Abstract
Background
Multiple sclerosis (MS) represents a risk factor for sleep disorders, but there are conflicting results about the prevalence and severity of sleep-related breathing disorders (SRBD) in MS. Most available data come from self-administered questionnaires.
Objective
To conduct a polysomnographic study in MS focused on SRBD, compared to a group of healthy controls (HC), also considering the neuroimaging findings. To evaluate the impact of SRBD on vigilance, fatigue and depression in MS.
Methods
In this cross-sectional, observational, instrumental study, 67 MS patients (men/women: 20/47; mean age: 50.6±8.2 years) underwent PSG and maintenance of wakefulness test. Findings were compared to 67 age-, sex-, BMI-matched HC, by using parametric (Student’s t-test) and nonparametric statistics (chi-squared test). A subgroup analysis was then performed, evaluating the influence of brainstem (mesencephalic, pontine and medullary) lesions at neuroimaging on instrumental and clinical data: MS patients with at least one brainstem lesion vs MS patients without vs HC.
Results
The frequency of SRBD was comparable in MS patients and HC. No MS patient had a central apnea index ≥2/h. The respiratory disturbance index (RDI) did not correlate to clinical parameters such as fatigue and depression. Patients with MS were drowsier than HC (47% vs 26%, p = 0.019) and showed a worse sleep pattern, in terms of duration, efficiency and architecture.
Conclusion
Our study does not provide evidence of an association between MS-specific symptoms such as fatigue, sleepiness, depression and central or obstructive apneas, even in the presence of brainstem lesions.
Introduction
Multiple sclerosis (MS) is an immune-mediated demyelinating and neurodegenerative disease of the central nervous system, which represents the leading cause of nontraumatic neurological disability in young adults. Up to 50% of patients with multiple sclerosis (MS) are at increased risk for sleep disorders.Citation1,Citation2 Insomnia and restless legs syndrome (RLS) represent the most common sleep complaintsCitation3 in MS, with significant impact on quality of life and critical symptoms, such as fatigue.Citation4 Despite this, sleep is yet underinvestigated in patients with MS. Although a growing amount of literature on sleep and MS has become available in the past decade, only a few studies have evaluated prospectively sleep by polysomnography (PSG).Citation5
MS and sleep-related breathing disorders (SRBD) have shared consequences, such as fatigue, sleepiness, cognitive impairment and depression, which may contribute to increased morbidity. Although some contend that the prevalence and severity of SRBD are higher in persons with MS, the current literature reports conflicting results,Citation5 and most of the available data come from self-administered questionnaires studies.
Little is known about the pathophysiological mechanisms that contribute to SRBD in people with MS and vice versa. Some speculate that obstructive sleep apnoea syndrome (OSAS) could worsen MS-related disability or progression through its association with local/systemic inflammation.Citation6 On the other hand, MS-related central nervous system lesions, in particular in areas critical for respiration, like the brainstem, might contribute to both obstructive and central sleep apnoea.Citation7
The primary aim of the current study was to compare prevalence, severity and PSG features of SRBD in patients with MS and healthy controls and their correlations with neuroimaging findings. Secondary aims were to look for clinical determinants of SRBD in MS and to evaluate the impact of SRBD on vigilance, fatigue and depression in MS.
Materials and Methods
Participants
A cross-sectional, observational study in a sample of patients older than 18 years old and affected by MS according to McDonald criteriaCitation8 or clinically isolated syndromes (CIS)Citation9 was carried out. Additional inclusion criteria were an Expanded Disability Status Scale (EDSS) score <7.0 (range 0–10),Citation10 and a brain magnetic resonance imaging (MRI) performed within the 12 months preceding the evaluation. MRI exams were performed using 3T Magnetom “Skyra” (Siemens, Erlangen, Germany) scanner with a 20-channel head coil. All participants were examined with a conventional protocol before and after contrast administration (0.1 mmol/Kg of Gadobutrolum). Sagittal 1-mm fluid-attenuated-inversion-recovery (FLAIR) 3D T2-weighted and axial 3 mm dual echo (T2/proton-density weighted) images were acquired and employed to assess the number of T2-visible lesions in the brainstem, subdivided in mesencephalic, pontine and medullary. All images were reviewed by a neuroradiologist (D.D.), blinded to clinical data.
Exclusion criteria were: Mini Mental Status Examination (MMSE) score lower than 24; clinical MS relapse within the last 3 months; radiologically isolated syndrome (RIS); history of drug and/or alcohol abuse; any serious general medical condition such as decompensated cardiopulmonary disease, cancer or decompensated renal failure, as well as any major neurological condition other than MS that could interfere with the correct execution of the study design. MS was classified as primary progressive, secondary progressive, or relapsing-remitting.
A group of healthy controls (HC), age-, sex- and body mass index (BMI)-matched with MS patients, was randomly selected by the HypnoLaus Sleep Cohort,Citation11 which is a large population-based cohort recruited in Lausanne (Switzerland). They had to be in general good health and were excluded if any of the following was present: a diagnosis of any significant sleep disorder(s) other than SRBD, major mental illness including any indications of cognitive problems as determined by history. A full-night home PSG had been previously recorded in the group of controls, in the same standard way adopted for MS patients.Citation12
All procedures performed in this study were in accordance with the Helsinki declaration and the protocol was approved by the Ethics Committee: Comitato etico cantonale (Ufficio di Sanità, 6501 Bellinzona, Switzerland), Trial No.: EOC.NSI.13.02. All participants signed an informed consent.
Questionnaires
Patients were interviewed concerning their medical history; received a complete clinical and neurological examination, including the Expanded Disability Status Scale (EDSS) score assessment. All participants filled in the following questionnaires: Epworth Sleepiness Scale (ESS) (range: 0–24; cutoff for normality: ≤10), Beck Depression Inventory (BDI) (range: 0–21; cutoff for normality: <9), Multiple Sclerosis Quality of Life (MSQoL) (range: 0–84; cutoff for normality: <38), Pittsburgh Sleep Quality Index (PSQI) (range: 0–21; cutoff for normality: <5), Fatigue Severity Scale (FSS) (range: 9–63; cutoff for normality: 36), Modified Fatigue Impact Scale (MFIS) (range: 0–84; cutoff for normality: 38).
Polysomnography and Maintenance of Wakefulness Test
Within one week from the screening visit, participants underwent a full night PSG by a portable device (Embletta ST + Proxy), and a maintenance of wakefulness test (MWT) the following day (the latter not available for the controls).
The PSG montage included the following: EEG, electrooculogram, electromyogram (EMG) of chin and both tibialis anterior muscles, electrocardiogram, body position; oro-nasal airflow (nasal pressure cannula), thoracic and abdominal movements, and oxygen saturation.
Apnea was scored in the presence of a drop in the nasal pressure signal peak amplitude by ≥90% of the pre-event baseline, lasting ≥10 s, while hypopnea when it drop by ≥30% of the pre-event baseline, in association with either ≥3% arterial oxygen desaturation or an arousal.Citation13 The diagnosis of OSAS was established in the presence of: 1) complaints of diurnal sleepiness (ESS ≥10) and an apnea/hypopnea index (AHI) >5/h; or 2) AHI ≥15/h.Citation14
The MWT quantify, under PSG recording, the ability to stay awake in sleep-promoting environmental conditions.Citation15 Patients were instructed to stay awake during the four sessions, each of 40 min of recording. Sleep latency was assessed in each of the four recordings and then the average of latencies was calculated.Citation16
Statistical Analysis
Descriptive statistics were used, followed by between-group comparisons by means of the Student’s t-test. Frequency data were analyzed by means of the chi-squared test. Correlations were assessed by means of the Pearson’s correlation coefficient; following the indications by Cohen,Citation17 we considered correlations 0.10, 0.30, and 0.50 as corresponding to small, medium, and large sizes, respectively. One-way between-groups analysis of variance (ANOVA) was conducted to explore the impact of brainstem lesions on instrumental and clinical parameters.
Results
Sixty-seven patients with MS (56 (86.3%) relapsing-remitting), whom 47 women (70.1%), with a mean age of 50.6±8.2 years and a mean BMI of 25.4±5.3 kg/m2, completed the study. Sixty-seven healthy subjects matched for sex, age and BMI served as a control group ().
Table 1 Demographic and Clinical Parameters
Among patients with MS, 64.2% were fatigued, with a mean FSS and MFIS score of, respectively, 41.7±16.9 and 42.7±22.4 points. 11.9% of patients had a severe disability with an EDSS ≥4, and 63.5% of all patients were depressed, according to the BDI score.
MS Vs Control Group
As illustrated in , the proportion of MS with a RDI >5/h, >15/h, >30/h was 40.3%, 16.4% and 4.5%, respectively, while corresponding figures were 58.2% (p = 0.057), 29.9% (p = 0.100), 11.9% (p = 0.210) in healthy controls.
Figure 1 Frequency of sleep-related breathing disorders in patients with multiple sclerosis and in healthy controls.
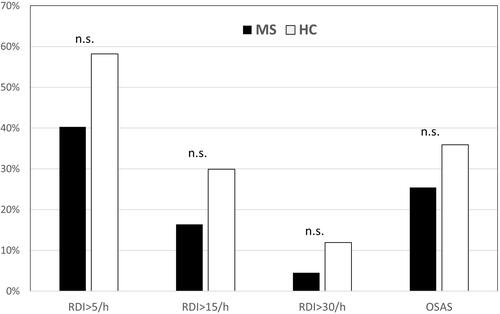
The diagnosis of OSAS was more frequent in healthy controls than in the MS group, without reaching statistical significance (35.9% vs 25.4%, p = 0.262). The percentage of patients with an AHI >15/h in the supine position was 22.4% in the MS group. AHI during REM sleep (AHI-REM) did not differ between healthy subjects and patients (p = 0.35). All subjects with an AHI >5/h had obstructive sleep apnea, no patient had a central or mixed AHI >2/h.
Patients had a higher sleep onset latency (p = 0.001), awakening index (p < 0.001), and a lower sleep efficiency (p < 0.001), total sleep time (p < 0.001), percentage of slow wave sleep (p = 0.07) and REM sleep (p < 0.001), when compared to controls (). Sleep quality impairment was also reflected by PSQI scores (p < 0.001, t = 7.465).
Table 2 Polysomnographic Parameters
Forty-seven percent of patients reported an ESS score higher than 10, of whom 16.7% and 40% had a pathological MWT, when considering the threshold of 20 and 30 minutes, respectively. The level of drowsiness was significantly lower in the control group: 25.8% had an ESS score >10 (p = 0.019, Phi = 0.23), for which MWT was not available ().
Table 3 Clinical Parameters and MWT
Correlation of SRBD with Clinical/Instrumental Variables in MS Group
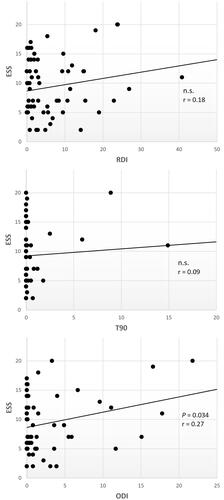
In the MS group, RDI was positively correlated with age (r = 0.39, moderate-to-large size, p =<0.001), BMI (r = 0.47, large size, p = < 0.001), and negatively with sleep efficiency (r = −0.30, moderate size, p = 0.014) and MSQoL54 (r = −0.27, moderate size, p = 0.042). No correlation was found with the disability status (EDSS), fatigue (FSS and MFIS), depression (BDI), and sleep quality (PSQI). AHIREM was higher in patients with EDSS ≥4 (t = 2.30; p = 0.025). The oxygen desaturation index (ODI), but not the RDI, correlated with subjective sleepiness (r = 0.27, moderate size, p = 0.034) (). There was no correlation between mean sleep latency at MWT and respiratory parameters.
Influence of the Symptomatic Treatment
Excessive daytime sleepiness (EDS) was associated with regular therapy with anti-depressants: 58.7% of patients with EDS received anti-depressants vs 27.3% of those without EDS (χ2 6.2, p = 0.025). A similar association was found with benzodiazepines (t = 2.18, p = 0.03). There was no correlation between anti-depressant/benzodiazepine consumption and respiratory parameters.
SRBD and MRI Findings in MS
Fifteen (22.4%) MS patients had lesions in the medulla, 21 (31.3%) in the pons and 12 (17.8%) in the midbrain. Sleep efficiency was significantly lower in MS with brainstem lesions than in MS without (t = −2.196, p = 0.032), while the respiratory variables (RDI, AHI-REM, central apnea index, ODI, percentage of sleep time with an SpO2 <90%) did not differ between MS patients with and without brainstem lesions, as well as between healthy controls and MS patients with brainstem plaques (). The number of lesions in the medulla, pons and midbrain (considered both together and individually) did not correlate with RDI.
Discussion
This is the largest PSG study focused on sleep-related breathing disorders in MS carried out so far. We did not find a higher RDI and OSAS frequency in MS patients compared to healthy controls and the percentages of subjects with RDI >5/h or >15/h were slightly, but not significantly, higher in the control group. No MS patient had a central apnea index ≥2/h, neither in the subgroup with brainstem lesions. As expected, sleep quality in MS was significantly lower than in healthy controls. However, in the MS group, RDI was not correlated with PSG markers of sleep quality, with the exception of sleep efficiency, neither with clinical parameters such as fatigue, depression, sleepiness, subjective sleep quality, and disability status. The presence of lesions in the brainstem was not correlated with any of the sleep-related parameters.
According to ESS, patients with MS were drowsier than healthy subjects. Only ODI and pharmacotherapy (benzodiazepines and anti-depressants) were correlated with drowsiness.
The current literature reports conflicting results concerning the prevalence and severity of SRBD in MS.Citation5,Citation18 Studies based on self-reported questionnaires suggested an elevated risk of OSA in MS patients.Citation19,Citation20 Nevertheless, the STOP-BANG test may not be an accurate tool to identify OSA patients in comorbidity with MS, where the high prevalence of fatigue might represent a confounding factor.Citation21 According to a recent review (2020),Citation5 there are only five instrumental case-control studies taking into account RDI or AHI, of which only one involved more than 50 patients (sample size = 62). AHI values varied considerably between studies, ranging from normal to moderately increased. Among these five studies, only Braley et alCitation7 found that the AHI and central apnea index (but not obstructive apnea index) were higher in MS patients than in controls, while the others did not find any differences.Citation22–Citation25 However, compared to our sample, the patients studied by Braley et alCitation7 had a higher disease severity, with 30% of patients having progressive MS with an EDSS ≥6, and with brainstem lesions in more than half of the group. A further study considered only obstructive apnea index, finding no difference between MS patients and controls.Citation23
Another studyCitation26 showed a higher AHI, AHI-REM and ODI in patients compared to controls. However, the sample differed from ours in terms of mean age (38.3 years) and sex (56% females). Moreover, the control group consisted of individuals (not affected by MS) who underwent PSG because of sleep complaints.
Two non-controlled studies partially addressed the relationship between SRBD and fatigue in MS, suggesting a positive correlation that diverge from the findings of our study. Nevertheless, the study by Veauthier et alCitation27 lacks of statistics due to the small sample size. The second study by Côté et alCitation28 only focused on the effect of treatment for OSAS (CPAP, positioning devices, weight loss) on fatigue, finding an improvement of fatigue after a successful intervention.
According to our findings, Veauthier et alCitation29 showed poor health-related QoL in MS patients with OSA, even if QoL was assessed with a different questionnaire (Nottingham Health Profile). Finally, no instrumental study provides information on the relationship between SRBD and depression in MS patients.Citation5
Our findings indicate that MS is not associated to an increased risk of SRBD, it seems therefore reasonable considering instrumental screening for SRBD only in MS patients with suspicious symptoms. Notably, RDI and AHI in this study do not account for drowsiness, fatigue and depression in MS patients. This suggests physicians should consider treating depression, MS-related fatigue and other sleep complaints, before assessing a possible underlying SRBD. Patients with an elevated ODI represent an exception, since this correlated to subjective sleepiness. However, it is known that in OSAS oxygen desaturations are more associated to drowsiness than AHI.Citation30
Patients with MS showed a worse sleep quality, in terms of duration, efficiency, and architecture compared to healthy subjects. As shown by Buratti et al,Citation31 the course of MS may be influenced by sleep quality, possibly because of the role of sleep in some oligodendrocyte functions, such as myelination. Of consequence, sleep quality assessment could be used to obtain prognostic information. In our study, sleep disruption was not imputable to the respiratory pattern during sleep but likely due to other factors, such as insomnia, RLS, periodic limb movements during sleep,Citation32 disease modifying treatments.Citation33
The presence of lesions within the brainstem, which harbors structures playing a critical role in breathing regulation, was not associated with a greater risk of SRBD or central apneas, but with a worse sleep quality. According to our results, the sole presence of either mesencephalic, pontine or medullary lesions in a patient with MS without SRBD-related symptoms should not per se justify the cost of an instrumental screening for SRBD, at least in the range of MS severity considered in our sample. Our results differ from those of another recent study, with a similar sample size (n = 65), which found an association between AHI and the number of lesions in the midbrain and pons, but not medulla.Citation34
Sleep latency at MWT did not correlate to any sleep respiratory parameter. The discrepancy between MWT and ESS is not surprising and has been described also in other disorders, such as OSAS and narcolepsy.Citation35
Limitations
This study has limitations. The exclusion of subjects with an EDSS ≥7 and the relatively low median EDSS (2.7) in this MS population limits the generalizability of our results to highly disabled MS. Spinal and cerebral magnetic resonance images were not fully evaluated and were used only for the assessment of the presence of brainstem lesions. Additionally, precise brainstem lesion extension and location were not considered. Cervico-thoracic spine dysfunction due to MS might also contribute to both obstructive and central sleep apnea severity.Citation6 Finally, the lack of data on oropharyngeal anatomy prevented us from assessing other causes of OSAS not linked to neuroinflammation and obesity.
Conclusions
Our study did not find evidence of an association between MS and central or obstructive apneas, even in the presence of brainstem lesions. In light of the so far small number of studies on this topic and their limitations, it seems currently reasonable not to screen the MS as well as the general population with PSG for central/obstructive apneas, and limit its use to suspicious cases.
Abbreviations
AHI, apnea/hypopnea index; BDI, Beck Depression Inventory; BMI, body mass index; CIS, clinically isolated syndrome; CPAP, continuous positive airways pressure; EDS, excessive daytime sleepiness; EDSS, Expanded Disability Status Scale; EMG, electromyogram; ESS, Epworth Sleepiness Scale; FSS, Fatigue Severity Scale; HC, healthy controls; MFIS, Modified Fatigue Impact Scale; MMSE, Mini Mental Status Examination; MRI, magnetic resonance imaging; MS, multiple sclerosis; MSQoL, Multiple Sclerosis Quality of Life; MWT, maintenance of wakefulness test; NS, not significant; ODI, oxygen desaturation index; OSAS, obstructive sleep apnea syndrome; PLMS, periodic limb movements during sleep; PLMSI, periodic limb movements during sleep index; PPMS, primary progressive multiple sclerosis; PSG, polysomnography; PSQI, Pittsburgh Sleep Quality Index; RDI, respiratory disturbance index; RIS, radiologically isolated syndrome; RLS, restless legs syndrome; RR-MS, relapsing-remitting multiple sclerosis; SD, standard deviation; SP-MS, secondary progressive multiple sclerosis; SRBD, sleep-related breathing disorders; T90, percentage of sleep time with an SpO2 <90%; WASO, wake after sleep onset.
Data Sharing Statement
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
Acknowledgments
Grant ABREOC from Ente Ospedaliero Cantonale (EOC); Grant Swiss MS Society (SMSS); Swiss National Science Foundation, grant number: 320030_160250. The authors want to thank all the patients and participants involved in the study. Special thanks are due to Ente Ospedaliero Cantonale (EOC), Swiss MS Society and Swiss National Science Foundation, for the financial support.
Disclosure
R.F. was partially supported by a fund of the Italian Ministry of Health (RC2764026). CZ reports grants from Swiss MS Society, during the conduct of the study; grants from Abbvie, Almirall, Biogen Idec, Bristol Meyer Squibb, Genzyme, Lundbeck, Merck, Novartis, Teva Pharma, and Roche, outside the submitted work. The authors report no other conflicts of interest in this work.
References
- Barun B. Pathophysiological background and clinical characteristics of sleep disorders in multiple sclerosis. Clin Neurol Neurosurg. 2013;115:S82–S85. doi:10.1016/j.clineuro.2013.09.028
- Melamud L, Golan D, Luboshitzky R, Lavi I, Miller A. Melatonin dysregulation, sleep disturbances and fatigue in multiple sclerosis. J Neurol Sci. 2012;314:37–40. doi:10.1016/j.jns.2011.11.003
- Caminero A, Bartolomé M. Sleep disturbances in multiple sclerosis. J Neurol Sci. 2011;309:86–91. doi:10.1016/j.jns.2011.07.015
- Aldughmi M, Huisinga J, Lynch SG, Siengsukon CF. The relationship between fatigability and sleep quality in people with multiple sclerosis. Mult Scler J. 2016. doi:10.1177/2055217316682774
- Tanioka K, Castelnovo A, Tachibana N, et al. Framing multiple sclerosis under a polysomnographic perspective. Sleep. 2020;43. doi:10.1093/sleep/zsz232
- Hensen HA, Krishnan AV, Eckert DJ. Sleep-disordered breathing in people with multiple sclerosis: prevalence, pathophysiological mechanisms, and disease consequences. Front Neurol. 2018;8. doi:10.3389/fneur.2017.00740
- Braley TJ, Segal BM, Chervin RD. Sleep-disordered breathing in multiple sclerosis. Neurology. 2012;79:929–936. doi:10.1212/WNL.0b013e318266fa9d
- Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011;69:292–302. doi:10.1002/ana.22366
- Miller DH, Chard DT, Ciccarelli O. Clinically isolated syndromes. Lancet Neurol. 2012;11:157–169. doi:10.1016/S1474-4422(11)70274-5
- Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology. 1983;33:1444. doi:10.1212/wnl.33.11.1444
- Haba-Rubio J, Marti-Soler H, Marques-Vidal P, et al. Prevalence and determinants of periodic limb movements in the general population. Ann Neurol. 2016;79:464–474. doi:10.1002/ana.24593
- Heinzer R, Vat S, Marques-Vidal P, et al. Prevalence of sleep-disordered breathing in the general population: the HypnoLaus study. Lancet Respir Med. 2015;3(4):310–318. doi:10.1016/S2213-2600(15)00043-0
- Berry RB, Budhiraja R, Gottlieb DJ, et al. AA of SM. Rules for scoring respiratory events in sleep: update of the 2007 AASM manual for the scoring of sleep and associated events. deliberations of the sleep apnea definitions task force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2012;8(5):597–619. doi:10.5664/jcsm.2172
- Sateia MJ. International classification of sleep disorders-Third Edition. Chest. 2014;146:1387–1394. doi:10.1378/chest.14-0970
- Doghramji K, Mitler MM, Sangal RB, et al. A normative study of the maintenance of wakefulness test (MWT). Electroencephalogr Clin Neurophysiol. 1997;103:554–562. doi:10.1016/S0013-4694(97)00010-2
- Littner MR, Kushida C, Wise M, et al. Practice parameters for clinical use of the multiple sleep latency test and the maintenance of wakefulness test. Sleep. 2005;28:113–121. doi:10.1093/sleep/28.1.113
- Cohen J. Statistical Power Analysis for the Behavioural Science. 2nd ed. Routledge; 1988.
- Foschi M, Rizzo G, Liguori R, et al. Sleep-related disorders and their relationship with MRI findings in multiple sclerosis. Sleep Med. 2019;56:90–97. doi:10.1016/j.sleep.2019.01.010
- Brass SD, Li CS, Auerbach S. The underdiagnosis of sleep disorders in patients with multiple sclerosis. J Clin Sleep Med. 2014;10:1025–1031. doi:10.5664/jcsm.4044
- Braley TJ, Segal BM, Chervin RD. Obstructive sleep apnea and fatigue in patients with multiple sclerosis. J Clin Sleep Med. 2014;10:155–162. doi:10.5664/jcsm.3442
- Sunter G, Omercikoglu Ozden H, Vural E, Ince Gunal D, Agan K. Risk assessment of obstructive sleep apnea syndrome and other sleep disorders in multiple sclerosis patients. Clin Neurol Neurosurg. 2021;207:106749. doi:10.1016/j.clineuro.2021.106749
- Braley TJ, Chervin RD, Segal BM. Fatigue, tiredness, lack of energy, and sleepiness in multiple sclerosis patients referred for clinical polysomnography. Mult Scler Int. 2012;2012:1–7. doi:10.1155/2012/673936
- Kaminska M, Kimoff RJ, Benedetti A, et al. Obstructive sleep apnea is associated with fatigue in multiple sclerosis. Mult Scler J. 2012;18:1159–1169. doi:10.1177/1352458511432328
- Kaynak H, Altintaş A, Kaynak D, et al. Fatigue and sleep disturbance in multiple sclerosis. Eur J Neurol. 2006;13:1333–1339. doi:10.1111/j.1468-1331.2006.01499.x
- Chen JH, Liu XQ, Sun HY, Huang Y. Sleep disorders in multiple sclerosis in China: clinical, polysomnography study, and review of the literature. J Clin Neurophysiol. 2014;31:375–381. doi:10.1097/WNP.0000000000000067
- Saçmacı H, Tanık N, Özcan SS, et al. Evaluation of sleep-related respiratory disorders in patients with multiple sclerosis. Acta Neurol Belg. 2020;120(5):1165–1171. doi:10.1007/s13760-020-01358-7
- Veauthier C, Radbru H, Gaede G, et al. Fatigue in multiple sclerosis is closely related to sleep disorders: a polysomnographic cross-sectional study. Mult Scler J. 2011;17:613–622. doi:10.1177/1352458510393772
- Côté I, Trojan DA, Kaminska M, et al. Impact of sleep disorder treatment on fatigue in multiple sclerosis. Mult Scler J. 2013;19:480–489. doi:10.1177/1352458512455958
- Veauthier C, Gaede G, Radbruch H, Wernecke KD, Paul F. Sleep disorders reduce health-related quality of life in multiple sclerosis (Nottingham health profile data in patients with multiple sclerosis). Int J Mol Sci. 2015;16:16514–16528. doi:10.3390/ijms160716514
- Rashid NHA, Zaghi S, Scapuccin M, Camacho M, Certal V, Capasso R. The value of oxygen desaturation index for diagnosing obstructive sleep apnea: a systematic review. Laryngoscope. 2021;131:440–447. doi:10.1002/lary.28663
- Buratti L, Iacobucci DE, Viticchi G, et al. Sleep quality can influence the outcome of patients with multiple sclerosis. Sleep Med. 2019;58:56–60. doi:10.1016/j.sleep.2019.02.020
- Sparasci D, Ferri R, Castelnovo A, et al. Restless legs syndrome and periodic limb movements in 86 patients with multiple sclerosis. Sleep. 2021;44. doi:10.1093/sleep/zsab066
- Rocchi C, Pulcini A, Vesprini C, et al. Sleep in multiple sclerosis patients treated with interferon beta: an actigraphic study. Neurol Res. 2020;42(9):744–748. doi:10.1080/01616412.2020.1773629
- Levit E, Bouley A, Baber U, Djonlagic I, Sloane JA. Brainstem lesions are associated with sleep apnea in multiple sclerosis. Mult Scler J. 2020;6(4):205521732096795. doi:10.1177/2055217320967955
- Erman M, Emsellem H, Black J, Mori F, Mayer G. Correlation between the Epworth Sleepiness Scale and the Maintenance of Wakefulness Test in patients with narcolepsy participating in two clinical trials of sodium oxybate. Sleep Med. 2017;38:92–95. doi:10.1016/j.sleep.2017.07.015