Abstract
Objective
To investigate the association between sleep disturbances and behavioral problems as well as quality of life (QOL) in Chinese children with epilepsy.
Methods
Caregivers of 167 epileptic children aged 3 to 12 years completed the Child Sleep Habits Questionnaire (CSHQ), the Strengths and Difficulties Questionnaire (SDQ), and the Pediatric Quality of Life Inventory (PedsQL™, 4.0 Core).
Results
The prevalence of sleep disturbances (CSHQ total score >41) in epileptic children was 73.7% [95% CI (66.9%.80.4%)]. Epileptic children with sleep disturbances demonstrated more behavioral problems and lower QOL compared to those without sleep disturbances. Sleep disturbances such as sleep anxiety and daytime sleepiness were associated with more behavioral problems and lower QOL (p <0.05). Linear regression analyses showed that higher disturbance in sleep duration domain were associated with more behavioral problems, while higher sleep disordered breathing domains was associated with lower QOL (p <0.05). The interaction between sleep disturbances and behavioral problems in predicting QOL was not significant. The sensitivity analysis using 48 as an alternative cutoff for CSHQ total score obtained consistent results.
Conclusion
Sleep disturbances occur frequently among Chinese children with epilepsy, and are associated with more behavioral problems and lower QOL. The sleep disturbance-QOL association is unlikely contingent on behavioral problems. This study highlights the necessity of evaluating and treating sleep disturbances multidimensionally among children with epilepsy to promote their whole health and wellbeing.
Introduction
Epilepsy is a common childhood neurological disease, affecting more than 10 million children worldwide.Citation1 Sleep disturbances are among the most prevalent comorbidities of epilepsy, with prevalence ranging 45–95% among children with epilepsy,Citation2,Citation3 as compared with 25–40% among typically developing children.Citation4 The most common sleep disturbances in children with epilepsy include excessive daytime sleepiness (76%), sleep disordered breathing (65%), and parasomnia (53%).Citation5 The relationship between epilepsy and sleep disturbances is likely bidirectional and complex.Citation6 Sleep can modulate seizure occurrence or interictal epileptiform discharges, and sleep disturbances may worsen epileptic symptoms. Conversely, epileptic discharges have been shown to disrupt sleep-wake cycles.Citation7 Therefore, epilepsy with the added sleep disturbances likely exacerbates children’s health and functioning.Citation7 Previous studies have independently reported high prevalence of sleep disturbances,Citation7,Citation8 behavioral problems,Citation2,Citation9 and poor QOL,Citation10 in children with epilepsy. Yet, there lacks a systematic examination of the association among sleep disturbances, behavioral problems, and QOL.
Emerging evidence suggested the association between sleep disturbances and behavioral problems in children with epilepsy. A study regarding the Rolandic epilepsy reported higher level of behavioral problems in epileptic children with sleep problems than those without.Citation11 Byars et al also found that in children with epilepsy, sleep disturbances were correlated with increased behavioral problems.Citation12 However, existing studies focused predominantly on Western children with epilepsy, and thus the results may not be generalizable to Chinese children due to the social and cultural differences in sleep practice and behaviour.Citation13 What’s more, for those having sleep disturbances or/and behavioral problems, impaired QOL may also be present. Yet, there are few studies on the relationship between sleep disturbance and QOL in children with epilepsy. A recent study showed that children with epilepsy and sleep disturbances had poorer QOL.Citation14 Given above evidence, it is possible that sleep disturbances may interact with behavioral problems in children with epilepsy to reduce QOL. However, such interaction has not been systematically investigated in empirical studies. Bridging such literature gap is crucial to furthering our understanding of the association among the three conditions and to inform clinical interventions.
Therefore, the current study was to explore the association among sleep disturbances, behavioral problems, and QOL in Chinese children with epilepsy. We hypothesized: 1) children with sleep disturbances would demonstrate more behavioral problems and poor QOL, 2) sleep disturbances would be associated with more behavioral problems and lower QOL, and 3) the sleep disturbance-QOL association would be contingent on behavioral problems in Chinese children with epilepsy.
Methods
Participants and Procedure
The current study is a cross-sectional design. We recruited children with epilepsy and their caregivers from a paediatric neurological clinic and inpatient ward in a tertiary children’s hospital in Shanghai. Children aged 3 to 12 years who met the International League Against Epilepsy (ILAE) 2017 criteriaCitation15 admitted for a diagnosis of epilepsy from July 2020 to October 2021. The exclusion criteria were: (1) children born at less than 37 weeks; (2) children with overt structural brain damage or cerebral palsy; (3) children with other confirmed physical and psychiatric diseases, such as haematologic tumours, congenital heart diseases, attention deficit hyperactivity disease (ADHD), autism spectrum disorder (ASD), and tic disorder (TD); or (4) parents unable to speak, read, and understand Chinese.
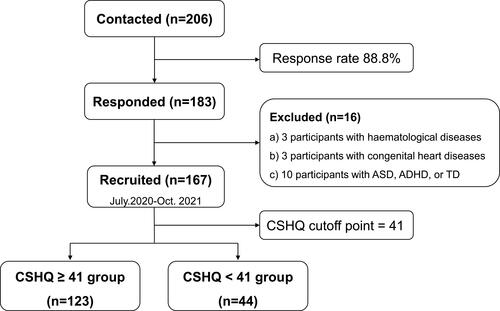
According to previous studies, sleep disturbances occurred 25–40% in typically developing childrenCitation4 and 45–95% of children with epilepsy.Citation2,Citation3 Using the two lowest rates (25% vs 45%) by PASS software, we estimated that a sample size of at least 115 would provide an effect size of 0.9. A total of 206 children with epilepsy were contacted, and caregivers of 183 agreed to participate and signed informed consent (response rate 88.8%). Investigators on site supervised the caregivers of children with epilepsy while completing the questionnaires in person and independently in a quiet hospital setting. Three participants with haematologic tumours, three with congenital heart diseases, and ten with other clearly diagnosed neurodevelopmental disorders (ie, ADHD, ASD or TD) were excluded; data from 167 participants ultimately were included in the analysis ().
Figure 1 Flowchart of study participants with data on inclusion, exclusion and distribution according to CSHQ.
The current study was approved by the Shanghai Children’s Medical Center Ethics Committee (SCMCIRB-K2020030-1) in accordance with the guidelines of the Declaration of Helsinki (1964).
Measures
Children’s sleep disturbances were assessed by the Children’s Sleep Habit Questionnaire (CSHQ), a 33-item parent-reported questionnaire initially designed for children aged 4 to 10 years.Citation16 More recent studies have extended its use to children aged 3–12 years.Citation17,Citation18 Each item was rated on a 3-point scale ranging from “usually” to “rarely”, reflecting sleep habits of children in the past week. The CSHQ evaluated sleep disturbances on eight subscales: bedtime resistance, sleep onset delay, sleep duration, sleep anxiety, night awaking, parasomnias, sleep disordered breathing, and daytime sleepiness. Higher total and subscale scores indicated more sleep problems. A total score of > 41 was suggested as the clinical cutoff for general sleep disturbance.Citation16 Considering the cutoff of 41 based on American children may overestimate sleep disturbances, especially for behavioral domains in Chinese children, a total score of 48 as an alternative cutoff point was utilized in the current study for a sensitivity analysis.Citation4,Citation19 The Chinese version of the CSHQCitation20 has been used in children with epilepsy.Citation8 The Cronbach’s α was 0.73 in the current study for the full questionnaire.
Behavioral problems were assessed by Strengths and Difficulties Questionnaire (SDQ).Citation21 This 25-item questionnaire was designed for children aged 4 to 16 years, with later studies extending its use to children aged as young as 3 years.Citation22 Each response is rated on a 3-point Likert scale (0 for “not true”, 1 for “partly true”, or 2 for “very true”). The SDQ consists of five subscales: emotional symptoms, conduct problems, hyperactivity/inattention, peer relationship problems, and prosocial behaviors. Score ranged from 0 to 10 for each of the five subscales. The total difficulties score ranging from 0 to 40 was calculated by totalling all subscales except for prosocial behaviors. The internalizing score was a sum of emotional symptoms and peer relationship scales (ranges from 0 to 20) while the externalizing score was a sum of conduct problems and hyperactivity/inattention scales (ranges from 0 to 20). A higher score was indicative of more severe behavioral symptoms. Total score ≥ 14 was suggested as the clinical cutoff point for behavioral difficulties.Citation21 The SDQ had been used in children with epilepsyCitation23 and prior to this was used within the Chinese population with good psychometrics.Citation24 The Cronbach’s α for the total difficulties was 0.61 in current study.
The Paediatric Quality of Life Inventory (PedsQL™, 4.0 Core) scale was used to assess the QOL in children.Citation25 The PedsQL is a 23-item generic measure including four age-group scales specific to children aged 2–4, 5–7, 8–12, and 13–18 years. It assesses the following 4 domains: physical functioning (8 items), emotional functioning (5 items), social functioning (5 items), and school functioning (5 items). Emotional, school, and social functioning can be combined into a psychosocial health domain. Each item was rated on a 5-point Likert scale from 0 to 4, subsequently reverse scored and linearly transformed to a 0 to 100 points scale (0 = 100, 1 = 75, 2 = 50, 3 = 25, 4 = 0). Thus, a higher converted score was indicative of a better QOL. The reliability and validity of the Chinese translations of the PedsQL 4.0 scale have been reported for Chinese children.Citation26 The Cronbach’s α for the full scale was 0.94 in the current study.
In addition to the above assessments, caregivers also reported the sociodemographic characteristics including age, sex, the only-child status, caregivers’ education level, family structure, family living environment, and their children’s seizure history.
Statistical Analysis
Descriptive statistics were used to characterize sociodemographic variables, and sleep and epilepsy-related variables. Continuous data were presented as mean ± standard deviation (SD), and categorical data as frequencies (percentages). To test for sample distribution, we used a Shapiro–Wilk test. As most of data were not distributed normally, we used the Mann–Whitney test to compare continuous variables between groups. The categorical variables from the two groups were compared using chi-square test. Spearman correlation analyses were used to measure the association between sleep variables and behavioral problems as well as QOL. Stepwise linear regression analyses were performed to determine sleep and epileptic parameters predicting behavior problems or lower QOL. We conducted a two-way ANOVA to examine the interaction between sleep disturbance and behavioral problems in predicting QOL, including CSHQ total score (below or above cutoff) and SDQ total difficulties (below or above cutoff) as independent variables, and some sociodemographic variables (ie, sex, age, being the only child at home, family structure, education of caregiver, and family living environment) as covariates. We also used 48 as the alternative cutoff point of CSHQ total score for sensitivity analysis to examine the robustness of our findings. All analyses were performed using Statistical Package for the Social Sciences (SPSS®), version 26.0, with a significance level of 0.05.
Results
Sample Characteristics
Demographic and sleep characteristics of the samples are summarized in . Of the 167 children, 123 (73.7%) were rated as having sleep disturbances (CSHQ total score > 41). There was a significant age difference between the group with sleep disturbances and the group without. As our preliminary analysis detected no difference in behavioral problems and QOL between children aged 3–6 years and 6–12 years, and thus we combined the age groups for all subsequent analyses. Children with epilepsy in the sleep disturbances group had significantly higher prevalence of bed-sharing and room-sharing than those without. Regarding epilepsy characteristics (), seizure duration of children with sleep disturbances were significantly longer than those without, and children with sleep disturbances had significantly more epileptic status during the duration of epilepsy than those in the other group. And significant difference in specified diagnosis was observed between the two groups, with a higher proportion of Benign Epilepsy with Centrotemporal Spikes (BECTS) in the group without sleep disturbance.
Table 1 Demographic Characteristics of Children with Epilepsy
Table 2 Epilepsy Related Characteristics
Differences of Behavioral Problems and QOL Between CSHQ > 41 Group and CSHQ ≤ 41 Group
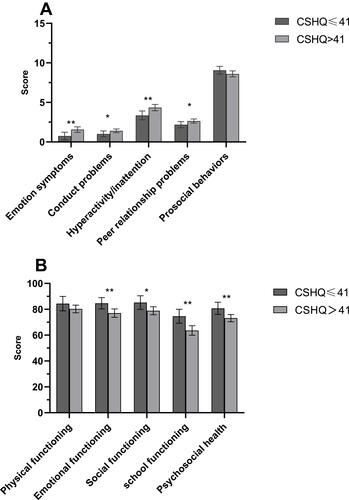
Compared with children without sleep disturbances, those with sleep disturbances had significantly higher score in SDQ total difficulty as well as the domains of emotional symptom, conduct problems, hyperactivity/inattention, and peer relationship (ps < 0.05). The domain of prosocial problems was not significantly different between the two groups (). Regarding QOL, children in the sleep disturbances group had significantly lower scores in emotional, social, and school functioning and psychosocial health domains (p < 0.05). Significant differences were not observed in physical functioning ().
Figure 2 Differences of SDQ subscale (A) and QOL subscale scores (B) between CSHQ > 41 group and CSHQ ≤ 41 group. Number of samples in each group are as follows: children with epilepsy in CSHQ ≤ 41 score group (n =44) and in CSHQ > 41 score group (n =123). Each column shows SDQ subscale scores and QOL subscale scores and error bar represent 95% confidence interval. *p < 0.05, **p < 0.01.
In the sensitivity analysis, consistent results showed that epileptic children with CSHQ total score > 48 had significantly higher SDQ total score and lower QOL total score.
Association Among Sleep Disturbances, Behavioral Problems, and QOL
A series of significant correlations were detected between sleep disturbances and behavioral problems or QOL in children with epilepsy (). Bedtime resistance, sleep onset delay, sleep duration, sleep anxiety, night waking, parasomnias, and daytime sleepiness were significantly correlated with SDQ total score (all rs < 0.5, p < 0.05). Sleep anxiety, sleep disordered breathing, daytime sleepiness, and total sleep disturbance scores were correlated significantly with QOL total score (all |rs| < 0.3, p < 0.05). Sleep disordered breathing and daytime sleepiness were significantly correlated with both physical functioning and psychosocial health scores (p < 0.05).
Table 3 Spearman Correlation Between Sleep Subscales and Behavioral Problems as Well as QOL
In the regression analyses (), the multicollinearity test found that the tolerance of each variable was between 0.81 and 0.98, and the variance expansion factor ranged between 1.01 and 1.23, indicating no multicollinearity. The SDQ total score was significantly associated with the CSHQ subscale score on sleep duration (β = 0.43, p < 0.001) and sleep anxiety (β = 0.16, p < 0.05), while QOL total score was significantly associated with the CSHQ subscale scores on sleep disordered breathing (β = −0.17, p < 0.05). In epilepsy-related parameters, diagnosis of BECTS was significantly correlated both higher SDQ total score (β = −0.17, p < 0.05) and lower QOL total scores (β = 0.24, p < 0.05). Duration of epilepsy ≤ 1 month (β = −0.18, p < 0.05) was only significantly correlated with SDQ total score, while the ≥2 anti-epileptic drugs (AEDs) was only significantly correlated lower QOL total score (β = −0.20, p < 0.01).
Table 4 Linear Regression Models for Epileptic and Sleep Parameters on Behavioral Problems and QOL in Children with Epilepsy
There was a strong correlation between sleep disturbances and behavioral problems, but the results of two-way ANOVA showed no significant interaction between CSHQ total score and SDQ total score (F (1,163) = 0.71, p > 0.05).
Discussion
Our study provided first evidence for the association among sleep disturbances, behavioral problems, and QOL among Chinese children with epilepsy. Sleep disturbances were highly prevalent in children with epilepsy (73.7%). Among children with epilepsy, sleep disturbances were significantly associated with more behavioral problems and lower QOL, and the sleep disturbance-QOL association was unlikely contingent on behavioral problems. The sensitivity analysis using an alternative cutoff of 48 for CSHQ total score obtained consistent results, ensuring the robustness of our findings. Results of the current study suggested that sleep disturbances, behavioral problems, and QOL should be integrated as part of a comprehensive assessment of overall life functioning for epileptic children to enhance clinicians’ awareness and promote children’s health.
The current study showed that sleep disturbances were common and severe in epileptic children, with a prevalence of 73.7% and a mean CSHQ total score of 45.92. In Hong Kong, CSHQ total score was 48.89 from a sample of epileptic children aged 4 to 12 years old.Citation8 A study among American children with epilepsy aged 2 to 10 years reported a CSHQ total score of 48.25.Citation7 The relatively lower severity level of sleep disturbances in the current sample compared to earlier reports in Hong Kong and US may due to sample’s heterogeneity and cross-cultural differences. In our study, nearly half of epileptic children reported no seizures within the last six months, which was higher than 41% in the Hong Kong study.Citation8 A prior study reported higher seizure frequency was related to higher scores for excessive sleepiness and disorders of sleep-wake transition.Citation11 Also, a polysomnographic study did not identify difference in sleep structures between epilepsy patients without seizures and healthy controls,Citation27 which may partially explain the lower prevalence of sleep disturbances in our sample. Additionally, the effect of antiepileptic drugs on sleep should be acknowledged. For example, benzodiazepines and barbiturates may reduce sleep latency, valproate may increase the stage 1 sleep, and lamotrigine may decrease slow wave sleep and insomnia.Citation28 Further, children on polytherapy presented with worse sleep problems than children on monotherapy.Citation28 In our study, 79% of the children with epilepsy took the monotherapy, which was higher than the American sample (50%)Citation7 and the Hong Kong sample (62%).Citation8 Regarding cross-cultural difference, unique school schedules and sleep practices may contribute to the differences in the sleep disturbances of children from different regions.Citation29 For example, previous studies showed that Chinese children had significantly later bedtimes, earlier morning wake time, and shorter sleep duration compared with those from the United States,Citation29 and Hong Kong children had significant later bedtime and shorter sleep duration than Shanghai children.Citation30 These factors may account for the lower prevalence of sleep disturbances in our study.
Consistent with previous studies,Citation11,Citation12 the current study showed that epileptic children with sleep disturbances presented with higher emotional symptoms, conduct problems, hyperactivity/attention, and peer relationship problems than those without.Citation12 The mechanisms of this relationship may involve the prefrontal cortex and its associated neurocognitive and neurobehavioral processes.Citation31 Additionally, our results revealed that epileptic children with sleep disturbances had lower scores on emotional, school, social functioning and psychosocial health of QOL, which corroborated with previous studies.Citation14 It is worth noting that discrepancies in the specific domain of QOL also existed. For instance, as previous studies reported significant differences in physical functioning of QOL between epileptic children with and without sleep disturbances,Citation14,Citation32 the current study did not. It may be possible that, as children in the current sample experienced shorter duration of illness, its deleterious influence on physical functioning has yet to manifest. Another conjectural reason may be that the low rate of seizure within the last six months may decrease the positive moderating role of active epileptic seizures on the relationship between sleep disturbances and physical functioning.Citation33 Also, patients with CHSQ > 41 in our study had more status epilepticus in which the massive epileptic discharges releasing from brain might have a different impact on physical and psychosocial health of QOL.Citation34
More importantly, we found an independent association of sleep disturbances and behavioral problems with QOL in children with epilepsy, without detecting a significant interaction effect. This finding expands our understanding of the complex relationship among sleep disturbances, behavioral problems, and QOL in children with epilepsy, and underlines comprehensive management of these comorbidities.
In analysing specific domains of sleep disturbances, we found sleep disordered breathing was significantly associated with QOL, but not with behavioral problems. However, previous studies have reported that children with sleep-disordered breathing displayed worse behavioral functioning and lower QOL.Citation35 It is possible that sleep-disordered breathing symptoms can affect QOL initially, but behavioral functioning over time. A prior study showed that remission of sleep breathing symptoms after adenotonsillectomy frequently improved QOL in short time but did not improve behavioral outcomes.Citation36 Hence, it is imperative to routinely screen for sleep disturbances and refer epileptic children for polysomnography (PSG) studies if necessary, particularly for those with sleep complaints and symptoms of sleep-disordered breathing.
Finally, the current study found that the diagnosis of BECTS was associated with both behavioral functioning and QOL. As a benign epilepsy, BECTS had lower seizure frequency than other epilepsy and seizures usually disappeared after puberty, so it had subtle impact on sleep, behavior, and QOL.Citation37 When viewed in combination with the studies of BECTS, behavioral problems may be slighter but more persistent in children with BECTS than in children with epilepsy in general, though direct comparisons between epilepsy syndromes are not available.Citation38
In our study, shorter duration of epilepsy was also correlated with more behavioral problems. Consistently, an Italian study suggested that the longer duration of the epilepsy was associated with behavioral problems and children with enduring epileptic manifestations were at higher risk for social failures.Citation39 Furthermore, epileptogenic networks with enduring changes in brain function and structure, may have resulted in emotional and behavioral alterations.Citation40 We also found the number of antiepileptic drugs was associated with lower QOL, which was similar with that being reported in previous studies.Citation41 The reason may be that the treatment of epilepsy may cause significant side effects, such as drowsiness, irritability, nausea, and headache, which had particular risk for diminished QOL.Citation41
Our study was strengthened by using validated instruments, covering a wide range of relevant factors, and conducting a sensitivity analysis. Nevertheless, the findings should be interpreted with caution due to the following limitations. Firstly, with the current sample being recruited from the same site, nearly half reported no seizures within the last six months, and the majority being on monotherapy, our findings may lack generalizability. Future studies with larger samples from multiple sites are encouraged to confirm and expand our findings. Secondly, the study did not include objective sleep measurements such as actigraphy or polysomnography, and thus the findings may be subject to reporting bias. Nevertheless, the CSHQ is a well-validated tool for screening sleep problems in children with neurodevelopmental disorders,Citation42 providing measurements that correspond moderately well with objective measurement results.Citation43 Similarly, the study could be enhanced by including clinical evaluation of sleep disorders, psychopathology, and neurodevelopmental disorders by experienced psychiatrist or developmental and behavioral pediatricians, which would provide more accurate information such as detecting more children with ADHD and ASD. Thirdly, parents, teachers and children (particularly for old children) might have different perspectives in terms of sleep disturbances, emotional and behavioral problem, and QOL, and thus future studies would better consider multiple informants. Fourthly, the small sample size of the group without sleep disturbances could potentially produce the type 2 error when comparing to the group with sleep disturbances. However, our sensitivity analysis using an alternative cutoff of 48 for CSHQ total score increased the sample size of the group without sleep disturbances, but still demonstrated consistent results, which ensured the robustness of our findings. Finally, we could not establish a causal relationship due to the cross-sectional design. Thus, longitudinal and intervention studies are warranted to fully unveil the relationship among sleep disturbances, behavioral problems and QOL in children with epilepsy.
Conclusion
In conclusion, sleep disturbances are prevalent in Chinese children with epilepsy. Among the epileptic children, sleep disturbances are significantly associated with worse behavioral functioning and lower QOL, and the sleep disturbance-QOL association is unlikely contingent on behavioral problems. Our study highlights the necessity of evaluating and treating sleep disturbances multidimensionally among children with epilepsy to promote their whole health and wellbeing. Future studies should investigate whether effective sleep interventions can improve behavioral functioning and QOL in children with epilepsy.
Abbreviations
QOL, quality of life; CSHQ, Children’s Sleep Habits Questionnaire; SDQ, the Strengths and Difficulties Questionnaire; PedsQL™, the Pediatric Quality of Life Inventory; ILAE, the International League Against Epilepsy; ADHD, Attention Deficit Hyperactivity Disease; ASD, Autism Spectrum Disorder; TD, Tic Disorder; AEDs, antiepileptic drugs; PSG, polysomnography; BECTS, Benign Epilepsy with Centrotemporal Spikes; ADNFLE, Autosomal Dominant Nocturnal Frontal Lobe Epilepsy.
Acknowledgment
Special thanks to the children and families who participated in the study. This work was supported by National Natural Science Foundation of China (82071493), Shanghai Science and Technology Commission (18JC1420305, 19QA1405800, 19411968800) and Shanghai Pudong District Technology Development Funds (PKJ2018-Y45).
Disclosure
The authors report no conflicts of interest in this work.
Additional information
Funding
References
- Guerrini R. Epilepsy in children. Lancet. 2006;367:499–524. doi:10.1016/S0140-6736(06)68182-8
- Tsai SY, Lee WT, Jeng SF, Lee CC, Weng WC. Sleep and behavior problems in children with epilepsy. J Pediatr Health Care. 2019;33:138–145. doi:10.1016/j.pedhc.2018.07.004
- Jain SV, Dye T, Kedia P. Value of combined video EEG and polysomnography in clinical management of children with epilepsy and daytime or nocturnal spells. Seizure. 2019;65:1–5. doi:10.1016/j.seizure.2018.12.009
- Wang G, Xu G, Liu Z, Lu N, Ma R, Zhang E. Sleep patterns and sleep disturbances among Chinese school-aged children: prevalence and associated factors. Sleep Med. 2013;14:45–52. doi:10.1016/j.sleep.2012.09.022
- Maganti R, Hausman N, Koehn M, Sandok E, Glurich I, Mukesh BN. Excessive daytime sleepiness and sleep complaints among children with epilepsy. Epilepsy Behav. 2006;8:272–277. doi:10.1016/j.yebeh.2005.11.002
- Gibbon FM, Maccormac E, Gringras P. Sleep and epilepsy: unfortunate bedfellows. Arch Dis Child. 2019;104:189–192. doi:10.1136/archdischild-2017-313421
- Larson AM, Ryther RC, Jennesson M, et al. Impact of pediatric epilepsy on sleep patterns and behaviors in children and parents. Epilepsia. 2012;53:1162–1169. doi:10.1111/j.1528-1167.2012.03515.x
- Chan B, Cheong EY, Ng SF, Chan YC, Lee QU, Chan KY. Evaluation of sleep disturbances in children with epilepsy: a questionnaire-based case-control study. Epilepsy Behav. 2011;21:437–440. doi:10.1016/j.yebeh.2011.05.007
- Graham L, Gossell-Williams M, Tapper J, Melbourne-Chambers R. Sleep disorders and behavioral disorders in Jamaican children with epilepsy: a case-control study. Epilepsy Behav. 2019;99:106477. doi:10.1016/j.yebeh.2019.106477
- Yong L, Chengye J, Jiong Q. Factors affecting the quality of life in childhood epilepsy in China. Acta Neurol Scand. 2006;113:167–173. doi:10.1111/j.1600-0404.2005.00567.x
- Samaitienė R, Norkūnienė J, Tumienė B, Grikinienė J. Sleep and behavioral problems in rolandic epilepsy. Pediatr Neurol. 2013;48:115–122. doi:10.1016/j.pediatrneurol.2012.10.012
- Byars AW, Byars KC, Johnson CS, et al. The relationship between sleep problems and neuropsychological functioning in children with first recognized seizures. Epilepsy Behav. 2008;13:607–613. doi:10.1016/j.yebeh.2008.07.009
- Mindell JA, Sadeh A, Kwon R, Goh DY. Cross-cultural differences in the sleep of preschool children. Sleep Med. 2013;14:1283–1289. doi:10.1016/j.sleep.2013.09.002
- Ekinci O, Isik U, Gunes S, Ekinci N. Understanding sleep problems in children with epilepsy: associations with quality of life, attention-deficit hyperactivity disorder and maternal emotional symptoms. Seizure. 2016;40:108–113. doi:10.1016/j.seizure.2016.06.011
- Fisher RS, Cross JH, French JA, et al. Operational classification of seizure types by the International League against epilepsy: position paper of the ILAE commission for classification and terminology. Epilepsia. 2017;58:522–530. doi:10.1111/epi.13670
- Owens JA, Spirito A, McGuinn M. The Children’s Sleep Habits Questionnaire (CSHQ): psychometric properties of a survey instrument for school-aged children. Sleep. 2000;23:1043–1051. doi:10.1093/sleep/23.8.1d
- Beebe DW, Lewin D, Zeller M, et al. Sleep in overweight adolescents: shorter sleep, poorer sleep quality, sleepiness, and sleep-disordered breathing. J Pediatr Psychol. 2007;32:69–79. doi:10.1093/jpepsy/jsj104
- Goodlin-Jones BL, Sitnick SL, Tang K, Liu J, Anders TF. The Children’s Sleep Habits Questionnaire in toddlers and preschool children. J Dev Behav Pediatr. 2008;29:82–88. doi:10.1097/dbp.0b013e318163c39a
- Liu Z, Wang G, Geng L, Luo J, Li N, Owens J. Sleep patterns, sleep disturbances, and associated factors among Chinese urban kindergarten children. Behav Sleep Med. 2016;14:100–117. doi:10.1080/15402002.2014.963581
- Tan TX, Wang Y, Cheah CSL, Wang GH. Reliability and construct validity of the children’s sleep habits questionnaire in Chinese kindergartners. Sleep Health. 2018;4:104–109. doi:10.1016/j.sleh.2017.10.008
- Goodman R. The strengths and difficulties questionnaire: a research note. J Child Psychol Psychiatry. 1997;38:581–586. doi:10.1111/j.1469-1610.1997.tb01545.x
- Du Y, Kou J, Coghill D. The validity, reliability and normative scores of the parent, teacher and self report versions of the strengths and difficulties questionnaire in China. Child Adolesc Psychiatry Ment Health. 2008;2:8. doi:10.1186/1753-2000-2-8
- Reilly C, Atkinson P, Memon A, et al. Autism, ADHD and parent-reported behavioural difficulties in young children with epilepsy. Seizure. 2019;71:233–239. doi:10.1016/j.seizure.2019.08.003
- Liu SK, Chien YL, Shang CY, Lin CH, Liu YC, Gau SS. Psychometric properties of the Chinese version of strength and difficulties questionnaire. Compr Psychiatry. 2013;54:720–730. doi:10.1016/j.comppsych.2013.01.002
- Varni JW, Seid M, Kurtin PS. PedsQL (TM) 4.0: reliability and validity of the pediatric quality of life Inventory (TM) Version 4.0 generic core scales in healthy and patient populations. Med Care. 2001;39:800–812. doi:10.1097/00005650-200108000-00006
- Duan X, Zhang S, Xiao N. Reliability and validity of the PedsQL generic core scales 4.0 for Chinese children with epilepsy. Epilepsy Behav. 2012;23:431–436. doi:10.1016/j.yebeh.2011.12.021
- Bazil CW. Sleep and Epilepsy. Semin Neurol. 2017;37:407–412. doi:10.1055/s-0037-1604352
- Al-Biltagi MA. Childhood epilepsy and sleep. World J Clin Pediatr. 2014;3:45–53. doi:10.5409/wjcp.v3.i3.45
- Liu X, Liu L, Owens JA, Kaplan DL. Sleep patterns and sleep problems among schoolchildren in the United States and China. Pediatrics. 2005;115:241–249. doi:10.1542/peds.2004-0815F
- Wang G, Zhang J, Lam SP, et al. Ten-year secular trends in sleep/wake patterns in shanghai and hong kong school-aged children: a tale of two cities. J Clin Sleep Med. 2019;15:1495–1502. doi:10.5664/jcsm.7984
- Owens J, Wang G, Lewin D, Skora E, Baylor A. Association between short sleep duration and risk behavior factors in middle school students. Sleep. 2017;40. doi:10.1093/sleep/zsw004
- Gutter T, Brouwer OF, de Weerd AW. Subjective sleep disturbances in children with partial epilepsy and their effects on quality of life. Epilepsy Behav. 2013;28:481–488. doi:10.1016/j.yebeh.2013.06.022
- Wirrell E, Blackman M, Barlow K, Mah J, Hamiwka L. Sleep disturbances in children with epilepsy compared with their nearest-aged siblings. Dev Med Child Neurol. 2005;47:754–759. doi:10.1017/S0012162205001581
- Chung S, Szaflarski JP, Choi EJ, et al. A systematic review of seizure clusters: prevalence, risk factors, burden of disease and treatment patterns. Epilepsy Res. 2021;177:106748. doi:10.1016/j.eplepsyres.2021.106748
- Hartmann S, Bruni O, Ferri R, Redline S, Baumert M. Cyclic alternating pattern in children with obstructive sleep apnea and its relationship with adenotonsillectomy, behavior, cognition, and quality of life. Sleep. 2021;44. doi:10.1093/sleep/zsaa145
- Constantin E, Kermack A, Nixon GM, Tidmarsh L, Ducharme FM, Brouillette RT. Adenotonsillectomy improves sleep, breathing, and quality of life but not behavior. J Pediatr. 2007;150:540–6, 546 e1. doi:10.1016/j.jpeds.2007.01.026
- Samaitienė R, Norkūnienė J, Jurkevičienė G, Grikinienė J. Behavioral problems in children with benign childhood epilepsy with centrotemporal spikes treated and untreated with antiepileptic drugs. Medicina. 2012;48:338–344.
- Tovia E, Goldberg-Stern H, Ben Zeev B, et al. The prevalence of atypical presentations and comorbidities of benign childhood epilepsy with centrotemporal spikes. Epilepsia. 2011;52:1483–1488. doi:10.1111/j.1528-1167.2011.03136.x
- Dal Canto G, Pellacani S, Valvo G, Masi G, Ferrari AR, Sicca F. Internalizing and externalizing symptoms in preschool and school-aged children with epilepsy: focus on clinical and EEG features. Epilepsy Behav. 2018;79:68–74. doi:10.1016/j.yebeh.2017.10.004
- Holmes GL. Effects of seizures on brain development: lessons from the laboratory. Pediatr Neurol. 2005;33:1–11. doi:10.1016/j.pediatrneurol.2004.12.003
- Miller V, Palermo TM, Grewe SD. Quality of life in pediatric epilepsy: demographic and disease-related predictors and comparison with healthy controls. Epilepsy Behav. 2003;4:36–42. doi:10.1016/S1525-5050(02)00601-7
- Gruber R, Somerville G, Santisteban JA. Using parental report to identify children at risk for poor sleep and daytime problems. Behav Sleep Med. 2020;18:460–476. doi:10.1080/15402002.2019.1613236
- Urfer-Maurer N, Brand S, Holsboer-Trachsler E, Grob A, Weber P, Lemola S. Correspondence of maternal and paternal perception of school-aged children’s sleep with in-home sleep-electroencephalography and diary-reports of children’s sleep. Sleep Med. 2018;48:180–186. doi:10.1016/j.sleep.2018.05.006
