. Dysbiosis of the intestinal flora, leads to a decrease in SCFA-producing flora, an increase in pathogenic bacteria in feces, and damage to the intestinal mucosa, which increases circulating LPS, which activates TLR4 receptors and activates NF-κB inflammatory channels triggering an inflammatory cascade of intestinal, circulatory, and neurological inflammatory response.
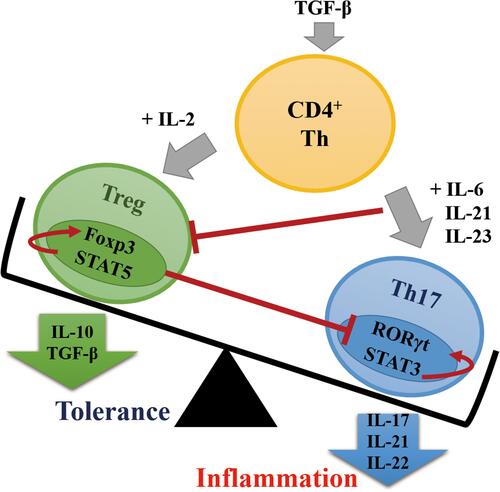
It has been shown that CD4+ T cells are important factors of intestinal homeostasis, and their differentiation is dependent on cytokines in the microenvironmentCitation55 (), the main subtypes of CD4+ T cells are effector T cells (T-helper cells, Th cells; including Th1, Th2, and Th17) and regulatory T cells (Treg), with Th17 and Treg, Th1 and Th2 are highly plastic and interconvertible.Citation56–Citation58 Antigen activates CD4+ T cells, which differentiate into different functional subtypes of Th cells and their corresponding cytokine profiles according to the body’s needs to suppress undesirable microorganisms. In the presence of low concentrations of transforming growth factor-β (TGF-β) and inflammatory cytokines IL-6, IL-21 and IL-23, CD4+ T cells differentiate into Th cells and activate CD4+CD25+ forkhead box P3 (Foxp3) to reprogram into Th17 cells, which played an anti-inflammatory and pathogen clearance role by secreting inflammatory cytokines IL-17A, IL-17F, IL-21, and IL22 under the regulation of retinoic acid-associated solitary nucleus receptor γt (ROR-γt).Citation56 After the initial effector T cell response, T cell receptor (TCR) induces high expression of Foxp3 in response to proinflammatory factor deficiency and high TGF-β concentration, promoting the differentiation of CD4+ T cells into Treg cells, and the transformation of Th cells into Treg cells, which maintain the body’s tolerance by releasing IL-10 and TGF-β and prevent damage to the organism from excessive inflammatory responses.Citation59 Foxp3+ Treg cells and Th17 cells are the most abundant cells in the mucosal barrier and found exclusively in the lamina propria, where they are functionally opposed and can be interconverted in different microenvironments.Citation60 The Th17/Treg cell balance is a vital consideration for “intestinal health”.Citation61 In OSA patients, the Th17/Treg ratio is increased.Citation54 The anti-inflammatory properties of Treg cells have been shown to prevent oxidative activation of vascular endothelial cells and migration and adhesion of leukocytes, thereby reducing the development of atherosclerosis; whereas, depletion of Treg cells promotes the progression of atherosclerotic lesions.Citation62–Citation65 Dietary fiber supplementation can reduce systemic inflammation and immune response, while low dietary fiber is associated with the increase in inflammatory diseases, and the main product of dietary fiber metabolism through intestinal bacteria is SCFAs.Citation66,Citation67 Butyrate in SCFAs enhances intestinal barrier function by reversing the abnormal expression of zonula occludens-1 (ZO-1), reducing endotoxin translocation, thereby inhibiting neutrophil infiltration, macrophage activation, and production of proinflammatory cytokines, and also increasing the release of IL-10 anti-inflammatory factor.Citation14
Figure 2 Differentiation of CD4+ T cells into Th17 or Treg cells under different cytokine environments. Foxp3 is highly expressed in the absence of proinflammatory factors and induced by high concentrations of TGF-β, promoting the differentiation of CD4+ T cells into Treg cells and CD4+ T cells into Th17 cells, when low concentrations of TGF-β are present together with the inflammatory cytokines IL-6, IL-21, and IL-23.
Systemic Immune Regulation by SCFAs
SCFAs also act on their specific GPRs to regulate host immunity (), among which the regulation of butyrate on the immune system is the most mature. Butyrate activates signal transducer and activator of transcription and the mammalian target of rapamycin (mTOR) on Th1 cells, upregulates transcription factor B lymphocyte-induced maturation protein-1, and induces Th1 cells to secrete IL-10 by acting on GPR43 to limit the excessive inflammatory response of Th cells. More than 50% of IL-10 is produced by well-differentiated Th1 cells mediated by butyrate through GPR43 on IECs.Citation68 Butyrate also acts on the GPR109a to induce the differentiation of Treg and T cells that produce IL-10 by increasing the anti-inflammatory properties of colon macrophages and DCs.Citation69 Gpr109a (encoded by niacin receptor 1) is activated not only by butyrate but also by niacin, which is also one of the metabolites of enterobacteria and inhibits intestinal inflammation.Citation70
Figure 3 SCFA regulate blood pressure through receptor channels and the immune system. Acetate and propionate mediate renin secretion via Olfr78 receptors to increase blood pressure and endothelium-dependent vasodilation via Gpr41 to decrease blood pressure; butyrate acts on Gpr43, upregulates Blimp-1, induces IL-10 secretion from Th1 cells, and on Gpr109a, induces Tregs differentiation and IL-10 secretion; butyrate and propionate inhibit HDAC activity through the mTOR-S6k pathway, suppress Th1 and Th17 differentiation, and induce CD4+CD25+ Treg differentiation with IL-10 secretion. Black arrow ![]()
 represents blood pressure regulation.
represents blood pressure regulation. , elevated;
, elevated;  , reduced; Blimp-1, B lymphocyte-induced maturation protein 1; BP, blood pressure; Olfr78, Olfactory receptor 78; vSMCs, vascular smooth muscle cells; HDAC, histone deacetylase; GPR, G-protein-coupled receptors; Th, effector T cells; Treg, regulatory T cells; Foxp3, forkhead box P3; IL, interleukin.
, reduced; Blimp-1, B lymphocyte-induced maturation protein 1; BP, blood pressure; Olfr78, Olfactory receptor 78; vSMCs, vascular smooth muscle cells; HDAC, histone deacetylase; GPR, G-protein-coupled receptors; Th, effector T cells; Treg, regulatory T cells; Foxp3, forkhead box P3; IL, interleukin.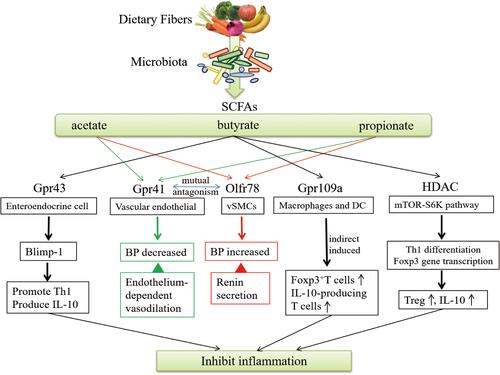
In colon cell studies, butyrate was found to strongly induce Foxp3 gene transcription and induce differentiation of naive CD4+ T cells into peripherally derived Tregs (pTregs), which secrete cytokine IL-10 and suppress excessive immune responses caused by Th1 and Th17.Citation68,Citation71–Citation74 An in vitro experiment showed that SCFAs could still regulate GPR41−/− or GPR43−/− cells obtained from mice, suggesting that its regulatory mechanism was independent of GPR41 and GPR43. Further studies showed that propionate and butyrate could effectively inhibit HDAC activity once entering activated T cells.
Propionate and butyrate can inhibit HDAC to regulate body immunity, while acetate does not possess this inhibitory activity on HDAC.Citation75 Propionate and butyrate mainly inhibited HDAC activity in T cells, enhanced the phosphorylation of ribosomal protein S6 (a target of mTOR pathway), and induced the acetylation of p70 S6 kinase (S6K), which regulates IL-10, interferon-gamma (IFN-γ), and IL-17 expressions ecreted by Th and Treg cells, according to the cytokine environment and immune background.Citation33 In vitro peripheral blood mononuclear cells (PBMCs) from healthy donors plus SCFAs intervention showed that butyrate, in particular, may promote upregulation of anti-inflammatory cytokines resulting in induction of CD4+CD25+ Treg.Citation73
Both CIH and SF of animal models simulating OSA demonstrated characteristic changes in the GM composition of induced animals, with a significant increase in the abundance of mucus-degrading Prevotella and Desulfovibrio, decreasing the relative abundance of SCFA-producing Ruminococcaceae family and butyrate-producing bacteria, and increasing lactate-producing bacteria.Citation34,Citation76,Citation77 Then tight junctions between colonic epithelium were destroyed, LPS increased and entered into circulation, stimulating DCs by activating TRL4 through the disrupted mucosal barrier,Citation49 The inflammatory environment was speculated to promote the differentiation of CD4+T cells into Th1 and Th17 and secrete the corresponding cytokines, as evidenced by increased levels of the inflammatory cytokines IL-1, IL-6, and tumor necrosis factor (TNF)-α in peripheral blood.Citation36 Excessive inflammatory response damages vascular endothelial cells and tissues. The endothelium-dependent vasodilator nitric oxide decreased, causing endothelium-dependent vasodilatation disorders. Furthermore, endothelial oxidative stress, resulting in increased low-density lipoprotein (LDL) and foam cells causing atherosclerosis, in turn, promoted the development of HTN, and increased coronary heart disease, stroke, sudden death, and other cardiovascular and cerebrovascular adverse complications.Citation1,Citation10,Citation78
Clinical studies have revealed that cardiovascular risk is significantly increased in patients with non-dipper HTN which is mostly combined in OSA patients.Citation79 OSA patients with HTN have significantly higher circulating Th1 and Th17 cells relative to OSA patients without HTN, which are accompanied by higher levels of IFN-γ, TNF-α, IL-6, IL-17 levels, and C-reactive protein (CRP) levels, and lower Th2, IL-4, and IL-10 levels.Citation78–Citation81 There is also increased inflammatory activity of the organism, decreased insulin sensitivity, increased dyslipidemia, and accelerated atheromatous plaque formation and vulnerability.Citation82,Citation83 Atherosclerosis, together with insulin resistance, constitute important factors in the development of secondary HTN in patients with OSA, while the anti-inflammatory effect of IL-10 facilitates the improvement of vascular endothelial function and the control of blood pressure.Citation84,Citation85 The chronic low-grade inflammation that developed in OSA patients is presumed to be an important factor contributing to HTN and terminal organ damage.
SCFAs Regulates Blood Pressure Through GPRs
The bacterial metabolites, SCFAs, are closely linked to HTN.Citation86 Significant declines in GM abundance and diversity were found in HTN animal models such as Dahl-sensitive rats,Citation87 spontaneously HTN rats,Citation88 angiotensin II–induced HTN rats,Citation23 deoxycorticosterone acetate salt miceCitation89 and HTN patientsCitation28 with an increased F/B ratio, decreased acetate and butyrate-producing bacteria, and increased lactate-producing bacteria. Lactate production positively correlated with systolic blood pressure (SBP). Meanwhile, Ruminococcaceae and Prevotellaceae families producing SCFAs negatively correlated with SBP.Citation88 Increased Gram-negative bacteria abundance and excess LPS enter the systemic circulation through damaged intestinal mucosa and promote systemic low endotoxemia. Oral administration of the broad-spectrum antibiotic minocycline or fecal microbiota transplantation (FMT) in angiotensin II–induced HTN rats model showed increased gut microbial diversity and reduced the F/B ratio and blood pressure.Citation23 Transplantation of fecal microbiota from spontaneously hypertensive rats (SHR) to normotensive Wistar-Kyoto (WKY) rats enhanced sympathetic activity, neuroinflammation, and elevated blood pressure. Correspondingly, transplantation of WKY fecal microbiota to SHR reduced neuroinflammation and sympathetic activity and lowered blood pressure.Citation90 Clinical evidence has also found that antibiotics reduce blood pressure in patients with refractory HTN.Citation91,Citation92 Above evidence suggesting that HTN is associated with gut microbiome dysbiosis.
Previous studies have confirmed that changes in the levels of butyrate, propionate, and lactate are intimately related to the regulation of blood pressure.Citation21,Citation93 It was found that SCFAs directly regulate blood pressure by acting on their specific receptors Gpr41 and Olfr78 (). Olfr78 is expressed in vascular smooth muscle cells of small resistance vessels, which are relatively enriched in the renal arterioles. Olfr78 knockout mice exhibited decreased blood pressure, suggesting that the Olfr78 agonist has an elevating effect on blood pressure in which acetate and propionate act on Olfr78 to regulate blood pressure by mediating renin secretion.Citation94 Gpr41 and Gpr43 are expressed in adipocytes, immune cells, allowing SCFA to modulate obesity and inflammation, and their expression on sympathetic, vascular endothelial and enteroendocrine cells allows SCFA to exert blood pressure-regulating effects.Citation95 Propionate directly regulates sympathetic activity through excitation of Gpr41 receptors leading to an increase in heart rate (HR),Citation95 and sympathetic nerves affect blood flow in the gastrointestinal tract by influencing the absorptive function of the epithelium and contraction of vascular smooth muscle, perhaps to some extent also affecting blood pressure levels in the host.Citation96 Intraperitoneal injection of acetate, propionate, and butyrate to unanesthetized live mice all significantly lowered mean arterial pressure and HR, and caused a negative inotropic effect by acting directly on cardiomyocytes; whereas, the hypotensive effect of intravenous injections was attenuated and had no effect on HR, possibly due to the rapid reduction in sympathetic tone by local SCFAs in the mesentery.Citation97,Citation98 In addition, Gpr41 knockout mice exhibited simple systolic HTN and were not accompanied by elevated plasma renin levels or salt sensitivity. Isolated vascular response assays revealed that acetate and propionate bound to the Gpr41 receptors on vascular endothelial cells, caused endothelium-dependent vasodilation and produced an acute hypotensive response in wildtype mice in a dose-dependent manner.Citation41
GM Dysbiosis Precede HTN Development
Evidence has shown a close relationship between OSA and HTN.Citation5,Citation10,Citation78 The longitudinal sleep cohort study in Wisconsin was the most representative; a linear relationship between AHI and 24h blood pressure was still observed after excluding the interference of body mass index (BMI), age, gender, and antihypertensive drugs. The severity of AHI was also associated with a new incidence of HTN four years later.Citation99 Thus, OSA was strongly proved to precede HTN, and is an independent risk factor for HTN.
A Chinese clinical study compared the GM in healthy controls (SBP ≤125 mmHg; diastolic blood pressure (DBP) ≤80 mmHg), pre-hypertension (preHTN; SBP, 125–139 mmHg; DBP, 80–89 mmHg) and HTN (SBP, ≥140 mmHg; DBP, ≥90 mmHg) patients. The microbiota/enterotype of preHTN subjects was very similar to HTN patients, indicating that changes in GM occurred in the prehypertensive state.Citation28 It proposed that changes in GM precede HTN, which might provide clinical evidence for the hypothesis that OSA causes GM dysbiosis and then leads to HTN. Transplanted cecum contents from SHR into normotensive WKY rats resulted in increased SBP of the latter,Citation88 similarly, transplanted cecum contents from HTN patients into germ-free (GM) mice also resulted in increased blood pressure of the latter.Citation28 These all reinforced the notion that GM play a role in blood pressure regulation.
A systematic review and meta-analysis showed that mild, moderate, and severe OSA were positively associated with the incidence of essential HTN, and that OSA significantly increased the risk of refractory HTN.Citation6 A study reviewed almost all the literature on HTN with GM and concluded that GM dysbiosis in OSA leads to intestinal mucosa barrier dysfunction resulting in a “leaky gut” phenomenon, leading to a state of systemic low-grade inflammation, and summary a hypothesis: neuroinflammation is a pathophysiological hallmark of OSA-induced HTNCitation100 in which links the gut-brain axis.
Consistent with previous studies, our preliminary studies found decreased relative abundance of SCFA-producing bacteria and increased relative abundance of pathogenic microbiota in OSA patients and IL-6 in the peripheral blood of OSA patients.Citation29 Detection of transcribed genes in fasting PBMCs from healthy controls, patients with severe OSA, and severe OSA combined with HTN, KEGG data revealed that differential genes in severe OSA combined with HTN are enriched in the mTOR pathway.Citation101 In our ongoing clinical study, we also found that excluding confounding factors such as age, BMI, other diseases, and medication history, the fecal 16sRNA microflora analysis showed that the abundance and diversity of intestinal microflora in OSA and OSA patients with HTN decreased as compared with the healthy control group, and the F/B ratio increased in OSA patients with HTN compared with severe OSA patients (data not published). Analyzed by gas chromatography and mass spectrometry (GC-LS) showed that the fecal contents of acetate and butyrate in the severe OSA group were decreased compared with the control group, also decreased in the severe OSA with HTN group (P<0.05). While serum GC-LS detection showed that level of propionate had statistical differences (P<0.05) in severe OSA patients and OSA combined with HTN patients, compared with the healthy control group (data not published). These data all suggest that GM and their major metabolite SCFA are closely related to blood pressure, and whether correcting abnormal SCFA levels in vivo can prevent or even treat hypertensive disease needs to be further explored.
Therapeutic Effects of SCFA on OSA-Induced HTN
Continuous positive airway pressure (CAPA) is the preferred treatment for moderate and severe OSA, reducing inflammatory cytokine levels and sympathetic nervous system activity, significantly reducing HTN, improving hypoxia and quality of life by reducing the AHI, and a more pronounced hypotensive effect in combined refractory hypertensive patients, but compliance with CAPA treatment is low.Citation102–Citation105 Our ongoing clinical study found differences in the relative abundance of GM in patients with severe OSA after CAPA treatment (data not published); the conclusion needs to be validated by a larger clinical sample and adherence to longer CAPA treatment.
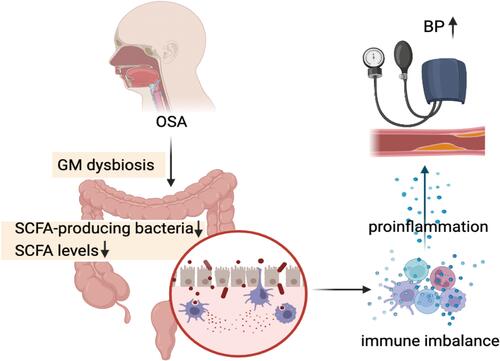
OSA patients suffer from repeated intermittent hypoxia and ischemia of the intestinal mucosa due to CIH, which in turn triggers GM dysbiosis. This includes a decrease in SCFA-producing bacteria and SCFA levels in the intestinal lumen, an increase in pathogenic bacteria as well as a rise in LPS in plasma, and impaired barrier function of intestinal epithelial tissue leading to “gut leaky”.Citation23,Citation29 At the same time, the weakened regulation of GPRs by SCFA leads to an inflammatory state, damaging the cells and tissues of the body.Citation10,Citation33,Citation68 The immune imbalance triggered by intestinal bacterial disorders and dysregulation of intestinal bacterial metabolites in OSA patients, ultimately leads to the development of hypertension ().
Figure 4 The postulated pathophysiology of OSA-associated hypertension mediating inflammation from gut microbiome dysbiosis crosstalk between with immune imbalance. In OSA patients, structural reorganization of the gut microbiota due to the altered intestinal lumen environment and the decrease of fecal SCFA lead to “leaky gut”, which triggers an intrinsic and adaptive immune response leading to a low-grade inflammatory state in the body that damages the vascular endothelium and causes vasodilatation disorders and atherosclerosis, ultimately leading to hypertension.
The composition of the GM is influenced by many factors, including age, gender, geography, genetics, lifestyle, disease, and so on, with diet structure being one of the most important. SCFAs are barely detectable in the plasma of germ-free (GF) mice, suggesting that SCFAs are primarily derived from the fermentation of indigestible carbohydrates by the gut microbiota.Citation106 A high-fiber-based Mediterranean diet has been shown to increase SCFAs, a key metabolite produced by the intestinal microbiome.Citation107 The CIH plus high-fat diet-induced HTN rat model was accompanied by a decrease in SCFA-producing bacteria and a decrease in acetate levels in the cecum. OSA-induced intestinal ecological imbalance, intestinal mucosal epithelial damage, and reduced activation of brain microglia could be repaired by supplementation with the probiotic C. butyricum or the prebiotic Hylon VII, and acetate levels in the cecum were restored with acetate injection, which reduced OSA-induced intestinal inflammation and restored blood pressure to normal in rats.Citation93 A study also found that administration of Lactobacillus rhamnosus in an animal model of CIH plus high-salt diet-induced HTN could regulate intestinal metabolite levels and CD4+ T cell-induced inflammation through the AKT (also known as protein kinase B, PKB)-mTOR pathway and alleviate the development of HTN, suggesting that GM, its metabolites, and the mTOR pathway are closely related with HTN.Citation108 Systemic inflammation decreased (Th cell levels decrease), blood pressure improved, and cardiovascular function was protected after propionic acid supplementation in Ang II–induced hypertensive mice.Citation21 Oral administration of acetate and propionate in SHR had a reduced F/B ratio, endotoxemia, and Th17/Treg ratios, and it prevented elevated blood pressure, presumably due to reduced activation of the LPS/TLR4 inflammatory pathway and vascular protection by IL-10 Treg cells.Citation109 Prebiotics, probiotics, and postbiotics (eg, acetate, propionate, and butyrate) supplementation may prevent or reduce HTN induced in rodent models, which provides a new insight for the treatment of clinically refractory HTN; subsequently, further validation in translational clinical studies are needed.
Conclusions
OSA shares many common pathogenic factors with hypertensive patients, and OSA remains an independent risk factor for HTN, along with gender, age, and obesity, which are the main common pathogenic factors found. Insufficient blood supply to the intestinal mucosa forms an ischemia-reperfusion-like injury; the hypoxic environment causes structural changes in the intestinal flora, a decrease in SCFA-producing bacteria and intestinal luminal SCFAs content, a weakened intestinal mucosal barrier function causes intestinal leakage, plasma LPS levels elevate, triggering an intrinsic immune response via TLRs, and the antigenic and inflammatory microenvironment in turn drives an adaptive immune response to activate lymphocytes and interact with the intrinsic immunity. The imbalance in intestinal flora thus triggers a disturbance in the body’s immunity, leading to insulin resistance and vascular endothelial damage, driving the process of atherosclerosis and a decrease in vascular compliance, ultimately leading to the development of HTN and cardiovascular and cerebrovascular complications. SCFA promote the expression of anti-inflammatory cells and cytokines by strengthening the tight junctions of IECs, activating specific GPRs, and inhibiting HDAC, which act as vascular protectors against atherosclerotic lesions and system inflammation and regulate blood pressure. In addition, SCFA reduce HR and BP through modulation of sympathetic nerves and myocardial contractility. The reduction of SCFAs in OSA patients, leads to immune dysregulation, neurological and vascular inflammation, and ultimately to increased blood pressure and the resulting complications.
GM dysbiosis plays an important role in the development of a chronic low-grade inflammatory state in OSA patients and also plays a vital role in the pathology of HTN secondary to OSA. The management of OSA combined with HTN requires comprehensive treatment. The GM as a potential therapeutic target for early reversal of GM dysbiosis and restoration of normal levels of SCFAs in the intestinal lumen through administration of prebiotics/probiotics/postbiotics or fecal flora transplantation, which is beneficial for strengthening the barrier function of the intestinal mucosa and optimizing immune regulation, which may open new therapeutic options for improving and preventing OSA-associated HTN, and combined CAPA and conventional drug therapy may lead to better control of refractory HTN to reduce target organ damage and adverse cardiovascular and cerebrovascular complications. However, more evidence is needed in animal experiments and clinical studies to elicit the effect SCFAs therapy OSA-associated HTN.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article was submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work. Zhang and Ko contributed equally to this paper. Ko and Zeng are co-corresponding authors and have contributed equally to this work.
Acknowledgments
The authors would like to thank the Research Project of Jinan Microecological BioMedicine Shandong Laboratory (JNL202220B) and the Fujian Province Science and Technology Project, China, under contract No. 2021J01267.
Disclosure
The authors report no conflicts of interest for this work and declare that they have no financial or personal relationships with others who might inappropriately influence the results or interpretation in this manuscript.
Additional information
Funding
References
- Zhang W, Si LY. Obstructive sleep apnea syndrome (OSAS) and hypertension: pathogenic mechanisms and possible therapeutic approaches. Ups J Med Sci. 2012;117:370–382. doi:10.3109/03009734.2012.707253
- Ye J. Emerging role of adipose tissue hypoxia in obesity and insulin resistance. Int J Obes (Lond). 2009;33:54–66. doi:10.1038/ijo.2008.229
- Seravalle G, Grassi G. Obesity and hypertension. Pharmacol Res. 2017;122:1–7. doi:10.1016/j.phrs.2017.05.013
- Sjöström C, Lindberg E, Elmasry A, Hägg A, Svärdsudd K, Janson C. Prevalence of sleep apnoea and snoring in hypertensive men: a population based study. Thorax. 2002;57:602–607. doi:10.1136/thorax.57.7.602
- He Q, Feng J, Zhang X, et al. Relationship of daytime blood pressure and severity of obstructive sleep apnea among Chinese: a multi-center investigation in China. Chin Med J. 2010;123:18–22.
- Hou H, Zhao Y, Yu W, et al. Association of obstructive sleep apnea with hypertension: a systematic review and meta-analysis. J Glob Health. 2018;8:10405. doi:10.7189/jogh.08.010405
- May AM, Van Wagoner DR, Mehra R. OSA and cardiac arrhythmogenesis: mechanistic insights. Chest. 2017;151:225–241. doi:10.1016/j.chest.2016.09.014
- Pace A, Iannella G, Rossetti V, et al. Diagnosis of obstructive sleep apnea in patients with allergic and non-allergic rhinitis. Medicina. 2020;56. doi:10.3390/medicina56090454
- Pollicina I, Maniaci A, Lechien JR, et al. Neurocognitive performance improvement after obstructive sleep apnea treatment: state of the art. Behav Sci. 2021;11. doi:10.3390/bs11120180
- Durgan DJ, Ganesh BP, Cope JL, et al. Role of the gut microbiome in obstructive sleep apnea-induced hypertension. Hypertens (Dallas, Tex 1979). 2016;67:469–474. doi:10.1161/HYPERTENSIONAHA.115.06672
- Bennett BJ, Vallim TQD, Wang ZN, et al. Trimethylamine-N-Oxide, a metabolite associated with atherosclerosis, exhibits complex genetic and dietary regulation. Cell Metab. 2013;17:49–60. doi:10.1016/j.cmet.2012.12.011
- Martin-Gallausiaux C, Marinelli L, Blottière HM, Larraufie P, Lapaque N. SCFA: mechanisms and functional importance in the gut. Proc Nutr Soc. 2020;80:37–49. doi:10.1017/S0029665120006916
- Hang S, Paik D, Yao L, et al. Author correction: bile acid metabolites control T17 and T cell differentiation. Nature. 2020;579:E7. doi:10.1038/s41586-020-2030-5
- Liu B, Qian J, Wang Q, Wang F, Ma Z, Qiao Y. Butyrate protects rat liver against total hepatic ischemia reperfusion injury with bowel congestion. PLoS One. 2014;9:e106184. doi:10.1371/journal.pone.0106184
- Layden BT, Angueira AR, Brodsky M, Durai V, Lowe WLJ. Short chain fatty acids and their receptors: new metabolic targets. Transl Res. 2013;161:131–140. doi:10.1016/j.trsl.2012.10.007
- Russo E, Giudici F, Fiorindi C, Ficari F, Scaringi S, Amedei A. Immunomodulating activity and therapeutic effects of short chain fatty acids and tryptophan post-biotics in inflammatory bowel disease. Front Immunol. 2019;10:2754. doi:10.3389/fimmu.2019.02754
- Schwiertz A, Taras D, Schäfer K, et al. Microbiota and SCFA in lean and overweight healthy subjects. Obesity. 2010;18:190–195. doi:10.1038/oby.2009.167
- Zhao L, Zhang F, Ding X, et al. Gut bacteria selectively promoted by dietary fibers alleviate type 2 diabetes. Science. 2018;359:1151–1156. doi:10.1126/science.aao5774
- Rose S, Bennuri SC, Davis JE, et al. Butyrate enhances mitochondrial function during oxidative stress in cell lines from boys with autism. Transl Psychiatry. 2018;8:42. doi:10.1038/s41398-017-0089-z
- Luu M, Visekruna A. Short-chain fatty acids: bacterial messengers modulating the immunometabolism of T cells. Eur J Immunol. 2019;49:842–848. doi:10.1002/eji.201848009
- Bartolomaeus H, Balogh A, Yakoub M, et al. Short-chain fatty acid propionate protects from hypertensive cardiovascular damage. Circulation. 2019;139:1407–1421. doi:10.1161/CIRCULATIONAHA.118.036652
- Zuo T, Ng SC. The gut microbiota in the pathogenesis and therapeutics of inflammatory bowel disease. Front Microbiol. 2018;9:2247. doi:10.3389/fmicb.2018.02247
- Yang T, Santisteban MM, Rodriguez V, et al. Gut dysbiosis is linked to hypertension. Hypertens (Dallas, Tex 1979). 2015;65:1331–1340. doi:10.1161/HYPERTENSIONAHA.115.05315
- Fu J, Bonder MJ, Cenit MC, et al. The gut microbiome contributes to a substantial proportion of the variation in blood lipids. Circ Res. 2015;117:817–824. doi:10.1161/CIRCRESAHA.115.306807
- Valles-Colomer M, Falony G, Darzi Y, et al. The neuroactive potential of the human gut microbiota in quality of life and depression. Nat Microbiol. 2019;4:623–632. doi:10.1038/s41564-018-0337-x
- Wang Q, Li F, Liang B, et al. A metagenome-wide association study of gut microbiota in asthma in UK adults. BMC Microbiol. 2018;18:114. doi:10.1186/s12866-018-1257-x
- Tang WHW, Kitai T, Hazen SL. Gut microbiota in cardiovascular health and disease. Circ Res. 2017;120:1183–1196. doi:10.1161/CIRCRESAHA.117.309715
- Li J, Zhao F, Wang Y, et al. Gut microbiota dysbiosis contributes to the development of hypertension. Microbiome. 2017;5:14. doi:10.1186/s40168-016-0222-x
- Ko C-Y, Liu -Q-Q, Su H-Z, et al. Gut microbiota in obstructive sleep apnea-hypopnea syndrome: disease-related dysbiosis and metabolic comorbidities. Clin Sci. 2019;133:905–917. doi:10.1042/CS20180891
- Qin J, Li R, Raes J, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464:59–65. doi:10.1038/nature08821
- Mariat D, Firmesse O, Levenez F, et al. The Firmicutes/Bacteroidetes ratio of the human microbiota changes with age. BMC Microbiol. 2009;9:123. doi:10.1186/1471-2180-9-123
- Corrêa-Oliveira R, Fachi JL, Vieira A, Sato FT, Vinolo MAR. Regulation of immune cell function by short-chain fatty acids. Clin Transl Immunol. 2016;5:e73. doi:10.1038/cti.2016.17
- Park J, Kim M, Kang SG, et al. Short-chain fatty acids induce both effector and regulatory T cells by suppression of histone deacetylases and regulation of the mTOR-S6K pathway. Mucosal Immunol. 2015;8:80–93. doi:10.1038/mi.2014.44
- Ko C-Y, Fan J-M, Hu A-K, et al. Disruption of sleep architecture in Prevotella enterotype of patients with obstructive sleep apnea-hypopnea syndrome. Brain Behav. 2019;9:e01287. doi:10.1002/brb3.1287
- Valentini F, Evangelisti M, Arpinelli M, et al. Gut microbiota composition in children with obstructive sleep apnoea syndrome: a pilot study. Sleep Med. 2020;76:140–147. doi:10.1016/j.sleep.2020.10.017
- Brahmakshatriya V, Kuang Y, Devarajan P, et al. IL-6 production by TLR-activated APC broadly enhances aged cognate CD4 Helper and B cell antibody responses in vivo. J Immunol. 2017;198:2819–2833. doi:10.4049/jimmunol.1601119
- Zeng X, Guo R, Dong M, Zheng J, Lin H, Lu H. Contribution of TLR4 signaling in intermittent hypoxia-mediated atherosclerosis progression. J Transl Med. 2018;16:106. doi:10.1186/s12967-018-1479-6
- Schellekens DHSM, Grootjans J, Dello SAWG, et al. Plasma intestinal fatty acid-binding protein levels correlate with morphologic epithelial intestinal damage in a human translational ischemia-reperfusion model. J Clin Gastroenterol. 2014;48:253–260. doi:10.1097/MCG.0b013e3182a87e3e
- Barceló A, Esquinas C, Robles J, et al. Gut epithelial barrier markers in patients with obstructive sleep apnea. Sleep Med. 2016;26:12–15. doi:10.1016/j.sleep.2016.01.019
- Heizati M, Li N, Shao L, et al. Does increased serum d-lactate mean subclinical hyperpermeability of intestinal barrier in middle-aged nonobese males with OSA? Medicine (Baltimore). 2017;96:e9144. doi:10.1097/MD.0000000000009144
- Natarajan N, Hori D, Flavahan S, et al. Microbial short chain fatty acid metabolites lower blood pressure via endothelial G protein-coupled receptor 41. Physiol Genomics. 2016;48:826–834. doi:10.1152/physiolgenomics.00089.2016
- Wrzosek L, Miquel S, Noordine M-L, et al. Bacteroides thetaiotaomicron and Faecalibacterium prausnitzii influence the production of mucus glycans and the development of goblet cells in the colonic epithelium of a gnotobiotic model rodent. BMC Biol. 2013;11:61. doi:10.1186/1741-7007-11-61
- Raqib R, Sarker P, Bergman P, et al. Improved outcome in shigellosis associated with butyrate induction of an endogenous peptide antibiotic. Proc Natl Acad Sci U S A. 2006;103:9178–9183. doi:10.1073/pnas.0602888103
- Raqib R, Sarker P, Mily A, et al. Efficacy of sodium butyrate adjunct therapy in shigellosis: a randomized, double-blind, placebo-controlled clinical trial. BMC Infect Dis. 2012;12:111. doi:10.1186/1471-2334-12-111
- Fukuda S, Toh H, Hase K, et al. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature. 2011;469:543–547. doi:10.1038/nature09646
- Sun L, Pan A, Yu Z, et al. Snoring, inflammatory markers, adipokines and metabolic syndrome in apparently healthy Chinese. PLoS One. 2011;6:e27515. doi:10.1371/journal.pone.0027515
- Kheirandish-Gozal L, Peris E, Wang Y, et al. Lipopolysaccharide-binding protein plasma levels in children: effects of obstructive sleep apnea and obesity. J Clin Endocrinol Metab. 2014;99:656–663. doi:10.1210/jc.2013-3327
- Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi:10.1038/nri1391
- Oyama J, Blais CJ, Liu X, et al. Reduced myocardial ischemia-reperfusion injury in toll-like receptor 4-deficient mice. Circulation. 2004;109:784–789. doi:10.1161/01.CIR.0000112575.66565.84
- Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol. 2010;72:219–246. doi:10.1146/annurev-physiol-021909-135846
- Kim CH, Park J, Kim M. Gut microbiota-derived short-chain Fatty acids, T cells, and inflammation. Immune Netw. 2014;14:277–288. doi:10.4110/in.2014.14.6.277
- Miller AH, Raison CL. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat Rev Immunol. 2016;16:22–34. doi:10.1038/nri.2015.5
- Huuskonen J, Suuronen T, Nuutinen T, Kyrylenko S, Salminen A. Regulation of microglial inflammatory response by sodium butyrate and short-chain fatty acids. Br J Pharmacol. 2004;141:874–880. doi:10.1038/sj.bjp.0705682
- Erny D, Hrabě de Angelis AL, Jaitin D, et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat Neurosci. 2015;18:965–977. doi:10.1038/nn.4030
- Boyton RJ, Altmann DM. Is selection for TCR affinity a factor in cytokine polarization? Trends Immunol. 2002;23:526–529. doi:10.1016/s1471-4906(02)02319-0
- Xu L, Kitani A, Fuss I, Strober W. Cutting edge: regulatory T cells induce CD4+CD25-Foxp3- T cells or are self-induced to become Th17 cells in the absence of exogenous TGF-beta. J Immunol. 2007;178:6725–6729. doi:10.4049/jimmunol.178.11.6725
- Veldhoen M, Uyttenhove C, van Snick J, et al. Transforming growth factor-beta “reprograms” the differentiation of T helper 2 cells and promotes an interleukin 9-producing subset. Nat Immunol. 2008;9:1341–1346. doi:10.1038/ni.1659
- Bending D, De la Peña H, Veldhoen M, et al. Highly purified Th17 cells from BDC2.5NOD mice convert into Th1-like cells in NOD/SCID recipient mice. J Clin Invest. 2009;119:565–572. doi:10.1172/JCI37865
- Ishikawa H, Tanaka K, Maeda Y, et al. Effect of intestinal microbiota on the induction of regulatory CD25+ CD4+ T cells. Clin Exp Immunol. 2008;153:127–135. doi:10.1111/j.1365-2249.2008.03668.x
- Atarashi K, Nishimura J, Shima T, et al. ATP drives lamina propria T(H)17 cell differentiation. Nature. 2008;455:808–812. doi:10.1038/nature07240
- Zhou L, Lopes JE, Chong MMW, et al. TGF-beta-induced Foxp3 inhibits T(H)17 cell differentiation by antagonizing RORgammat function. Nature. 2008;453:236–240. doi:10.1038/nature06878
- Maganto-García E, Bu D-X, Tarrio ML, et al. Foxp3+-inducible regulatory T cells suppress endothelial activation and leukocyte recruitment. J Immunol. 2011;187:3521–3529. doi:10.4049/jimmunol.1003947
- Foks AC, Frodermann V, Ter Borg M. Differential effects of regulatory T cells on the initiation and regression of atherosclerosis. Atherosclerosis. 2011;218:53–60. doi:10.1016/j.atherosclerosis.2011.04.029
- van Es T, van Puijvelde GHM, Foks AC, et al. Vaccination against Foxp3(+) regulatory T cells aggravates atherosclerosis. Atherosclerosis. 2010;209:74–80. doi:10.1016/j.atherosclerosis.2009.08.041
- Ait-Oufella H, Taleb S, Mallat Z, Tedgui A. Cytokine network and T cell immunity in atherosclerosis. Semin Immunopathol. 2009;31:23–33. doi:10.1007/s00281-009-0143-x
- Macfarlane S, Cleary S, Bahrami B, Reynolds N, Macfarlane GT. Synbiotic consumption changes the metabolism and composition of the gut microbiota in older people and modifies inflammatory processes: a randomised, double-blind, placebo-controlled crossover study. Aliment Pharmacol Ther. 2013;38:804–816. doi:10.1111/apt.12453
- Morrison DJ, Preston T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes. 2016;7:189–200. doi:10.1080/19490976.2015.1134082
- Sun M, Wu W, Chen L, et al. Microbiota-derived short-chain fatty acids promote Th1 cell IL-10 production to maintain intestinal homeostasis. Nat Commun. 2018;9:3555. doi:10.1038/s41467-018-05901-2
- Singh N, Gurav A, Sivaprakasam S, et al. Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity. 2014;40:128–139. doi:10.1016/j.immuni.2013.12.007
- Blad CC, Tang C, Offermanns S. G protein-coupled receptors for energy metabolites as new therapeutic targets. Nat Rev Drug Discov. 2012;11:603–619. doi:10.1038/nrd3777
- Candido EPM, Reeves R, Davie JR. Sodium butyrate inhibits histone deacetylation in cultured cells. Cell. 1978;14:105–113. doi:10.1016/0092-8674(78)90305-7
- Sealy L, Chalkley R. The effect of sodium butyrate on histone modification. Cell. 1978;14:115–121. doi:10.1016/0092-8674(78)90306-9
- Asarat M, Apostolopoulos V, Vasiljevic T, Donkor O. Short-chain fatty acids regulate cytokines and Th17/Treg cells in human peripheral blood mononuclear cells in vitro. Immunol Invest. 2016;45:205–222. doi:10.3109/08820139.2015.1122613
- Chen L, Sun M, Wu W, et al. Microbiota metabolite butyrate differentially regulates Th1 and Th17 cells’ differentiation and function in induction of colitis. Inflamm Bowel Dis. 2019;25:1450–1461. doi:10.1093/ibd/izz046
- Davie JR. Inhibition of histone deacetylase activity by butyrate. J Nutr. 2003;133:2485S–2493S. doi:10.1093/jn/133.7.2485S
- Moreno-Indias I, Torres M, Sanchez-Alcoholado L. Intermittent hypoxia alters gut microbiota diversity in a mouse model of sleep apnoea. Eur Respir J. 2015;45:1055–1065. doi:10.1183/09031936.00184314
- Arumugam M, Raes J, Pelletier E, et al. Enterotypes of the human gut microbiome. Nature. 2011;473:174–180. doi:10.1038/nature09944
- Durgan DJ. Obstructive sleep apnea-induced hypertension: role of the gut microbiota. Curr Hypertens Rep. 2017;19.doi: 10.1007/S11906-017-0732-3
- Sapiña-Beltrán E, Santamaria-Martos F, Benítez I, et al. Normotensive patients with obstructive sleep apnoea: changes in 24-h ambulatory blood pressure monitoring with continuous positive airway pressure treatment. J Hypertens. 2019;37:720–727. doi:10.1097/HJH.0000000000001934
- Binar M, Akcam T, Karakoc O, Sagkan RI, Musabak U, Gerek M. A new surgical technique versus an old marker: can expansion sphincter pharyngoplasty reduce C-reactive protein levels in patients with obstructive sleep apnea? Eur Arch Oto-Rhino-L. 2017;274:829–836. doi:10.1007/s00405-016-4290-0
- Maniaci A, Di Luca M, Lechien JR, et al. Lateral pharyngoplasty vs. traditional uvulopalatopharyngoplasty for patients with OSA: systematic review and meta-analysis. Sleep Breath. 2022. doi:10.1007/s11325-021-02520-y
- Qian X, Yin T, Li T, et al. High levels of inflammation and insulin resistance in obstructive sleep apnea patients with hypertension. Inflammation. 2012;35:1507–1511. doi:10.1007/s10753-012-9464-3
- Dyugovskaya L, Lavie P, Lavie L. Lymphocyte activation as a possible measure of atherosclerotic risk in patients with sleep apnea. Ann N Y Acad Sci. 2005;1051:340–350. doi:10.1196/annals.1361.076
- Didion SP, Kinzenbaw DA, Schrader LI, Chu Y, Faraci FM. Endogenous interleukin-10 inhibits angiotensin II-induced vascular dysfunction. Hypertens (Dallas, Tex 1979). 2009;54:619–624. doi:10.1161/HYPERTENSIONAHA.109.137158
- Kassan M, Galan M, Partyka M, Trebak M, Matrougui K. Interleukin-10 released by CD4(+) CD25(+) natural regulatory T cells improves microvascular endothelial function through inhibition of NADPH oxidase activity in hypertensive mice. Arterioscler Thromb Vasc Biol. 2011;31:2534–2542. doi:10.1161/ATVBAHA.111.233262
- Oyama J-I, Node K. Gut microbiota and hypertension. Hypertens Res. 2019;42:741–743. doi:10.1038/s41440-018-0203-5
- Mell B, Jala VR, Mathew AV. Evidence for a link between gut microbiota and hypertension in the Dahl rat. Physiol Genomics. 2015;47:187–197. doi:10.1152/physiolgenomics.00136.2014
- Adnan S, Nelson JW, Ajami NJ, et al. Alterations in the gut microbiota can elicit hypertension in rats. Physiol Genomics. 2017;49:96–104. doi:10.1152/physiolgenomics.00081.2016
- Marques FZ, Nelson E, Chu P-Y, et al. High-fiber diet and acetate supplementation change the gut microbiota and prevent the development of hypertension and heart failure in hypertensive mice. Circulation. 2017;135:964–977. doi:10.1161/CIRCULATIONAHA.116.024545
- Toral M, Robles-Vera I, de la Visitación N, et al. Critical role of the interaction gut microbiota - sympathetic nervous system in the regulation of blood pressure. Front Physiol. 2019;10:231. doi:10.3389/fphys.2019.00231
- Qi Y, Aranda JM, Rodriguez V, Raizada MK, Pepine CJ. Impact of antibiotics on arterial blood pressure in a patient with resistant hypertension - A case report. Int J Cardiol. 2015;201:157–158. doi:10.1016/j.ijcard.2015.07.078
- Yellowlees Douglas J, Bhatwadekar AD, Li Calzi S, et al. Bone marrow-CNS connections: implications in the pathogenesis of diabetic retinopathy. Prog Retin Eye Res. 2012;31:481–494. doi:10.1016/j.preteyeres.2012.04.005
- Ganesh BP, Nelson JW, Eskew JR, et al. Prebiotics, probiotics, and acetate supplementation prevent hypertension in a model of obstructive sleep apnea. Hypertension. 2018;72:1141–1150. doi:10.1161/HYPERTENSIONAHA.118.11695
- Pluznick JL, Protzko RJ, Gevorgyan H, et al. Olfactory receptor responding to gut microbiota-derived signals plays a role in renin secretion and blood pressure regulation. Proc Natl Acad Sci U S A. 2013;110:4410–4415. doi:10.1073/pnas.1215927110
- Kimura I, Inoue D, Maeda T, et al. Short-chain fatty acids and ketones directly regulate sympathetic nervous system via G protein-coupled receptor 41 (GPR41). Proc Natl Acad Sci U S A. 2011;108:8030–8035. doi:10.1073/pnas.1016088108
- Furness JB, Costa M. The adrenergic innervation of the gastrointestinal tract. Ergeb Physiol. 1974;69:2–51.
- Onyszkiewicz M, Gawrys-Kopczynska M, Konopelski P, et al. Butyric acid, a gut bacteria metabolite, lowers arterial blood pressure via colon-vagus nerve signaling and GPR41/43 receptors. Pflugers Arch. 2019;471:1441–1453. doi:10.1007/s00424-019-02322-y
- Poll BG, Xu J, Jun S, et al. Acetate, a short-chain fatty acid, acutely lowers heart rate and cardiac contractility along with blood pressure. J Pharmacol Exp Ther. 2021;377:39–50. doi:10.1124/jpet.120.000187
- Young T, Palta M, Dempsey J, Peppard PE, Nieto FJ, Hla KM. Burden of sleep apnea: rationale, design, and major findings of the Wisconsin sleep cohort study. Wis Med J. 2009;108:246–249.
- Mashaqi S, Gozal D. Obstructive sleep apnea and systemic hypertension: gut dysbiosis as the mediator? J Clin Sleep Med. 2019;15:1517–1527. doi:10.5664/jcsm.7990
- Ko C, Su H, Zhang L, Zeng Y. Disturbances of the gut microbiota, sleep architecture, and mTOR signaling pathway in patients with severe obstructive sleep apnea-associated hypertension. Int J Hypertens. 2021;2021:e43.
- Campos-Rodriguez F, Asensio-Cruz MI, Cordero-Guevara J, et al. Effect of continuous positive airway pressure on inflammatory, antioxidant, and depression biomarkers in women with obstructive sleep apnea: a randomized controlled trial. Sleep. 2019;42. doi:10.1093/sleep/zsz145
- Hall AB, Ziadi MC, Leech JA, et al. Effects of short-term continuous positive airway pressure on myocardial sympathetic nerve function and energetics in patients with heart failure and obstructive sleep apnea: a randomized study. Circulation. 2014;130:892–901. doi:10.1161/CIRCULATIONAHA.113.005893
- Iftikhar IH, Valentine CW, Bittencourt LRA, et al. Effects of continuous positive airway pressure on blood pressure in patients with resistant hypertension and obstructive sleep apnea: a meta-analysis. J Hypertens. 2014;32:2341–50; discussion 2350. doi:10.1097/HJH.0000000000000372
- Pengo MF, Ratneswaran C, Berry M, et al. Effect of continuous positive airway pressure on blood pressure variability in patients with obstructive sleep apnea. J Clin Hypertens. 2016;18:1180–1184. doi:10.1111/jch.12845
- Perry RJ, Peng L, Barry NA, et al. Acetate mediates a microbiome-brain-β-cell axis to promote metabolic syndrome. Nature. 2016;534:213–217. doi:10.1038/nature18309
- De Filippis F, Pellegrini N, Vannini L, et al. High-level adherence to a Mediterranean diet beneficially impacts the gut microbiota and associated metabolome. Gut. 2016;65:1812–1821. doi:10.1136/gutjnl-2015-309957
- Liu J, Li T, Wu H, et al. Lactobacillus rhamnosus GG strain mitigated the development of obstructive sleep apnea-induced hypertension in a high salt diet via regulating TMAO level and CD4 + T cell induced-type I inflammation. Biomed Pharmacother. 2019;112:108580. doi:10.1016/j.biopha.2019.01.041
- Robles-Vera I, Toral M, de la Visitación N, et al. Probiotics prevent dysbiosis and the rise in blood pressure in genetic hypertension: role of short-chain fatty acids. Mol Nutr Food Res. 2020;64:e1900616. doi:10.1002/mnfr.201900616
