Abstract
Purpose
Obstructive sleep apnea (OSA) is common in hypertrophic cardiomyopathy (HCM) patients and is related to worse adverse prognosis in HCM patients. However, there are no acknowledged warning characteristics to help to identify OSA in HCM patients.
Methods
Seventy-one HCM patients and forty-nine hypertensive (HTN) patients as control group underwent polysomnography (PSG) examination at the Second Affiliated Hospital of Nanchang University from January 2015 to December 2019 patients were consecutively enrolled. The characteristics were analyzed and compared between HCM patients with OSA and without OSA.
Results
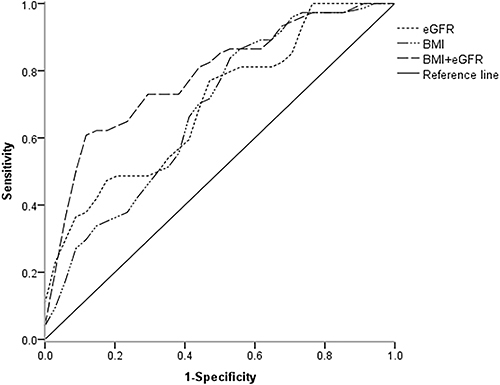
A total of 37 (52%) HCM patients and 25 (51%) HTN patients were diagnosed with OSA. High body mass index (BMI) (OR = 1.228, 95% CI: 1.032,1.461, P = 0.020) and low estimated glomerular filtration rate (eGFR) (OR = 0.959, 95% CI: 0.931,0.989, P = 0.007) independently correlated with the occurrence of OSA in HCM patients, respectively. Multiplicative interaction was shown between high BMI and low eGFR on the risk of OSA in HCM patients (OR: 6.050, 95% CI: 1.598, 22.905, P = 0.008). The additive interaction analysis further suggested that 70.1% of HCM patients developed OSA due to the additive interaction between BMI and eGFR. The identification ability of OSA in HCM patients was significantly enhanced by using both BMI and eGFR (area under receiver-operating characteristic analysis curve 0.785; P = 0.000038) as compared with BMI (area under curve 0.683, P = 0.008) or eGFR (area under curve 0.700, P = 0.004), respectively.
Conclusion
High BMI or low eGFR independently related to the occurrence of OSA in HCM patients, and the multiplicative and additive interactions between BMI and eGFR increased the identification ability of OSA in HCM patients.
Introduction
Hypertrophic cardiomyopathy (HCM) is the most common hereditary cardiomyopathy,Citation1 and the prevalence is at least 0.2% in populations.Citation2,Citation3 Obstructive sleep apnea (OSA) is the most common type of sleep apnea,Citation4 which is characterized by partial (hypopnea) or complete (apnea) airway obstruction during sleep despite respiration labored, leading to upper airway structure collapse. HCM has been a known cause of cardiac morbidity and mortality, and OSA is a significant reversible cause of many cardiovascular diseases including hypertension,Citation5 myocardial infarction,Citation6 atrial fibrillation (AF),Citation7 sudden cardiac death (SCD)Citation8 and renal disease.Citation9 OSA has been seen to occur concurrently in several cardiovascular diseases including heart failure, and indeed in HCM. Since 2004, Banno et al firstly reported that OSA might be a common disease in HCM patients, there have been many clinical studies on the clinical characteristics and incidence of HCM patients with OSA, suggesting that 32% to 80% HCM patients may have OSA.Citation10–15 As a common complication, OSA is known to increase the risk that contributes to morbidity and mortality in HCM including arrhythmias, myocardial hypertrophy, and SCD.Citation16
Considering the correlation between HCM and OSA, researchers had tried to find predictive indicators to identify the risk of OSA in HCM patients. One study indicated that age larger than 45 years old and the presence of atrial fibrillation helped to identify OSA in HCM patients.Citation17 Another study showed that male, age, body mass index, hypertension and left ventricular outflow tract obstruction were significantly correlated with OSA in HCM patients.Citation18 We previously found that HCM patients with OSA had a higher risk of eGFR decline compared with HCM patients without OSA, indicating low eGFR might be a warning characteristic identifying the occurrence of OSA in HCM patients.Citation19 We therefore investigated the characteristics including eGFR in a cohort of HCM patients to find the potential risk factors and their potential interactions on the occurrence of OSA in HCM patients.
Methods
Patients
We included 71 consecutive patients diagnosed with HCM (no relatives of the index cases were included) and 49 HTN patients as control group at the Second Affiliated Hospital of Nanchang University from January 2015 to December 2019. HTN patients were matched for BMI, sex, age, systolic and diastolic blood pressure by using propensity score matching from the patients admitted to the hospital at the same time as HCM patients. All patients received polysomnography (PSG) and were divided into OSA+ and OSA- according to apnea-hypopnea index (AHI) ≥15 or <15 events/h. OSA was diagnosed according to Chinese Guidelines for the Diagnosis and Treatment of Obstructive Sleep Apnea Hypopnea Syndrome (Revised Edition 2011).Citation20 HCM was diagnosed in accordance with the 2014 ESC Guidelines on the diagnosis and management of HCM.Citation21 For patients with hypertension, HCM was diagnosed only when the patients met the following criterions: 1) family history of HCM; 2) maximum LV wall thickness ≥15 mm; 3) marked repolarization abnormalities, conduction disease or Q-waves on 12 lead electrocardiogram.Citation21
Exclusion criteria were patients who have already received continuous positive airway pressure (CPAP) therapy, central sleep apnea, acute myocardial infarction, acute heart failure, severe respiratory insufficiency, severe liver disease, systemic or local inflammatory, cancer and patients who refused to participate in the study.
The study was approved by the Medical Research Ethics Committee of Second Affiliated Hospital of Nanchang University and a signed informed consent was obtained from each patient before participation. All methods were performed in accordance with the relevant guidelines and regulations. The study was carried out by complying with the Declaration of Helsinki.
Clinical Indices
Doppler echocardiography was used to evaluate the cardiac structure and function as described previously.Citation22 All echocardiographic examinations were performed on a Siemens-Acuson SequoiaTM 512 ultrasound machine (Siemens, Erlangen, Germany) with a curved array multifrequency transducer (2.25–4.25MHz) by experienced sonographers who were blinded to the patients’ clinical characteristics. Two-dimensional and two-dimensionally guided M-mode images were recorded from standardized views. Left atrial diameter, right atrial diameter, left ventricular end-diastolic diameter (LVIDd), left ventricular end-diastolic posterior wall thickness (PWTd), right ventricular end-diastolic diameter, interventricular septal wall end-diastolic thickness (SWTd), ascending aorta diameter, left ventricular mass index (LVMI), left ventricular ejection fraction, left ventricular outflow tract obstruction were measured. Left ventricular outflow tract obstruction is defined as an instantaneous peak Doppler LV outflow tract pressure gradient ≥30 mm Hg at rest or during physiological provocation such as Valsalva manoeuvre, standing and exercise. LV mass was calculated by the formula: LV mass = 0.8 × {1.04[(LVIDd + PWTd +SWTd)3–(LVIDd)3]} + 0.6 g.Citation23 LVMI was calculated by dividing LV mass by body surface area. The body surface area was calculated as follows: 0.0073×(height in centimeter)+0.0127×(weight in kilogram)-0.2106 (for female), 0.0057×(height in centimeter)+0.0121×(weight in kilogram)+0.0882 (for male).Citation24
All participants underwent overnight PSG (PHILIPS RESPIRONICS, Alice PDx, 1001 Murry Ridge Lane Murrysville, PA 15668). PSG was performed and scored according to the American Academy of Sleep Medicine (AASM) practice standards. An OSA was defined as a drop in airflow to ≤90% of baseline for ≥10s as recorded with the oronasal sensor with continued respiratory effort. Apnea was defined as the complete cessation of airflow or a clear decrease in airflow ≥90%, lasting for ≥10s. Hypopnea was defined as a clear decrease in airflow ≥50% lasting for ≥10s accompanied by a decrease in blood oxygen saturation (SpO2) of at least 3% or a clear decrease in airflow ≥30% lasting for ≥10s accompanied by a decrease in SpO2 of at least 4% and/or associated with arousal. The average number of apneas and hypopneas per hour of sleep was defined as the AHI. Nocturnal oxygen desaturation was assessed as the minimum O2 saturation during sleep. The diagnosis and severity of OSA were based on the definitions recommended by AASM as follows: non-OSA (AHI < 5), mild OSA (5 ≤ AHI < 15), moderate OSA (15 ≥ AHI < 30) and severe OSA (AHI ≥ 30). Patients with AHI ≥ 15 were recruited in the present study.
eGFR was calculated on the basis of Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI):Citation25 serum creatinine of woman ≤62 μmol/L: eGFR = 144×(serum creatinine/62)−0.329×(0. 993)age; serum creatinine of woman >62 μmol/L:eGFR= 144×(serum creatinine/62)−1.209×(0. 993)age; serum creatinine of man ≤80 μmol/L: eGFR = 141×(serum creatinine/80)−0.411×(0.993)age; serum creatinine of man >80 μmol/L: eGFR= 141 ×(serum creatinine/80)−1.209×(0. 993)age.
A standardized medical history and accurate physical examination were obtained from all patients. Smoking status and medications were recorded. Height and weight were measured in a standing position without shoes. Body mass index (BMI) was calculated as weight (kg) divided by height (m) squared. After a 12-h fasting (no alcohol), a peripheral blood sample was collected. Creatinine, uric acid, urea nitrogen, fasting plasma glucose, glycosylated hemoglobin, total cholesterol (TC), triglycerides (TG), high-density lipoprotein (HDL) cholesterol and low-density lipoprotein (LDL) cholesterol were measured with standard assays for all patients. All the biochemical variables were measured using an auto-analyzer (OLYMPUS AU-2700) at the central laboratory of the Second Affiliated Hospital of Nanchang University.
Statistical Analysis
t-test was used for normally distributed results, and the results were expressed as mean ± standard deviation. Mann–Whitney U-test was used for those who did not normally distribute, the result is expressed by quartile spacing. Differences between groups were evaluated using one-way analysis of variance (ANOVA) followed by the post hoc test with Least-Significant difference (LSD) correction, for the continuous variables, and the chi-square test, for the categorical variables, and the results were described by composition ratio. Binary Logistic regression analysis was used to identify the risk factors associated with the occurrence of OSA in HCM. Continuous variables with a skewed distribution were natural logarithm (ln)-transformed to attain normal distributions.
Body mass index (BMI) was stratified into two levels with a boundary of 24 (non-High BMI <24kg/m2; High BMI ≥ 24 kg/m2); Estimate glomerular filtration rate (eGFR) was stratified into two levels with a boundary of 90 (non-Low eGFR ≥90 mL/min per 1.73m2; Low eGFR <90 mL/min per 1.73m2). ROC curve was constructed for BMI and eGFR. The area under the curve (AUC), sensitivity, and specificity were calculated to predict the ability to detect the occurrence of OSA in HCM, with an AUC value of 0.50 indicating no accuracy and a value of 1.00 indicating maximal accuracy.
We performed multinomial logistic regression analyses to identify the multiplicative interaction of high BMI and low eGFR on the occurrence of OSA. In multinomial logistic regression analysis, the group without high BMI and low eGFR was used for reference. The crude model was the primary model. The model 1 was adjusted for current smoking, hypertension and atrial fibrillation. The model 2 was adjusted for current smoking, hypertension, atrial fibrillation, fasting plasma glucose, and glycosylated hemoglobin. The model 3 was adjusted for current smoking, hypertension, atrial fibrillation, fasting plasma glucose, glycosylated hemoglobin, total cholesterol, triglyceride, low-density lipoprotein and high-density lipoprotein.
Three measures of biological interaction: the relative excess risk due to interaction (RERI); the attributable proportion due to interaction (AP); and the synergy index (S) were used to evaluate the interaction of high BMI and low eGFR on the occurrence of OSA. If there is no biological interaction, RERI and AP are equal to 0 and S is equal to 1. The biological interaction was accessed by the methods set up by Tomas Andersson.Citation26 Results were adjusted for current smoking, hypertension, atrial fibrillation, fasting plasma glucose, glycosylated hemoglobin, total cholesterol, triglyceride, low-density lipoprotein and high-density lipoprotein.
A two-sided P value of <0.05 was considered significant. All statistical analyses were performed using SPSS software for Windows, version 21.0 (SPSS, Chicago, IL, USA).
Results
Baseline Characteristics of the Patients
details the baseline characteristics of patients. Total 71 consecutive HCM patients and 49 HTN patients were enrolled in this study. OSA was present in 37 patients (52%) with HCM and 25 patients (51%) with HTN. Compared with HCM with OSA group (HCM OSA +) and HTN with OSA group (HTN OSA +), the BMI and systolic blood pressure were significantly lower in HCM without OSA group (HCM OSA -) (P < 0.05). AHI was significantly higher in HTN OSA + than that in HCM OSA + (P < 0.05). Compared with HCM OSA +, HTN OSA + had a significantly decreased minimum O2 saturation (P < 0.05). Compared with HCM OSA -, the minimum O2 saturation in HTN without OSA group (HTN OSA -) was also significantly lower (P < 0.05). HTN patients administrated fewer beta-blockers than HCM patients (P < 0.001). HCM OSA + had a significantly higher creatinine and lower eGFR than HCM OSA - (P < 0.001); however, no differences of creatinine and eGFR were seen between HTN OSA+ and HTN OSA -. There was no significant difference between the two groups in gender, age, current smoking, diastolic blood pressure, glycosylated hemoglobin, total cholesterol, triglyceride, low-density lipoprotein and high-density lipoprotein.
Table 1 Clinical Characteristics of the Study Groups
Echocardiography
details the echocardiographic data of the two groups. The HCM with OSA group had significantly wider ascending aorta diameter (P < 0.05) than the HCM group. There were no significant differences in left atrial diameter, right atrial diameter, left ventricular end-diastolic diameter, right ventricular end-diastolic diameter, interventricular septal wall end-diastolic thickness, left ventricular end-diastolic posterior wall thickness, left ventricular mass index, left ventricular ejection fraction, and left ventricular outflow tract obstruction between the two groups.
Table 2 Echocardiographic Data of the Study Groups
BMI and eGFR Were Independently Associated with the Occurrence of OSA in HCM
details the univariate and multivariate logistic regression analyses in the HCM patients with and without OSA. The univariate logistic regression analysis showed that BMI (OR=1.249,95% CI: 1.064, 1.466, P = 0.006), SBP (OR=1.028,95% CI: 1.005, 1.052, P = 0.015), Ln Creatinine (OR = 16.190, 95% CI: 2.475, 105.917, P=0.004), eGFR (OR=0.959,95% CI: 0.934, 0.986,P=0.003) were significantly correlated with the occurrence of OSA. Considering the causal relationship between eGFR and creatinine, creatinine values were not included in the multivariate analysis. The multivariate regression analysis further identified that BMI (OR=1.228,95% CI: 1.032,1.461, P = 0.020) and eGFR (OR=0.959,95% CI: 0.931,0.989, P = 0.007) were independently correlated with the occurrence of OSA.
Table 3 Logistic Regression Analysis of Clinical Factors for the Presence of OSA in HCM Patients
ROC Analysis for BMI and eGFR to Predict the Occurrence of OSA in HCM Patients
To determine the ability of BMI and eGFR to predict the occurrence of OSA in HCM patients, ROC analysis was performed, and the results are shown in and . The AUC for BMI and eGFR to predict the occurrence of OSA was 0.683, 0.700, respectively. The highest Youden’s index was 0.336, 0.316 respectively and the sensitivity and specificity were 0.865 and 0.471, 0.559 and 0.757, respectively. Then the multivariate logistic regression analysis of BMI and eGFR values was included in the SPSS software to obtain the predicted probability values of both, and then the values were included in the ROC curve analysis. After the combination of BMI and eGFR, the cutoff value was 0.646 which had the maximum value of the Youden’s index of 0.507, and the corresponding sensitivity and specificity were 0.595 and 0.912. The AUC was 0.785.
Table 4 ROC Analysis for BMI and/or eGFR to Predict OSA in HCM Patients
Interaction of BMI and eGFR on the Occurrence of OSA in HCM Patients
Interaction of BMI and eGFR on the occurrence of OSA in HCM patients was assessed both on multiplicative interaction and on additive interaction in the present study. The multiplicative interaction was further identified by multinomial logistic regression analysis (). Results showed that compared with patients without High BMI and Low eGFR, patients with High BMI (OR: 1.100, 95% CI: 0.192, 6.286, P = 0.915) or with Low eGFR (OR: 1.711, 95% CI: 0.403, 7.271, P = 0.467) had no significantly increased risk on the occurrence of OSA in HCM patients. However, there was a significantly higher risk of the occurrence of OSA in HCM patients with both high BMI and low eGFR (OR: 6.050, 95% CI: 1.598, 22.905, P = 0.008). This effect was persisted after adjusting for different confounders in models 1 to 3.
Table 5 Relation of Study Groups to the Occurrence of OSA in HCM Patients
Further, we analyzed the additive effect of biological interactions, which was set up by Tomas Andersson.Citation25 details the measurement results of the three biological interactions. According to AP (0.701, 95% CI: 0.221, 1.181), it was concluded that 70.1% of patients with HCM developed OSA due to the biological interaction of the High BMI and Low eGFR. AP values were more significant after adjusting for confounding factors (0.754, 95% CI: 0.354, 1.155).
Table 6 Interaction Between BMI and eGFR in Relation to OSA in HCM Patients
Discussion
A main finding in this study was that there were significant multiplicative and additive interactions of BMI and eGFR on the occurrence of OSA in patients with HCM, and the combination of the two factors had a better predictive effect on the occurrence of OSA in HCM patients. This finding was supported by the following results: 1) BMI and eGFR were respectively and independently correlated with the occurrence of OSA in HCM patients (); 2) BMI and eGFR had significant multiplicative () and additive interactions () in patients with HCM and OSA; 3) the combination of BMI and eGFR had a better predictive effect on the occurrence of OSA in HCM patients ( and ).
HCM is the most common inherited cardiomyopathy and OSA is a significant reversible cause of many cardiovascular diseases including hypertension,Citation5 myocardial infarction,Citation6 atrial fibrillation (AF),Citation7 sudden cardiac death (SCD)Citation8 and renal disease.Citation9 OSA has been seen to occur concurrently in several cardiovascular diseases including heart failure, and indeed in HCM. In fact, a high prevalence of OSA with a range from 32% to 80% has been previously reported in HCM patients,Citation10–15 and OSA is known to increase the risk that contribute to morbidity and mortality in HCM including arrhythmias, myocardial hypertrophy, and SCD.Citation16 A significant reduction in major adverse cardiovascular events with effective treatment of OSA can be seen in both normal populations and in populations with established cardiovascular diseases.Citation27 In patients with both HCM and OSA, heart functional improvement, left atrial volume reduction, and left ventricular outflow tract obstruction amelioration were shown after continuous positive airway pressure (CPAP) treatment, a most used therapy for OSA.Citation28 It has also been reported that CPAP treatment could terminate recurrent episodes of ventricular tachycardia.Citation29 Therefore, the two conditions occurring together pose a particular challenge in that the identification and appropriate management of OSA in HCM patients.
Though researchers tried to find a predictor to identify the risk of OSA in HCM patients, they discovered that patients with HCM and OSA had no typical clinical symptoms, such as fatigue and excessive daytime sleepiness.Citation17 Male, age, BMI, hypertension and left ventricular outflow tract obstruction were previously reported correlated with OSA in HCM patients.Citation18 Although study had shown that the co-presence of these factors might suggest the presence of OSA in patients with HCM,Citation18 this association had not been constant in other study,Citation17 including the present study. Obese has been recognized as a risk factor for OSA,Citation30,Citation31 however, it was indicated that patients with HCM and OSA were not as obese as the typical OSA patients,Citation32 which was consistent with our previous study showing that OSA patients alone had higher BMI than patients with both HCM and OSA.Citation19
Interestingly, we previously found that HCM patients with OSA had a higher risk of eGFR decline compared with HCM patients without OSA, indicating eGFR decline could be a characteristic identifying HCM patients with OSA.Citation19 In the present study, we matched HTN patients who received PSG with HCM patients and found that there was no difference of eGFR between HTN patients with and without OSA. We further found that combination of higher BMI and lower eGFR had a better identification ability of OSA in HCM patients, which seems to be correlated with a multiplicative and additive interaction of high BMI and low eGFR in HCM patients with OSA. These findings might afford the opportunity to increase the alertness of OSA in HCM patients.
In HCM patients, high BMI was shown to be associated with increased left ventricular outflow tract obstruction, which increased the incidence of heart failure and atrial fibrillation.Citation33 High BMI changes the structure and function of the upper respiratory tract, reduces the power in the central respiratory system and causes hypoxemia.Citation34 These underlying pathogeneses may contribute to the OSA in obesity populations. The mechanism underlying the correlation between eGFR and OSA in HCM patients is not very clear. However, in previous study, nocturnal rostral fluid shift was found to be independently related to the severity of OSA in patients with end-stage renal disease.Citation35 This may be due to the fluid accumulated in the lower limbs during the day would enter the neck slowly during sleep, causing the neck dilated large veins and/or edema of the pharyngeal soft tissue and being prone to upper airway obstruction.Citation35 Such a phenomenon of nocturnal rostral fluid shift could lead to increased neck circumference and upper airway resistance even in non-obese healthy subjects.Citation36
The present study has certain limitations. Firstly, as a major limitation, we were unable to construct a clinical prediction model based on the available data to effectively address the prediction of OSA onset in HCM patients. The development of a clinical prediction model based on the TRIPOD statement should become an important research direction in the future. Secondly, our study is a cross-sectional design, and we could not identify the cause-and-effect association between BMI, eGFR and OSA in the patients with HCM. Thirdly, considering the mildly altered GFR and almost normal BMI in the HCM population of this study, this may somewhat attenuate the performance of both factors in predicting OSA in clinical HCM cases. Fourthly, we did not investigate the effect of weight loss and kidney protection treatment on severity of OSA in HCM patients. Fifthly, the study investigated a relatively small number of patients in a single center. Finally, the mechanism underlying the effect of BMI and eGFR on OSA in HCM patients remains unclear.
Nevertheless, based on our previous finding, the current study further provided the first evidence that a multiplicative and additive interaction of BMI and eGFR in HCM patients could increase the identification ability of OSA in this population. Therefore, warning of high BMI and low eGFR may increase the opportunity for identification and appropriate management of OSA in HCM patients.
Disclosure
The authors report no conflicts of interest in this work.
Additional information
Funding
References
- Van Driest SL, Ommen SR, Tajik AJ, Gersh BJ, Ackerman MJ. Yield of genetic testing in hypertrophic cardiomyopathy. Mayo Clin Proc. 2005;80(6):739–744. doi:10.1016/S0025-6196(11)61527-9
- Maron BJ, Gardin JM, Flack JM, Gidding SS, Kurosaki TT, Bild DE. Prevalence of hypertrophic cardiomyopathy in a general population of young adults. echocardiographic analysis of 4111 subjects in the CARDIA study. coronary artery risk development in (Young) adults. Circulation. 1995;92(4):785–789. doi:10.1161/01.CIR.92.4.785
- Maron BJ, Mathenge R, Casey SA, Poliac LC, Longe TF. Clinical profile of hypertrophic cardiomyopathy identified de novo in rural communities. J Am Coll Cardiol. 1999;33(6):1590–1595. doi:10.1016/S0735-1097(99)00039-X
- Kapur VK, Auckley DH, Chowdhuri S, et al. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: an American Academy of sleep medicine clinical practice guideline. J Clin Sleep Med. 2017;13(3):479–504. doi:10.5664/jcsm.6506
- Venkataraman S, Vungarala S, Covassin N, Somers VK. Sleep apnea, hypertension and the sympathetic nervous system in the adult population. J Clin Med. 2020;9(2):591. doi:10.3390/jcm9020591
- Lee CH, Khoo SM, Chan MY, et al. Severe obstructive sleep apnea and outcomes following myocardial infarction. J Clin Sleep Med. 2011;7(6):616–621. doi:10.5664/jcsm.1464
- Linz D, Schotten U, Neuberger HR, Böhm M, Wirth K. Negative tracheal pressure during obstructive respiratory events promotes atrial fibrillation by vagal activation. Heart Rhythm. 2011;8(9):1436–1443. doi:10.1016/j.hrthm.2011.03.053
- Priori SG, Blomström-Lundqvist C, Mazzanti A, et al. 2015 ESC guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: the task force for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Eur Heart J. 2015;36(41):2793–2867. doi:10.1093/eurheartj/ehv316
- Somers VK, White DP, Amin R, et al. Sleep apnea and cardiovascular disease: an American Heart Association/American College of Cardiology foundation scientific statement from the American Heart Association Council for High Blood Pressure Research professional education committee, Council on Clinical Cardiology, Stroke Council, and Council on Cardiovascular Nursing. J Am Coll Cardiol. 2008;52(8):686–717. doi:10.1016/j.jacc.2008.05.002
- Banno K, Shiomi T, Sasanabe R, et al. Sleep-disordered breathing in patients with idiopathic cardiomyopathy. Circ J. 2004;68(4):338–342. doi:10.1253/circj.68.338
- Eleid MF, Konecny T, Orban M, et al. High prevalence of abnormal nocturnal oximetry in patients with hypertrophic cardiomyopathy. J Am Coll Cardiol. 2009;54(19):1805–1809. doi:10.1016/j.jacc.2009.07.030
- Konecny T, Brady PA, Orban M, et al. Interactions between sleep disordered breathing and atrial fibrillation in patients with hypertrophic cardiomyopathy. Am J Cardiol. 2010;105(11):1597–1602. doi:10.1016/j.amjcard.2010.01.023
- Pedrosa RP, Lima SG, Drager LF, et al. Sleep quality and quality of life in patients with hypertrophic cardiomyopathy. Cardiology. 2010;117(3):200–206. doi:10.1159/000321718
- Prinz C, Bitter T, Oldenburg O, Horstkotte D, Faber L. Incidence of sleep-disordered breathing in patients with hypertrophic cardiomyopathy. Congest Heart Fail. 2011;17(1):19–24. doi:10.1111/j.1751-7133.2010.00196.x
- Patel SI, Shamoun FE, Esser H, et al. Sleep disordered breathing in hypertrophic cardiomyopathy. Sleep Vigil. 2020;4(1):3. doi:10.1007/s41782-019-00080-6
- Javaheri S, Barbe F, Campos-Rodriguez F, et al. Sleep apnea: types, mechanisms, and clinical cardiovascular consequences. J Am Coll Cardiol. 2017;69(7):841–858. doi:10.1016/j.jacc.2016.11.069
- Nerbass FB, Pedrosa RP, Genta PR, et al. Lack of reliable clinical predictors to identify obstructive sleep apnea in patients with hypertrophic cardiomyopathy. Clinics. 2013;68(7):992–996. doi:10.6061/clinics/2013(07)17
- Xu H, Wang J, Yuan J, et al. Clinical predictors of the presence of obstructive sleep apnea in patients with hypertrophic cardiomyopathy. Sci Rep. 2021;11(1):13528. doi:10.1038/s41598-021-93039-5
- Wang SY, Luo J, Dong YF, et al. Risk of glomerular filtration rate decline in patients with hypertrophic cardiomyopathy and obstructive sleep apnoea. Sci Rep. 2017;7(1):17399. doi:10.1038/s41598-017-17818-9
- Sleep Disordered-breathing Committee, Respiratory Society, Chinese Medical Association. Guidelines for the diagnosis and treatment of obstructive sleep apnea hypopnea syndrome (Revised Edition 2011). Chin J Tuberc Respir Dis. 2012;35:9–12.
- Elliott PM, Anastasakis A, Charron P, et al.; Authors/Task Force members. 2014 ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy: the task force for the diagnosis and management of hypertrophic cardiomyopathy of the European Society of Cardiology (ESC). Eur Heart J. 2014;35(39):2733–2779.
- Cao C, Hu JX, Dong YF, et al. Association of endothelial and mild renal dysfunction with the severity of left ventricular hypertrophy in hypertensive patients. Am J Hypertens. 2016;29(4):501–508. doi:10.1093/ajh/hpv128
- Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18(12):1440–1463. doi:10.1016/j.echo.2005.10.005
- Hu YM, Wu XL, Hu ZH, et al. [Study of formula for calculating body surface areas of the Chinese adults]. Sheng Li Xue Bao. 1999;51(1):45–48. Chinese.
- Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi:10.7326/0003-4819-150-9-200905050-00006
- Andersson T, Alfredsson L, Källberg H, Zdravkovic S, Ahlbom A. Calculating measures of biological interaction. Eur J Epidemiol. 2005;20(7):575–579. doi:10.1007/s10654-005-7835-x
- Milleron O, Pillière R, Foucher A, et al. Benefits of obstructive sleep apnoea treatment in coronary artery disease: a long-term follow-up study. Eur Heart J. 2004;25(9):728–734. doi:10.1016/j.ehj.2004.02.008
- Sengupta PP, Sorajja D, Eleid MF, et al. Hypertrophic obstructive cardiomyopathy and sleep-disordered breathing: an unfavorable combination. Nat Clin Pract Cardiovasc Med. 2009;6(1):14–15. doi:10.1038/ncpcardio1401
- Shimada YJ, Sato K, Hanon S, Schweitzer P. Termination of recurrent ventricular tachycardia by continuous positive airway pressure ventilation. Ann Noninvasive Electrocardiol. 2009;14(4):404–406. doi:10.1111/j.1542-474X.2009.00331.x
- Young T, Peppard PE, Taheri S. Excess weight and sleep-disordered breathing. J Appl Physiol. 2005;99(4):1592–1599. doi:10.1152/japplphysiol.00587.2005
- Shah N, Roux F. The relationship of obesity and obstructive sleep apnea. Clin Chest Med. 2009;30(3):455–465. doi:10.1016/j.ccm.2009.05.012
- Nerbass FB, Pedrosa RP, Danzi-Soares NJ, Drager LF, Arteaga-Fernández E, Lorenzi-Filho G. Obstructive sleep apnea and hypertrophic cardiomyopathy: a common and potential harmful combination. Sleep Med Rev. 2013;17(3):201–206. doi:10.1016/j.smrv.2012.06.006
- Fumagalli C, Maurizi N, Day SM, et al. Association of obesity with adverse long-term outcomes in hypertrophic cardiomyopathy. JAMA Cardiol. 2020;5(1):65–72. doi:10.1001/jamacardio.2019.4268
- Strobel RJ, Rosen RC. Obesity and weight loss in obstructive sleep apnea: a critical review. Sleep. 1996;19(2):104–115. doi:10.1093/sleep/19.2.104
- Elias RM, Bradley TD, Kasai T, Motwani SS, Chan CT. Rostral overnight fluid shift in end-stage renal disease: relationship with obstructive sleep apnea. Nephrol Dial Transplant. 2012;27(4):1569–1573. doi:10.1093/ndt/gfr605
- Redolfi S, Yumino D, Ruttanaumpawan P, et al. Relationship between overnight rostral fluid shift and Obstructive sleep apnea in nonobese men. Am J Respir Crit Care Med. 2009;179(3):241–246. doi:10.1164/rccm.200807-1076O