Abstract
Purpose
Mental stress induced myocardial ischemia (MSIMI) is regarded as the primary cause of the angina with no obstructive coronary artery disease (ANOCA). Obstructive sleep apnea (OSA) is autonomously linked to obstructive coronary heart disease, hypertension, and sudden cardiac death. Similar to the impact of psychological stress on the cardiovascular system, individuals with OSA experience periodic nocturnal hypoxia, resulting in the activation of systemic inflammation, oxidative stress, endothelial dysfunction, and sympathetic hyperactivity. The contribution of OSA to MSIMI in ANOCA patients is unclear. To explore the prevalence of OSA in ANOCA patients and the correlation between OSA and MSIMI, a prospective cohort of female ANOCA patients was recruited.
Patients and Methods
We recruited female patients aged 18 to 75 years old with ANOCA and evaluated MSIMI using positron emission tomography-computed tomography. Subsequently, Level III portable monitors was performed to compare the relationship between OSA and MSIMI.
Results
There is higher REI (7.8 vs 2.6, P=0.019), ODI (4.7 vs 9.2, P=0.028) and percentage of OSA (67.74% vs 33.33%, P=0.004) in MSIMI patients. The patients diagnosed with OSA demonstrated higher myocardial perfusion imaging scores (SSS: 1.5 vs 3, P = 0.005, SDS: 1 vs 3, P = 0.007). Adjusted covariates, the risk of developing MSIMI remained 3.6 times higher in OSA patients (β=1.226, OR = 3.408 (1.200–9.681), P = 0.021).
Conclusion
Patients with MSIMI exhibit a greater prevalence of OSA. Furthermore, the myocardial blood flow perfusion in patients with OSA is reduced during mental stress.
Introduction
Roughly 66% of females experiencing Angina with no obstructive coronary artery disease (ANOCA).Citation1 Notwithstanding the absence of noteworthy coronary obstruction, these patients frequently exhibit persistent symptoms, recurrent hospitalizations, diminished functional status, heightened adverse cardiovascular events, and utilize healthcare resources with repeated medical evaluations and interventions.Citation2,Citation3 Therefore, a profound investigation into the risk factors and pathogenesis of ANOCA is of paramount importance.
Our previous studies have reported that the prevalence of mental stress induced myocardial ischemia (MSIMI) in patients with ANOCA was much higher than health control (42.9% VS 2.4%). MSIMI is a temporary ischemic response of the myocardium to mental stress, indicating an impaired cardiovascular response to mental stress and preclinical functional impairment of coronary heart disease in individuals with ANOCA.Citation4,Citation5 The occurrence of MSIMI may be attributed to increased vulnerability of the cardiovascular system to stress due to long-term chronic psychological stress. The underlying mechanisms include sympathetic nervous system hyperactivity, endothelial dysfunction, and enhanced inflammatory responses.Citation6,Citation7 Prior research has demonstrated that MSIMI possesses the capacity to incite angina, myocardial infarction, arrhythmia, and left ventricular dysfunction.Citation8–11
There exists evidence that obstructive sleep apnea (OSA) is autonomously linked to obstructive coronary heart disease, hypertension, and sudden cardiac death.Citation12–16 OSA is typified by recurrent upper airway blockage during sleep, leading to reduced arterial oxygen saturation, and apnea/hypopnea events are frequently terminated by micro arousals.Citation13,Citation17,Citation18 Due to periodic nocturnal hypoxia,Citation19,Citation20 patients with OSA experience systemic inflammatory activation,Citation21 oxidative stress,Citation22 endothelial dysfunction,Citation23 and sympathetic nervous system overactivity,Citation24,Citation25 which subsequently impairs the cardiovascular system. To date, only a small-scale clinical study has reported the prevalence of OSA in patients with ischemia with no obstructive coronary artery disease,Citation26 and no research has explored the correlation between OSA and MSIMI in ANOCA patients. Given the similarities in the mechanisms of cardiovascular damage due to OSA and MSIMI, it is crucial to investigate the prevalence of OSA and its association with MSIMI in ANOCA patients.
The third edition of the International Classification of Sleep Disorders (ICSD-3) defines OSA as an obstructive respiratory disturbance index (RDI) of ≥ 5 events per hour determined by polysomnography (PSG), along with typical symptoms of OSA, such as unrefreshing sleep, daytime somnolence, fatigue or insomnia, awakening with gasping or choking sensations, loud snoring, or witnessed apneas, or an obstructive RDI of ≥ 15 events per hour even in the absence of symptoms.Citation27,Citation28 Due to the high prevalence of OSA, evaluating all patients suspected of having OSA with PSG, currently considered the gold standard diagnostic test, entails significant costs. Additionally, opportunities for laboratory testing might be limited in certain areas.Citation29,Citation30 Home Sleep Apnea Testing (HSAT) is an alternative method for diagnosing adult OSA, which may be more cost-effective and efficient in certain populations. Furthermore, compared to PSG, Level III portable monitors (PM), which are user-friendly and economically efficient, offer similar accuracy for the diagnosis of OSA.Citation31,Citation32
Consequently, this study recruited a cohort of female patients with ANOCA. Positron Emission Tomography-Computed Tomography (PET-CT) was utilized to assess the occurrence of MSIMI in ANOCA via myocardial perfusion imaging (MPI). Concurrently, sleep data of the subjects were collected using a Level III PM, aiming to explore the prevalence of OSA in ANOCA patients and the correlation between OSA and MSIMI.
Materials and Methods
Research Registration
This study is currently taking place at the Guangdong Provincial People’s Hospital in Guangzhou, Guangdong, China. The study protocol and procedures were approved by the Ethics Committee at Guangdong Provincial People’s Hospital (number: GDREC2019298H(R3)), and its procedures conformed to standards set by the Declaration of Helsinki.
Study Population
Our study focused on female Outpatient and inpatient patients aged between 18 and 75 years old who visited the cardiovascular clinic of the Guangdong Provincial People’s Hospital from 2018 to 2020. Inclusion Criteria involve voluntary informed consent, angina in women with coronary artery stenosis less than 50%, ages between 18 and 75 years, and the ability to cooperate with evaluation and examination procedures. Exclusion Criteria encompass chest pain originating from non-cardiovascular or non-pulmonary diseases, pulmonary embolism, aortic dissection, life-threatening severe arrhythmias, concurrent cardiomyopathy or severe valvular disease, New York Heart Association (NYHA) Class IV heart failure, myocardial infarction within the last month, severe psychiatric illness like schizophrenia with a risk of suicide, history of alcohol, medication abuse or drug abuse within the last year, serious primary diseases or dysfunctions in lungs, liver, kidneys, hematopoietic or immune systems, use of antidepressants or anxiety medications in the four weeks prior to the study, participation in any clinical drug trials within the last 12 weeks, and cognitive impairments or inability to comply with study requirements. Qualified participants will receive comprehensive verbal instructions regarding the study’s objectives and nature, allowing them sufficient time to make an informed decision about their participation. Prior to enrollment, written informed consent will be obtained from all participants, and strict confidentiality measures will be implemented for all data collected.
Collection of baseline demographic and clinical characteristics was performed for the participants. This included assessing the Canadian Cardiovascular Society angina grade, as well as identifying the presence of hyperlipidemia, hypertension, or diabetes, alongside a review of medication history.
Mental Stress Test
Prior to the commencement of study assessments, patients are required to maintain hemodynamic and clinical stability for a minimum of 48 hours. The consumption of any food or drugs containing caffeine must be ceased for a minimum of 12 hours prior to the stress test. As the protocol previously published, the patient are required to wear virtual reality (VR) goggles and undergo a 12-minute mental stress test after being adequately rested. The mental stress test comprised of three tasks: (1) the Stroop Color and Word Test; (2) an event that elicits negative affect - participants will be requested to deliver a three-minute speech on a situation that has caused them anger, sadness, or fear to three virtual reality physicians, with only one minute of preparation time. The participants will receive notification that their performance, encompassing the lucidity of their explanation, cognitive and behavioral processes, and outcome of the scenario, will undergo assessment. In the event that they are unable to provide a response, the investigators will pose inquiries to elicit additional data and fulfill the three-minute speech; (3) mental arithmetic task, which involves the sequential subtraction of seven from a three-digit numeral, with emphasis on expeditious computation. Criteria for terminating the stress tests are in Supplementary Methods.
PET-CT Scan and Myocardial Perfusion Imaging
In PET-CT imaging, ammonia labeled with 13NH3 is used to assess myocardial perfusion. Following its entry into the heart with the bloodstream, 13NH3 is rapidly absorbed by myocardial cells and converted into metabolites within the cells. The half-life of 13NH3 is 10 minutes, and its decay produces positrons that annihilate with electrons to generate gamma rays, which are captured by the PET scanner to construct an activity map of the heart. By comparing the uptake of 13NH3 in myocardial regions, it is possible to evaluate the uniformity of blood flow and identify ischemic areas, providing support for the diagnosis of MSIMI.
Each subject underwent two PET-CT scans, one at rest and another during a mental stress test. The schematic representation of the mental stress and PET scan procedure are in Supplemental Figure 1. Seven minutes after the start of the mental stress test, 13NH3 was administered, and the patient continued with the stress test until completion. Following the injection, PET scanning was conducted for a duration of 10 minutes. The images were evaluated using a 17-segment model and a five-point scoring system. The commercially available and previously validated Cedars-Sinai software was utilized to calculate the summed stress score (SSS), summed rest score (SRS), and summed difference score (SDS). A myocardial perfusion defect was identified when the SDS ≥3, indicating the occurrence of MSIMI in the patient.Citation33 For further details on the PET-CT scans, refer to the Supplementary Methods.
Screening for OSA
Within seven days of study enrollment, patients were having HSAT to assess for OSA, utilizing the Level III PM (Alice PDx or NightOne; Philips China Investment Co., Ltd., Shanghai, China). Level III PM measures cardiopulmonary parameters, which include oxygen saturation at the fingertip, respiratory variables (eg, effect to breathe and nasal airflow), and a cardiac variable (eg, heart rate and electrocardiogram). An effective event interpretation required a recorded time of at least four hours. Apnea events were documented when there was a reduction of respiratory airflow by ≥ 90% for a duration exceeding 10 seconds. Hypopnea events were documented when two criteria were met: (1) a reduction of respiratory airflow by over 30% for more than 10 seconds and (2) a 3% decrease in oxygen saturation compared to the baseline. The respiratory event index (REI) was calculated by dividing the total number of apnea and hypopnea events by the duration of recorded data in hours. The oxygen desaturation index (ODI) defined as the number of times per hour during the sleep period when the blood oxygen saturation drops by more than 3% on average. We used the REI for the ultimate scoring of OSA severity. Depending on the value of the REI, OSA is classified into three categories: mild (5–14 events/hour), moderate (15–29 events/hour), and severe (≥30 events/hour). A cutoff REI ≥5 events/h was used to define the existence of OSA, and those with REI < 5 events/h were considered without OSA.
Statistical Analysis
Continuous variables that follow a normal distribution are typically represented by Means ± SD, whereas non-normally distributed variables are described by the Median (Q1/Q3). Categorical variables are typically reported as numbers and percentages. During univariate analysis, differences between groups for continuous variables were assessed using either the One Way ANOVA or Mann–Whitney U-test, while differences between groups for categorical variables were evaluated using either the Chi-Square test or Fisher’s exact test. The Pearson correlation test is employed to analyze continuous grade data, followed by the correction of covariates through Logistic Regression Analysis subsequent to univariate analysis. Three multivariable models were conducted: Model one only include OSA; in Model two, BMI was adjusted; Model three was adjusted for the variables in model two plus a combination variable called cardiovascular disease (CVD) risk factors (which combined smoking, hypertension, diabetes, and hyperlipidemia). The statistical analysis was conducted utilizing SPSS 26.0 and R Studio.
Result
Baseline Characteristics of Patients in MSIMI and Non-MISMI Groups
Among the cohort of 70 patients presenting with chest pain and no epicardial coronary stenosis, 55.7% (n=39) were diagnosed with myocardial ischemia and mental stress-induced myocardial ischemia (MSIMI), while the remaining 44.3% (n=31) did not exhibit MSIMI. There was no significant difference in mean age and height between the MSIMI and non-MSIMI groups.
The study findings indicate that MSIMI patients exhibit higher body mass and body mass index (BMI) compared to non-MSIMI patients. However, there is no significant difference in chest pain levels (CCS scale) between the two groups, and other cardiovascular risk factors, including hypertension, hyperlipidemia, diabetes, and history of arrhythmia, do not exhibit any statistical differences. Furthermore, the total cholesterol, triglycerides, and low-density lipoprotein, which are known risk factors for coronary heart disease, do not exhibit any significant differences between the two groups.
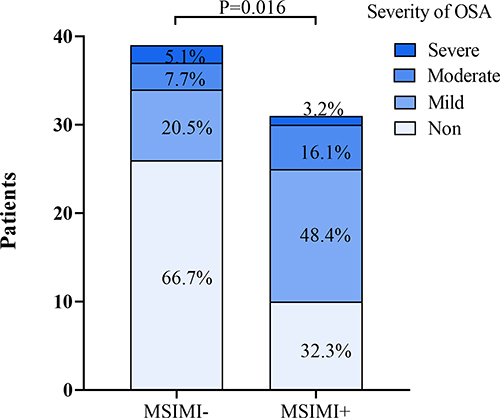
In a total sample of 70 people, the prevalence of OSA was very high. Notably, a higher percentage of OSA patients were observed among MSIMI patients (). Furthermore, a larger percentage of MSIMI patients exhibit mild to moderate OSA ().
Table 1 Baseline Characteristics of Patients in MSIMI and Non-MISMI Groups
Figure 1 The proportion of individuals with OSA in both cohorts. In the MSIMI group, there is a higher proportion of patients with OSA (P = 0.004), with a greater percentage being classified as mild to moderate (P = 0.016).
The Distinction in Sleep Monitoring Between the MSIMI and Non-MSIMI Cohorts
Upon comparing the occurrence of sleep-disordered breathing events between the MSIMI and Non-MSIMI groups, it was discovered that patients with MSIMI exhibited a greater respiratory event (Apnea and Hypopnea, AH times) and a higher REI. Additionally, there was a tendency towards a higher frequency of apnea events and AI, with a greater number of hypopnea events and HI. Furthermore, there were more occurrences of oxygen desaturation events and a higher ODI ().
Table 2 The Distinction in Sleep Monitoring Between the MSIMI and Non-MSIMI Cohorts
Correlation Between OSA and MSIMI
Following univariate analysis, variables exhibiting differences were progressively incorporated into the Logistic regression model. Within the population under investigation, patients with OSA exhibited a 4.2-fold increase in the risk of MSIMI. Notably, even after adjusting for BMI (Model 2), OSA remained a significant factor, while BMI did not exhibit significance within the model. Upon correction for common cardiovascular risk factors (Model 3), the risk persisted ().
Table 3 Logistic Regression Analysis of MSIMI Predictive Factors
Characteristics of Patients with OSA
The study findings indicate that patients diagnosed with OSAS exhibited advanced age and higher BMI compared to those patients without. However, no significant differences were observed in other cardiovascular risk factors, including chest pain, hypertension, hyperlipidemia, and diabetes. In terms of blood test results, patients with OSA had elevated hemoglobin levels. Additionally, mild increases in liver enzymes and bilirubin were noted, and plasma protein S levels were decreased ().
Table 4 Characteristics of Patients in OSA and Non-OSA Groups
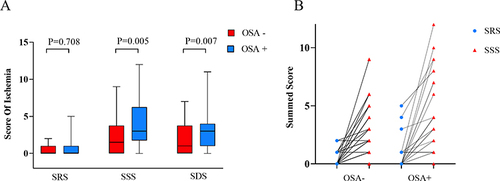
In a state of rest, there exists no statistically significant variance in SRS between patients afflicted with OSA and those without. However, subsequent to undergoing mental stress testing, a significant difference in SSS and SDS was observed between the two patient groups (). Furthermore, prior to and following mental stress, patients with OSA exhibited a notably greater prevalence of myocardial perfusion defects ().
Figure 2 Myocardial Perfusion in OSA and Non-OSA patients. (A) There was no discernible variation in myocardial perfusion levels between the two cohorts of patients in a state of rest (SRS). Nevertheless, a noteworthy dissimilarity in myocardial perfusion levels was observed subsequent to mental stress (SSS), and the variance in the degree of alteration before and after was statistically significant (SDS). (B) The myocardial perfusion summed score in the OSA group showed a significant improvement after mental stress.
The results indicate a positive correlation between SSS and REI, as well as between HI and hemoglobin (Hb), and HI and Hb were also found to be positively correlated (Supplemental Table 1).
Discussion
The present findings suggest that there exists an autonomous correlation between OSA and MSIMI among female patients with ANOCA. In terms of sleep monitoring parameters, MSIMI patients exhibit a greater prevalence of respiratory sleep events and REI compared to those without MSIMI, with the most notable difference being observed in hypoventilation events and HI. However, there was no statistically significant difference in the number of apnea events and AI between the two groups. Furthermore, MSIMI patients display a higher occurrence of oxygen desaturation events and ODI. Simultaneously, our findings indicate that female ANOCA patients with OSA exhibit functional impairment of the heart prior to the emergence of significant coronary stenosis, as evidenced by the deficiency of myocardial blood perfusion (SDS ≥ 3) during heightened mental stress. The correlation between OSA and MSIMI is intricate due to various confounding factors, particularly BMI. Several extensive clinical studies have indicated a strong association between OSA and obstructive coronary heart disease. Moreover, our findings reveal that OSA remains a significant independent risk factor for MSIMI in patients with non-obstructive angina pectoris, even after accounting for common cardiovascular risk factors such as BMI.
Individuals may experience mental stress during the day, while OSA manifests during nocturnal hours. With repeated occurrences over extended periods, a variety of pathophysiological mechanisms may link OSA and MSIMI.Citation12 Prolonged and recurrent psychological stress may result in heightened sympathetic nervous system activity, diminished parasympathetic cardiac control, activation of the hypothalamic-pituitary-adrenal (HPA) axis, and increased systemic inflammation. The activation of these physiological systems may result in hemodynamic alterations, modifications in coronary vascular reactivity, platelet activation, and endothelial impairment, ultimately culminating in myocardial ischemia, infarction, or arrhythmia.Citation6,Citation34 Obstructive apnea events have the potential to induce systemic hypoxemia. The recurring reduction in oxygen saturation and potential hypercapnia can trigger a chemical reflex, resulting in heightened vascular sympathetic nerve activity and serum catecholamines. The tachycardia and blood pressure surge that occur at the conclusion of an apneic episode can increase myocardial oxygen demand at the nadir of oxygen saturation, potentially leading to myocardial ischemia and arrhythmic sequelae.Citation12,Citation35
Cardiac autonomic dysfunction may account for the heightened susceptibility to MSIMI observed in patients with OSA. OSA disrupts the mechanisms responsible for regulating heart rate variability, such as the coupling between cardiac and respiratory parasympathetic inputs within the central nervous system, the arterial baroreflex, and the feedback from lung distraction receptors.Citation36 Consequently, heart rate variability is diminished in individuals with OSA.Citation37 Furthermore, OSA patients exhibit a persistent elevation in sympathetic nerve activity during their waking hours.Citation38 Tamisier et al observed that healthy individuals exposed to chronic intermittent hypoxia (IH) for a period of two weeks experienced an increase in sympathetic nerve outflow during the daytime.Citation39 While the exact correlation between autonomic nervous function and MSIMI remains largely unclear, chronic sympathetic overdrive has been identified as a potential risk factor for MSIMI.Citation6
The present study underscores the significance of OSA as a risk factor for preclinical cardiovascular disease, characterized by functional alterations in the heart prior to the manifestation of observable obstructive coronary stenosis. Additionally, our findings highlight the potential for OSA and MSIMI to synergistically exacerbate cardiovascular damage via a shared mechanism, despite their occurrence at distinct temporal intervals during daily life. These results offer novel insights into the early prevention of cardiovascular diseases.
This report presents several potential limitations. Firstly, the cross-sectional design employed in this study precludes the ability to establish a causal relationship between OSA and MSIMI. Secondly, the research sample is restricted to female ANOCA patients. Nonetheless, this limitation is not deemed significant given that the prevalence of OSA is higher among males than females. Thirdly, the sleep monitoring data utilized in this study was obtained from wearable sleep monitoring devices and corresponds to Level III sleep testing. While laboratory PSG is considered the gold standard for diagnosing OSA, it is relatively expensive and technically complex. As a result, PM has been employed as an alternative diagnostic test for OSA. In 2014, a meta-analysis was conducted to synthesize the available evidence regarding the use of portable monitoring devices as a viable substitute for laboratory PSG in comparative studies aimed at assessing the accuracy of sleep tests for adults with suspected OSA at both level III and level I. The findings of this study confirmed that level III portable devices demonstrate good diagnostic efficacy.
Conclusions
In brief, our investigation demonstrates an autonomous correlation between OSA and MSIMI in female ANOCA patients. Individuals with MSIMI exhibit a greater prevalence of OSA. The myocardial blood flow perfusion in patients with OSA is reduced during mental stress. Nonetheless, additional research is necessary to investigate the fundamental mechanisms and prognostic implications of this association.
Ethics Approval and Consent to Participate
Ethical approval was given by the medical ethics committee of Guangdong General Hospital with the following reference number: No. 2019-298H-5. All participants gave written informed consent.
Clinical Trial Registration
Trial name: Effect of Mental Stress on Myocardial Perfusion in Women (MS in women); ClinicalTrials.gov Identifier: NCT03982901, https://clinicaltrials.gov/ct2/show/NCT03982901.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
Data Sharing Statement
All data will be shared upon reasonable request to the corresponding authors.
Additional information
Funding
References
- Ma H, Guo L, Fei H, et al. Assessing mental stress on myocardial perfusion and myocardial blood flow in women without obstructive coronary disease: protocol for a mechanistic clinical trial. BMJ open. 2020;10(12). doi:10.1136/bmjopen-2020-038362
- Johnson BD, Shaw LJ, Buchthal SD, et al. Prognosis in Women With Myocardial Ischemia in the Absence of Obstructive Coronary Disease. Circulation. 2004;109(24):2993–2999. doi:10.1161/01.CIR.0000130642.79868.B2
- Chaudhry S, Kumar N, Behbahani H, et al. Abnormal heart-rate response during cardiopulmonary exercise testing identifies cardiac dysfunction in symptomatic patients with non-obstructive coronary artery disease. Int J Cardiol. 2017;228:114–121. doi:10.1016/j.ijcard.2016.11.235
- Wokhlu A, Pepine CJ. Mental Stress and Myocardial Ischemia: young Women at Risk. J Am Heart Assoc. 2016;5(9):e004196. doi:10.1161/JAHA.116.004196
- Arri SS, Ryan M, Redwood SR, Marber MS. Mental stress-induced myocardial ischaemia. Heart. 2016;102(6):472–480. doi:10.1136/heartjnl-2014-307306
- Vancheri F, Longo G, Vancheri E, Henein MY. Mental Stress and Cardiovascular Health—Part I. J Clin Med. 2022;11(12):3353. doi:10.3390/jcm11123353
- Henein MY, Vancheri S, Longo G, Vancheri F. The Impact of Mental Stress on Cardiovascular Health—Part II. J Clin Med. 2022;11(15):4405. doi:10.3390/jcm11154405
- Steptoe A, Kivimäki M. Stress and cardiovascular disease. Nat Rev Cardiol. 2012;9(6):360–370. doi:10.1038/nrcardio.2012.45
- Strike PC, Steptoe A. Behavioral and emotional triggers of acute coronary syndromes: a systematic review and critique. Psychosom Med. 2005;67(2):179–186. doi:10.1097/01.psy.0000155663.93160.d2
- Lampert R, Jain D, Burg MM, Batsford WP, McPherson CA. Destabilizing effects of mental stress on ventricular arrhythmias in patients with implantable cardioverter-defibrillators. Circulation. 2000;101(2):158–164. doi:10.1161/01.cir.101.2.158
- Sun JL, Boyle SH, Samad Z, et al. Mental stress-induced left ventricular dysfunction and adverse outcome in ischemic heart disease patients. Eur J Prev Cardiol. 2017;24(6):591–599. doi:10.1177/2047487316686435
- McNicholas WT, Bonsigore MR, Bonsignore MR. Management Committee of EU COST ACTION B26. Sleep apnoea as an independent risk factor for cardiovascular disease: current evidence, basic mechanisms and research priorities. Eur Respir J. 2007;29(1):156–178. doi:10.1183/09031936.00027406
- Wei. A narrative review on obstructive sleep apnea in China: a sleeping giant in disease pathology. Available from: https://www.heartmindjournal.org/article.asp?issn=2468-6476;year=2022;volume=6;issue=4;spage=232;epage=241;aulast=Wei. Accessed June 11, 2023.
- Polecka A, Olszewska N, Danielski L, Olszewska E. Association between Obstructive Sleep Apnea and Heart Failure in Adults-A Systematic Review. J Clin Med. 2023;12(19):6139. doi:10.3390/jcm12196139
- Yeghiazarians Y, Jneid H, Tietjens JR, et al. Obstructive Sleep Apnea and Cardiovascular Disease: a Scientific Statement From the American Heart Association. Circulation. 2021;144(3):e56–e67. doi:10.1161/CIR.0000000000000988
- Bhatt P, Patel V, Motwani J, et al. Insomnia and Cardiovascular Health: exploring the Link Between Sleep Disorders and Cardiac Arrhythmias. Curr Cardiol Rep. 2023;25(10):1211–1221. doi:10.1007/s11886-023-01939-x
- Jordan AS, McSharry DG, Malhotra A. Adult obstructive sleep apnoea. Lancet. 2014;383(9918):736–747. doi:10.1016/S0140-6736(13)60734-5
- Sum-Ping. Impact of Sleep on Cardiovascular Health: a Narrative Review. Available from: https://www.heartmindjournal.org/article.asp?issn=2468-6476;year=2022;volume=6;issue=3;spage=120;epage=126;aulast=Sum-Ping. Accessed June 11, 2023.
- Foster GE, Brugniaux JV, Pialoux V, et al. Cardiovascular and cerebrovascular responses to acute hypoxia following exposure to intermittent hypoxia in healthy humans. J Physiol. 2009;587(Pt 13):3287–3299. doi:10.1113/jphysiol.2009.171553
- Gilmartin GS, Lynch M, Tamisier R, Weiss JW. Chronic intermittent hypoxia in humans during 28 nights results in blood pressure elevation and increased muscle sympathetic nerve activity. Am J Physiol Heart Circ Physiol. 2010;299(3):H925–931. doi:10.1152/ajpheart.00253.2009
- Ryan S, Taylor CT, McNicholas WT. Selective activation of inflammatory pathways by intermittent hypoxia in obstructive sleep apnea syndrome. Circulation. 2005;112(17):2660–2667. doi:10.1161/CIRCULATIONAHA.105.556746
- Lavie L. Obstructive sleep apnoea syndrome--an oxidative stress disorder. Sleep Med Rev. 2003;7(1):35–51. doi:10.1053/smrv.2002.0261
- Carlson JT, Rångemark C, Hedner JA. Attenuated endothelium-dependent vascular relaxation in patients with sleep apnoea. J Hypertens. 1996;14(5):577–584. doi:10.1097/00004872-199605000-00006
- Carlson JT, Hedner J, Elam M, Ejnell H, Sellgren J, Wallin BG. Augmented Resting Sympathetic Activity in Awake Patients With Obstructive Sleep Apnea. Chest. 1993;103(6):1763–1768. doi:10.1378/chest.103.6.1763
- Carneiro ER, Azoubel LA, Dias RC, et al. Correlation of sleep quality and cardiac autonomic modulation in hemodialysis patients. Sleep Sci. 2022;15(Spec 1):59–64. doi:10.5935/1984-0063.20200126
- Ooi EL, Rajendran S, Munawar DA, et al. The Association of Obstructive Sleep Apnea in Ischemia with No Obstructive Coronary Artery Disease - A Pilot Study. Curr Probl Cardiol. 2023;48(5):101111. doi:10.1016/j.cpcardiol.2022.101111
- Sateia MJ. International classification of sleep disorders-third edition: highlights and modifications. Chest. 2014;146(5):1387–1394. doi:10.1378/chest.14-0970
- Kapur VK, Auckley DH, Chowdhuri S, et al. Clinical Practice Guideline for Diagnostic Testing for Adult Obstructive Sleep Apnea: an American Academy of Sleep Medicine Clinical Practice Guideline. J Clin Sleep Med. 2012;13(03):479–504. doi:10.5664/jcsm.6506
- Park KS, Choi SH, Yoon H. Modulation of sleep using noninvasive stimulations during sleep. Biomed Eng Lett. 2023;13(3):329–341. doi:10.1007/s13534-023-00298-4
- Souza de LFF, Paineiras-Domingos LL, Melo-Oliveira de ME, et al. The impact of COVID-19 pandemic in the quality of sleep by Pittsburgh Sleep Quality Index: a systematic review. Cien Saude Colet. 2021;26(4):1457–1466. doi:10.1590/1413-81232021264.45952020
- de Oliveira ACT, Martinez D, Vasconcelos LFT, et al. Diagnosis of obstructive sleep apnea syndrome and its outcomes with home portable monitoring. Chest. 2009;135(2):330–336. doi:10.1378/chest.08-1859
- El Shayeb M, Topfer LA, Stafinski T, Pawluk L, Menon D. Diagnostic accuracy of level 3 portable sleep tests versus level 1 polysomnography for sleep-disordered breathing: a systematic review and meta-analysis. CMAJ. 2014;186(1):E25–E51. doi:10.1503/cmaj.130952
- Ma H, Guo L, Fei H, et al. Assessing mental stress on myocardial perfusion and myocardial blood flow in women without obstructive coronary disease: protocol for a mechanistic clinical trial. BMJ Open. 2020;10(12):e038362. doi:10.1136/bmjopen-2020-038362
- Pereira VH, Cerqueira JJ, Palha JA, Sousa N. Stressed brain, diseased heart: a review on the pathophysiologic mechanisms of neurocardiology. Int J Cardiol. 2013;166(1):30–37. doi:10.1016/j.ijcard.2012.03.165
- Lévy P, Kohler M, McNicholas WT, et al. Obstructive sleep apnoea syndrome. Nat Rev Dis Primers. 2015;1:15015. doi:10.1038/nrdp.2015.15
- Hall AB, Ziadi MC, Leech JA, et al. Effects of short-term continuous positive airway pressure on myocardial sympathetic nerve function and energetics in patients with heart failure and obstructive sleep apnea: a randomized study. Circulation. 2014;130(11):892–901. doi:10.1161/CIRCULATIONAHA.113.005893
- Roche F, Xuong ANT, Court-Fortune I, et al. Relationship among the severity of sleep apnea syndrome, cardiac arrhythmias, and autonomic imbalance. Pacing Clin Electrophysiol. 2003;26(3):669–677. doi:10.1046/j.1460-9592.2003.00116.x
- Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest. 1995;96(4):1897–1904.
- Tamisier R, Pépin JL, Rémy J, et al. 14 nights of intermittent hypoxia elevate daytime blood pressure and sympathetic activity in healthy humans. Eur Respir J. 2011;37(1):119–128. doi:10.1183/09031936.00204209
