Abstract
Purpose
Sleep state misperception, which is the discrepancy between subjective and objective sleep, is often observed in patients with depression. This phenomenon may delay the remission of depression. Previous studies have focused on the total sleep time (TST) misperception, with many of these studies using actigraphy. Thus, our study investigated depressed patients with the exploratory aim of clarifying factors associated with the sleep state misperception including the wake after sleep onset (WASO) misperception, with their objective sleep additionally evaluated by polysomnography (PSG).
Patients and Methods
We conducted a cross-sectional study. Before undergoing overnight PSG monitoring, 40 patients with depression completed questionnaires that included the Beck Depression Inventory (BDI), Epworth sleepiness scale, Temperament and Character Inventory, and the Pittsburgh sleep quality index. Patients were also asked to estimate their subjective sleep duration after they woke up in the morning. Based on this data, we calculated the misperception using the following formula: subjective sleep duration minus objective sleep duration. We compared each factor between negative and positive misperception groups and the multiple regression analysis was performed for TST and WASO misperception, respectively.
Results
Although sleep architectures, age, severity of depression and obstructive sleep apnea (OSA) exhibited differences in underestimating or overestimating the WASO, only sex differences were associated with underestimating or overestimating their total sleep time (TST). Moreover, BDI, the severity of OSA, sleep architectures (N1% and N2%), and benzodiazepine (BZD) use were significantly correlated with WASO misperception, whereas only OSA severity was significantly correlated with TST misperception. A subsequent multiple regression analysis demonstrated the BDI was independently correlated with the WASO misperception (β=0.341, p=0.049).
Conclusion
In clinical practice, interventions especially for OSA, and the reduction of depressive symptoms are an important method for improving patient sleep perception. Moreover, current results suggest that BZD prescriptions should be avoided as well.
Introduction
Sleep state misperception is a discrepancy between subjective sleep and objective sleep, with the prevalence ranging from 9.2% to 56% in patients with insomnia.Citation1–Citation4 Generally, most patients tend to underestimate their total sleep time (TST) and overestimate their sleep onset latency (SOL) and wake after sleep onset (WASO).Citation5 Previous studies have shown that insomnia patients who complain about sleep often have anxiety about sleep arousals during sleep along with daytime cognitive impairment, in addition to stating that they could not achieve sleep satisfaction even though they were getting adequate objective sleep.Citation6,Citation7 Since sleep state misperception interferes with diagnosis and evaluation of sleep,Citation5 it is essential to clarify associated factors in order to better determine the course of treatment.
Recently, sleep state misperception has been reported in patients with depression, along with discussions of associational factors.Citation8–Citation10 A cross-sectional study of major depression revealed that subjects in the TST underestimation group were more depressed and exhibited less slow wave sleep as compared to the TST overestimation group. Furthermore, this study also revealed that individuals in the TST underestimation group had a significantly higher introvert trait index as measured by the Maudsley personality inventory as compared to the overestimation group.Citation8 Other studies of bipolar disorders have reported finding that the severity of depression, but not manic symptoms, was positively correlated with the inaccuracy of the TST.Citation9,Citation10 However, most of these studies only focused on the TST misperception and did not fully investigate other factors, such as the presence of comorbid sleep disorders or the use of psychiatric drugs. Furthermore, many of these studies used actigraphy to estimate the objective sleep duration, even though the validity of calculating sleep parameters, especially WASO, is inferior to polysomnography (PSG).Citation11
Furthermore, a recent retrospective study demonstrated that OSA was comorbid with depression and bipolar disorder at a high rate, with this comorbidity observed in more than half of these patients.Citation12 Obstructive sleep apnea (OSA) is one of the factors associated with the direction and degree of sleep state misperception. Previous research have showed that patients with OSA greatly overestimate SOL while they are more likely to underestimate TST and WASO, which is associated with insomnia severity, age, and the severity of OSA.Citation13–Citation15 These results implied the importance of OSA, as it is a crucial factor for determining the direction of WASO and TST misperception. In addition, many patients with depression are additionally being administered various types of pharmacotherapies.Citation16,Citation17 Although these medications may help to improve sleep conditions, they may also have negative effects on the overall sleep architectures.Citation16–Citation19
Therefore, our current study explored the psychosocial and physiological factors associated with the sleep state misperception, which included WASO misperception among depressive patients, but did not exclude sleep disorders.
Materials and Methods
Study Participants
We conducted a cross-sectional single-center study. We recruited participants from inpatients being examined at Nagoya University Hospital in Japan between 2016 and 2020. Inclusion criteria were participants of 18 years and older with depression, including major depressive disorder and depressive phase of bipolar disorders based on DSM-5 along with having insomnia symptoms. Other criterion used was the availability of subjective and objective sleep data. Exclusion criteria included 1) participants who were diagnosed for dementia, neurodegenerative disease, Parkinson disease, fibromyalgia, major vascular disease, arrhythmia and cerebrovascular disease, 2) who had previously undergone continuous positive airway pressure (CPAP) and/or oral appliance, 3) who work night shift or day/night shifts. We categorized medications that particularly influenced sleep into 3 groups: benzodiazepines (BZDs), antidepressants and antipsychotics. BZDs such as triazolam and etizolam were converted to an equivalent dose of diazepam (DZP). Antidepressants and antipsychotics were also converted to equivalent doses of imipramine (IP) and chlorpromazine (CP), respectively.Citation20 Eventually, we recruited a total of 52 participants with depression, with all diagnoses made by multiple skilled psychiatrists. This study was conducted in accordance with the Declaration of Helsinki and all procedures were approved by the Nagoya University Ethics Review Committee. All participants agreed to the purpose and procedures of this study and provided written consent.
Questionnaires
Interview questionnaires
Hamilton Rating Scale for Depression (HAM-D)
The HAM-D, which is an objective depressive symptom rating scale,Citation21 is one of the most widely used depression scales in clinical settings. Skilled psychiatrists or psychologists use this scale to assess depressed symptoms of all patients. A total score of 7 or higher indicates that the patient is clinically depressed.
Young Mania Rating Scale (YMRS)
The YMRS was used as a scale to assess the objective manic symptoms of the participants.Citation22 A total score of 12 or less indicates that the patient is in remission while more than 12 indicates the patient is clinically manic.
Self-Reported Questionnaires
Beck Depression Inventory (BDI)
The BDI was used for evaluating the severity of depression, and is a widely used self-reported scale.Citation23 The BDI is a 21-item self-report questionnaire that is based on a four Likert scale with the score ranging from 0 to 63 points. A score of 0 to 9 indicates minimal depression, 10 to 18 indicates mild depression, 19–29 indicates moderate depression and 30–63 indicates severe depression.
Epworth Sleepiness Scale (ESS)
The ESS was used to measure daytime sleepiness. This test is a questionnaire that asks subjects about sleepiness during eight situations in daily living routines.Citation24 The questionnaire consists of eight items for a four-point scale, with the total scores ranging from 0 to 24. A score of 11 or higher indicates excessive sleepiness during the day.
Temperament and Character Inventory (TCI)-Harm Avoidance
TCI is a personality test developed by CloningerCitation25 that consists of 125 items that are used to measure four temperament dimensions (novelty seeking: NS, harm avoidance: HA, reward dependence: RD, and persistence: P) and three personality dimensions (self-directedness: SD, cooperativeness: C, and self-transcendence: ST). We chose to use the HA from these seven dimensions, as HA has been previously reported to be significantly associated with insomnia severity and depression.Citation26
Pittsburgh Sleep Quality Index (PSQI)
The PSQI is a 19-item instrument capable of assessing subjective sleep quality, sleep onset latency, and total sleep time.Citation27 The questionnaire is a sum of all items, with the scores ranging from 0 to 27. Previous reports have indicated that subjects with a score of 6 or higher are poor sleepers.
PSG
This study used the standard attended PSG (EMBLA N7000, Nates Pleasanton, CA, USA or PSG-1100, Nihon Kohden Co., Tokyo, Japan). Results were analyzed based on version 2.1 of the American Academy of Sleep Medicine scoring manual.Citation28 These methods have been previously described in detail.Citation29 We evaluated the following parameters: TST, sleep efficiency (% of TST to total time in bed), rapid eye movement (REM) sleep latency, stage REM (%TST), non-REM sleep stage N1 (%TST), N2 (%TST), N3 (%TST), WASO, apnea–hypopnea index (AHI), and arousal index.
Sleep State Misperception
The index of sleep state misperception was the value between the subjective sleep index and minus the objective sleep index.Citation8 In the morning, the PSG was ended, with the participants then asked to rate their subjective TST, and WASO using the following questions:“How long did you sleep last night?” and “How long did you awake after initially falling asleep?”
Statistical Analysis
All data were reported as the mean and standard deviation (SD). We divided participants into two groups based on the underestimation or overestimation of TST or WASO. When comparing each factor between the two groups, analysis was performed using a non-paired t-test for those factors that could be assumed to normally distributed, while a Mann–Whitney U-test was performed for those factors that were non-normally distributed. Fisher’s exact test was also used to evaluate the significant difference of the categorical factors. Pearson and Spearman correlation analyses of each factor were performed to analyze the TST and WASO misperception. Subsequently, we then performed a multiple regression analysis to determine the factors that were independently associated with TST and WASO misperception. Independent variables were selected based on previous research and clinical insights. We put all variables into the model with the forced entry method. Models were determined from a probability value, adjust R-squared and checking the normality of residuals. All statistical analyses were performed using R and R commander.
Results
Although there were 52 participants who met the inclusion criteria, 12 participants were subsequently found to meet the exclusion criteria or had missing values in their questionnaires. The missing values were treated by the listwise deletion. Therefore, a total of 40 participants were included in the final analysis. lists the demographic characteristics of participants.
Table 1 Summary of Demographic, PSG, Subjective Sleep, and Symptoms Rating Data
WASO State Misperception (Underestimation vs Overestimation)
No significant difference was found for the demographic factors between the two groups other than for age. Average age of the underestimation group was older compared to that of the overestimation group (55.4±13.8 vs 45.7±16.1, p=0.047). There were several differences in the sleep architectures between the two groups. Although N1% was significantly higher in participants with WASO underestimation versus that for the WASO overestimation group (49.3±21.4 vs 36.5±20.6, p=0.044), the N2% was lower for participants in the underestimation group compared to the overestimation group (35.0±17.0 vs 49.0±15.7, p=0.014). Participants in the underestimation group also had a significantly higher AHI as compared to those in the overestimation group (23.9±18.4 vs 16.6±24.3, p=0.03). In contrast, the BDI and HAM-D scores were significantly higher in the overestimation group as compared to the underestimation group, respectively (BDI: 12.8±10.3 vs 20.9±12.1, p=0.031, HAM-D: 10.6±6.5 vs 14.8±6.4, p=0.047). Furthermore, the PSQI score was higher in the overestimation group versus the underestimation group (8.7±2.7 vs 12.0±4.1, p=0.006) ().
Table 2 Underestimation vs Overestimation in WASO
TST State Misperception (Underestimation vs Overestimation)
There was no significant difference found between the groups with respect to age, diagnosis, BMI, subjective WASO, sleep stages, BDI, HA, ESS, or psychiatric drugs (). The percentage of females in the underestimation group (50%,13/26) was significantly higher than that found in the overestimation group (14%, 2/14). These findings confirm that there was a sex difference between the two groups (p=0.01). In addition, the PSQI score was significantly higher in the TST underestimation group (11.5±4.0 vs 8.5±2.5, p=0.017).
Table 3 Underestimation vs Overestimation in TST
Correlation Analysis
and present the correlation analysis results. Only AHI was positively correlated with the TST misperception (r=0.483 p=0.005), with none of the other factors exhibiting any correlation. However, the BDI score and DZP were positively correlated with WASO misperception (BDI: r=0.319, p=0.045, DZP: r=0.338, p=0.033). N1% and N2% were also correlated with WASO state misperception, respectively (N1%: r=−0.367, p=0.019, N2%: r=0.463, p=0.002). AHI was negatively correlated with WASO misperception (r=−0.412, p=0.025).
Table 4 Results of Correlation Analysis with Each Factor
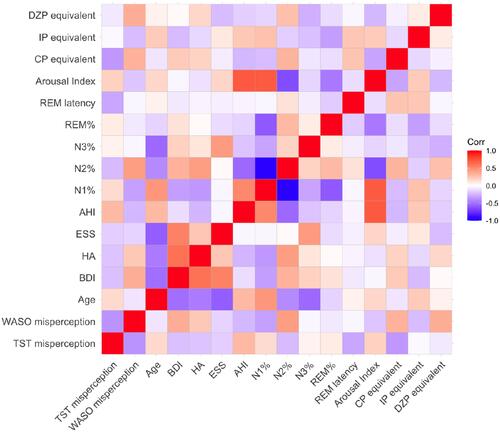
Figure 1 Heatmap of correlation analysis. The results of the correlation analysis described in are visualized in this heatmap. The darker the color, the stronger the correlation. A positive correlation is indicated by red, whereas a negative correlation is indicated by blue.
Multiple Regression Analysis for TST Misperception and WASO Misperception
The multiple regression analysis results showed that none of the factors were independently related to the TST misperception (AHI: β=0.271, p=0.385, sex: β=0.139, p=0.095)(). In contrast, a significant relationship was found between the BDI score and WASO misperception (β=0.341, p=0.049) (). The adjusted R2 was 0.229 and the significance level of this model was 0.021. None of the other factors were directly associated with WASO misperception.
Table 5 Multiple Regression Analysis for TST Misperception
Table 6 Multiple Regression Analysis for WASO Misperception
Discussion
Our current study was designed as an attempt to explore and investigate the factors associated with TST misperception as well as WASO misperception, which have yet to be fully evaluated among patients with depression. The severity of depression, the severity of OSA, N1%, N2%, and BZDs were all found to be associated with WASO misperception, with the severity of depression independently associated with WASO overestimation. In contrast, only the severity of OSA was positively correlated with TST misperception. To the best of our knowledge, this is the first study to determine factors associated with not only TST but also WASO misperception in patients with depression.
WASO Misperception
Our current findings demonstrated that the severity of OSA was associated with WASO underestimation, which is consistent with a previous study that examined insomnia patients with OSA.Citation13 These results could be explained by the sleep fragmentations, which are more likely to occur with increasing severity of OSA.Citation30 Although the underlying psychophysiological mechanism remains unknown, previous studies have indicated that the micro sleep architectures, memory process, and glucose metabolism in the brain appear to be interrelated and can interfere with sleep state misperception.Citation6,Citation31,Citation32 Based on these findings in conjunction with the results of our current study, we believe that even though it has been shown that OSA can help amplify wakefulness during sleep, the repetition of wakefulness and sleep state may be an obstacle with regard to the perception of their state, thereby resulting in an underestimation of the WASO. In contrast, the severity of depression was higher in the WASO overestimation group, with the multiple regression analysis indicating that the severity of depression might be independently associated with WASO overestimation. Previous reports have shown that higher depression was associated with a longer subjective WASO.Citation33,Citation34 A meta-analysis has demonstrated that time perception was slow in the examined clinical group, which implies that the subjective time perception of depressed patients might be longer as compared to the control group.Citation35 Thus, in our current study, depression may have caused a dysfunction with regard to the sense of time and thus, the subjects may have estimated the actual time of their awake state to be longer than it actually was.
Furthermore, we showed that the use of psychiatric drugs and sleep architectures was associated with WASO misperception. Our analysis indicated that the use of BZDs and N2% were associated with WASO overestimation. BZDs are widely prescribed in clinical settings for not only treating anxiety disorders, but also treating insomnia symptoms that make it especially difficult to fall asleep.Citation16,Citation36 However, BZDs could have potentially increased the objective WASO, thereby causing a discrepancy between subjective and objective sleep.Citation19 In addition, it could also induce dependency and increase N2%.Citation36,Citation37 Therefore, considering the influence of BZDs on N2%, it is possible that N2% may be a mediating factor between BZDs and WASO overestimation, with the use of BZDs interfering with the perception of sleep throughout the N2%.
In contrast, N1%, which is the light sleep stage that is related to arousals, was negatively correlated with WASO misperception. One potential explanation for this is that the high AHI enhanced N1% rather than N1% being associated with WASO underestimation by itself. It is well known that the severity of OSA is positively correlated with N1%.Citation38 Considering the magnitude of AHI in our current study, the severity of OSA could have potentially influenced N1%. Furthermore, age was higher in the WASO underestimation group. This result is in line with a previous report demonstrating that aging was one of the risk factors for the underestimation of WASO among insomniacs.Citation39
TST Misperception
The only factors that differed between the two groups were sex and PSQI score. Prior research has implied the possibility that women tend to underestimate their TST as compared with men.Citation40 Furthermore, a meta-analysis demonstrated that while women report more subjective sleep complaints,Citation40 PSG often does not confirm this difference between the sexes.Citation41 Our current study revealed that while there was an association between TST overestimation and the severity of OSA, our multiple regression analysis did not demonstrate any significant relationship. While the overestimation of TST is expected to be influenced by electroencephalography frequencies during sleep, especially beta and gamma bands, the mechanism responsible for this remains unclear.Citation42 Further research will need to be undertaken in order to determine how OSA affects sleep misidentification in depressed patients.
Contrary to prior reports, personality traits were not associated with TST misperception and there were no differences found for these between the two groups. Previous studies of depressed patients have shown that anxiety tendency is high in TST underestimation,Citation8 however, this study was not confirmed. One possible explanation for this agreement is that in our study, the average BDI score was mild to moderate while in this previous study, it was reported to be moderate to severe.Citation8 Thus, differences in the severity of depression would be related to this result.
Limitations and Future Research
There are some limitations in our current study. First, interpretation requires caution due to the relatively small sample size. A future study with a larger number of samples will be needed in order to confirm the present results. Second, this study was not of a longitudinal design. Due to this, the causal relationship between sleep state misperception and these factors cannot be inferred at the present time. Third, the severity of depression in our subjects ranged from mild to moderate. Therefore, our results may not be representative of depressive patients in general. A future study that recruits more severe cases will be required. Fourth, although this result would reflect actual clinical practice, we did not control all confounding factors affecting sleep and PSG. Furthermore, most of participants were hospitalized one day before the PSG for prevention of first-night effect while some participants did not. Future research in more controlled settings will elucidated the mechanism clearly. Fifth, although several participants take anti-epileptic drugs the number of them was not enough to analyze. In future research, since performing PSG can be a heavy burden for patients with severe mental disorders,Citation11 there is a need to find a better way to measure their sleep using less burdensome instruments, such as home-based simple objective measurement devices.Citation43
Conclusion
Although there were several limitations, as listed above, this study was able to reflect a more clinically relevant situation by taking into consideration OSA, which has a high comorbidity with depression. Among patients with depressive symptoms, a greater severity of depression can be a marker of potential WASO overestimation. Moreover, BZDs and OSA, which affect sleep architectures, could also be a risk factor interrupting the subject’s WASO perception. In clinical settings, clinicians need to intervene in cases of depression and OSA while also considering tapering or withdrawing BZDs when there is subjective prolonged sleep arousal detected among such patients.
Abbreviations
AHI, apnea-hypopnea index; BD, bipolar disorders; BDI, Beck Depression Inventory; BMI, body mass index; CP, chlorpromazine; DZP, diazepam; ESS, Epworth sleepiness index; HA, harm avoidance; HAM-D, Hamilton Rating Scale for Depression; IP, imipramine; MDD, major depressive disorders; PSG, polysomnography; CPAP, continuous positive airway pressure; SE, sleep efficacy; SOL, sleep onset latency; TST, total sleep time; WASO, wake after sleep onset latency; YMRS, Young Mania Rating Scale.
Data Sharing Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Acknowledgment
This research was supported in part by research grants from the Ministry of Education, Culture, Sports, Science and Technology of Japan, a Grant-in-Aid for Comprehensive Research on Disability Health and Welfare from the Ministry of Health, Labour and Welfare of Japan, a Grant-in-Aid for the center of Innovation Program by the Japan Science and Technology Agency, JSPS KAKENHI Grant No. 17K10272 and No. 20K11577, AMED under grant No. JP21dk0307103 and No. JP21dk0307099.
Disclosure
KI has received speakers’ honoraria from Eisai, Kyowa, Meiji Seika Pharma, Otsuka, Sumitomo Dainippon, Taisho, Takeda, and Towa. NO has received research support or speakers’ honoraria from, or has served as a consultant to, Sumitomo Dainippon, Eisai, Otsuka, KAITEKI, Mitsubishi Tanabe, Shionogi, Eli Lilly, Mochida, Daiichi Sankyo, Nihon Medi-Physics, Takeda, Boehringer Ingelheim, Meiji Seika Pharma, EA Pharma, Pfizer, MSD, Lundbeck Japan, Taisho Pharma, Kyowa Kirin, Janssen, UCB, Shionogi, Nihon Medi-Physics, Tsumura, Novartis, Astellas, Ono, Viatris, Medical Review, Yoshitomi Yakuhin, and Astellas. In addition, NO has a patent 19874599.4 with royalties paid to Woolsey; a patent 2020-552954 with royalties paid to Sumitomo Pharma co., Ltd. The authors report no other conflicts of interest in this work.
References
- Coleman RM, S J Kennedy, C Guilleminault, et al. Sleep-wake disorders based on a polysomnographic diagnosis. A national cooperative study. JAMA. 1982;247(7):997–1003. doi:10.1001/jama.1982.03320320033026
- Edinger JD, Krystal AD. Subtyping primary insomnia: is sleep state misperception a distinct clinical entity? Sleep Med Rev. 2003;7(3):203–214. doi:10.1053/smrv.2002.0253
- Fernandez-Mendoza J, Calhoun S, Bixler EO, et al. Insomnia with objective short sleep duration is associated with deficits in neuropsychological performance: a general population study. Sleep. 2010;33(4):459–465. doi:10.1093/sleep/33.4.459
- Castelnovo A, Ferri R, Punjabi NM, et al. The paradox of paradoxical insomnia: a theoretical review towards a unifying evidence-based definition. Sleep Med Rev. 2019;44:70–82. doi:10.1016/j.smrv.2018.12.007
- Rezaie L, Fobian AD, McCall WV, Khazaie H. Paradoxical insomnia and subjective-objective sleep discrepancy: a review. Sleep Med Rev. 2018;40:196–202. doi:10.1016/j.smrv.2018.01.002
- Harvey AG, Tang NK. (Mis)perception of sleep in insomnia: a puzzle and a resolution. Psychol Bull. 2012;138(1):77–101. doi:10.1037/a0025730
- Mercer JD, Bootzin RR, Lack LC. Insomniacs’ perception of wake instead of sleep. Sleep. 2002;25(5):564–571. doi:10.1093/sleep/25.5.559
- Tsuchiyama K, Nagayama H, Kudo K, Kojima K, Yamada K. Discrepancy between subjective and objective sleep in patients with depression. Psychiatry Clin Neurosci. 2003;57(3):259–264. doi:10.1046/j.1440-1819.2003.01114.x
- Gonzalez R, Tamminga C, Tohen M, Suppes T. Comparison of objective and subjective assessments of sleep time in subjects with bipolar disorder. J Affect Disord. 2013;149(1–3):363–366. doi:10.1016/j.jad.2013.02.013
- Krishnamurthy V, Mukherjee D, Reider A, et al. Subjective and objective sleep discrepancy in symptomatic bipolar disorder compared to healthy controls. J Affect Disord. 2018;229:247–253. doi:10.1016/j.jad.2017.12.100
- Baandrup L, Jennum PJ. A validation of wrist actigraphy against polysomnography in patients with schizophrenia or bipolar disorder. Neuropsychiatr Dis Treat. 2015;11:2271–2277. doi:10.2147/NDT.S88236
- Okada I, Miyata S, Iwamoto K, Fujishiro H, Noda A, Ozaki N. Prevalence of obstructive sleep apnea as assessed by polysomnography in psychiatric patients with sleep-related problems. Sleep Breath. 2022. doi:10.1007/s11325-022-02566-6
- Bianchi MT, Williams KL, McKinney S, Ellenbogen JM. The subjective-objective mismatch in sleep perception among those with insomnia and sleep apnea. J Sleep Res. 2013;22(5):557–568. doi:10.1111/jsr.12046
- Castillo J, Goparaju B, Bianchi MT. Sleep-wake misperception in sleep apnea patients undergoing diagnostic versus titration polysomnography. J Psychosom Res. 2014;76(5):361–367. doi:10.1016/j.jpsychores.2014.03.001
- Lee SA, Im K, Yang HR. Factors associated with sleep state misperception in patients with obstructive sleep apnea. Sleep Breath. 2022. doi:10.1007/s11325-021-02543-5
- Ogawa Y, Takeshima N, Hayasaka Y, et al. Antidepressants plus benzodiazepines for adults with major depression. Cochrane Database Syst Rev. 2019;6(6):1–65. doi:10.1002/14651858.CD001026.pub2
- Alexander GC, Gallagher SA, Mascola A, Moloney RM, Stafford RS. Increasing off-label use of antipsychotic medications in the United States, 1995–2008. Pharmacoepidemiol Drug Saf. 2011;20(2):177–184. doi:10.1002/pds.2082
- DeMartinis NA, Winokur A. Effects of psychiatric medications on sleep and sleep disorders. CNS Neurol Disord Drug Targets. 2007;6(1):17–29. doi:10.2174/187152707779940835
- Mazza M, Losurdo A, Testani E, et al. Polysomnographic findings in a cohort of chronic insomnia patients with benzodiazepine abuse. J Clin Sleep Med. 2014;10(1):35–42. doi:10.5664/jcsm.3354
- Inada T, Inagaki A. Psychotropic dose equivalence in Japan. Psychiatry Clin Neurosci. 2015;69(8):440–447. doi:10.1111/pcn.12275
- Hamilton M. Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol. 1967;6(4):278–296. doi:10.1111/j.2044-8260.1967.tb00530.x
- Young RC, Ziegler VE, Meyer DA, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133(5):429–435. doi:10.1192/bjp.133.5.429
- Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation; 1996.
- Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540–545. doi:10.1093/sleep/14.6.540
- Cloninger CR. A systematic method for clinical description and classification of personality variants. A proposal. Arch Gen Psychiatry. 1987;44(6):573–588. doi:10.1001/archpsyc.1987.01800180093014
- de Saint Hilaire Z, Straub J, Pelissolo A. Temperament and character in primary insomnia. Eur Psychiatry. 2005;20(2):188–192. doi:10.1016/j.eurpsy.2004.04.009
- Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi:10.1016/0165-1781(89)90047-4
- Berry RB, Brooks R, Gamaldo CE, et al. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications, version 2.1. Illinois. American Academy of Sleep Medicine; 2014.
- Fujishiro H, Okuda M, Iwamoto K, et al. Early diagnosis of Lewy body disease in patients with late-onset psychiatric disorders using clinical history of rapid eye movement sleep behavior disorder and [(123) I]-metaiodobenzylguanidine cardiac scintigraphy. Psychiatry Clin Neurosci. 2018;72(6):423–434. doi:10.1111/pcn.12651
- Bianchi MT, Cash SS, Mietus J, Peng CK, Thomas R. Obstructive sleep apnea alters sleep stage transition dynamics. PLoS One. 2010;5(6):1–12. doi:10.1371/journal.pone.0011356
- Kay DB, Karim HT, Soehner AM, et al. Subjective-objective sleep discrepancy is associated with alterations in regional glucose metabolism in patients with insomnia and good sleeper controls. Sleep. 2017;40(11):1–11. doi:10.1093/sleep/zsx155
- Krystal AD, Edinger JD, Wohlgemuth WK, Marsh GR. NREM sleep EEG frequency spectral correlates of sleep complaints in primary insomnia subtypes. Sleep. 2002;25(6):630–640.
- O’Callaghan VS, Couvy-Duchesne B, Strike LT, McMahon KL, Byrne EM, Wright MJ. A meta-analysis of the relationship between subjective sleep and depressive symptoms in adolescence. Sleep Med. 2021;79:134–144. doi:10.1016/j.sleep.2021.01.011
- Paterson LM, Nutt DJ, Wilson SJ. NAPSAQ-1: national patient sleep assessment questionnaire in depression. Int J Psychiatry Clin Pract. 2009;13(1):48–58. doi:10.1080/13651500802450498
- Thönes S, Oberfeld D. Time perception in depression: a meta-analysis. J Affect Disord. 2015;175:359–372. doi:10.1016/j.jad.2014.12.057
- Lader M. Dependence and withdrawal: comparison of the benzodiazepines and selective serotonin re-uptake inhibitors. Addiction. 2012;107(5):909–910. doi:10.1111/j.1360-0443.2011.03736.x
- Wu CS, Wang SC, Chang IS, Lin KM. The association between dementia and long-term use of benzodiazepine in the elderly: nested case-control study using claims data. Am J Geriatr Psychiatry. 2009;17(7):614–620. doi:10.1097/JGP.0b013e3181a65210
- Shahveisi K, Jalali A, Moloudi MR, Moradi S, Maroufi A, Khazaie H. Sleep architecture in patients with primary snoring and obstructive sleep apnea. Basic Clin Neurosci. 2018;9(2):147–156. doi:10.29252/NIRP.BCN.9.2.147
- Kushida CA, Chang A, Gadkary C, Guilleminault C, Carrillo O, Dement WC. Comparison of actigraphic, polysomnographic, and subjective assessment of sleep parameters in sleep-disordered patients. Sleep Med. 2001;2(5):389–396. doi:10.1016/s1389-9457(00)00098-8
- Zhang B, Wing YK. Sex differences in insomnia: a meta-analysis. Sleep. 2006;29(1):85–93. doi:10.1093/sleep/29.1.85
- Voderholzer U, Al-Shajlawi A, Weske G, Feige B, Riemann D. Are there gender differences in objective and subjective sleep measures? A study of insomniacs and healthy controls. Depress Anxiety. 2003;17(3):162–172. doi:10.1002/da.10101
- Trajanovic NN, Radivojevic V, Kaushansky Y, Shapiro CM. Positive sleep state misperception - a new concept of sleep misperception. Sleep Med. 2007;8(2):111–118. doi:10.1016/j.sleep.2006.08.013
- Miyata S, Iwamoto K, Banno M, et al. Performance of an ambulatory electroencephalogram sleep monitor in patients with psychiatric disorders. J Sleep Res. 2020:1–7. doi:10.1111/jsr.13273
