Abstract
Purpose
This feasibility study examined the initial-use safety and effectiveness of a new noninvasive oral pressure therapy (OPT) system developed to treat obstructive sleep apnea (OSA).
Methods
The OPT system consists of a console that connects with flexible tubing to a premanufactured polymer mouthpiece. Through the mouthpiece, a pump in the console creates oral vacuum intended to move the soft palate anteriorly to decrease obstruction of the airway during sleep. The mouthpiece was produced in ten different sizes to accommodate a range of oral dimensions. Subjects with OSA in this single-center, single-night study underwent a polysomnography (PSG) study at baseline, followed by PSG during use of OPT.
Results
Fifty-six men and 20 women, aged 50.8 ± 12.0 years (mean ± standard deviation [SD]), had OSA with apnea–hypopnea indices (AHI) greater than five events per hour at baseline. Body weight averaged 98.0 ± 18.2 kg (mean ± SD), body mass index ranged from 22.6 kg/m2 to 57.9 kg/m2 and averaged 32.5 ± 5.8 kg/m2 (mean ± SD). OPT was generally well tolerated without any serious adverse events. Baseline AHI was 38.7 ± 27.5 events/hour (mean ± SD) and was reduced with treatment to 24.6 ± 25.7 events/hour (P < 0.001, Cohen’s d 0.53). Treatment produced AHI less than or equal to ten events/hour in 38% of the subjects. Oxygen desaturation index was 30.1 ± 23.7 events/hour at baseline versus 15.8 ± 19.1 events/hour with treatment (P < 0.001, Cohen’s d 0.66). The minimum oxygen saturation increased with treatment from 77.9 ± 8.3 to 82.2 ± 7.9 (P < 0.001, Cohen’s d 0.53). Stage-N1 sleep shifts, total sleep-stage shifts, and awakenings were significantly reduced with treatment.
Conclusion
This single-center, single-night feasibility study demonstrates that OPT can improve OSA in certain subjects identifiable by PSG during systematic usage. In appropriately responsive patients, OPT shows potential as a clinically useful new alternative for treatment of OSA without the need for custom manufacture of an oral device component.
Introduction
Obstructive sleep apnea (OSA) is a common clinical problem characterized by periods of reduction and cessation of airflow during sleep. Desaturation of arterial blood oxygen occurs in connection with diminished ventilation. Chronic OSA is associated with important medical problems including hypertension, coronary artery disease, cardiac dysrhythmia, cerebral vascular accident, and diabetes.Citation1–Citation3
Various treatment methods are available for the management of OSA. Positive airway pressure (PAP) is the most commonly prescribed treatment. PAP is a noninvasive therapy that creates a pressure gradient between the posterior oropharynx and the anterior oropharynx to move tissues anteriorly and improve gas flow in the airway. While the mechanism of action of PAP is well characterized and PAP is understood as an effective treatment, tolerability issues with PAP limit the use of this therapy by some patients.Citation4 Other noninvasive treatments less commonly employed include mandibular repositioning appliances, positional therapy, and weight loss.Citation5–Citation7 A variety of surgical treatments are available as well.Citation8,Citation9 Results with these alternative noninvasive and invasive treatments have been disappointing for some patients, especially those with severe sleep apnea. Some therapies require significant investment of effort and expense before effectiveness can be adequately established in an individual patient.
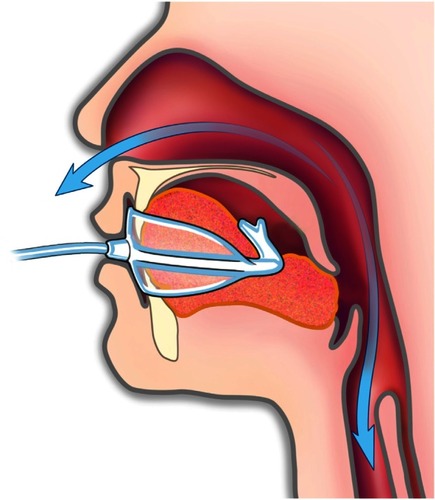
Recognizing that some patients remain untreated or under-treated despite these important current therapeutic options, oral pressure therapy (OPT) was developed recently. The OPT system consists of a bedside console containing a pump, a soft polymer mouthpiece, and a flexible tube connecting the mouthpiece to the console (). The console creates vacuum in the oral cavity intended to pull the soft palate anteriorly to decrease airway obstruction during sleep. A previous study evaluated this approach using mouthpieces customized for each subject.Citation10 A new version of the OPT mouthpiece has been developed and is now available in ten standard sizes to accommodate a range of oral dimensions in individuals without requiring a custom production process for each patient. The system is designed to be easy to trial and evaluate before a decision is made about chronic therapy. This single-night-use feasibility study examined the safety and initial effectiveness of this new noninvasive OPT system with premanufactured mouthpieces.
Methods
Study participants were recruited from the patient database of the Peninsula Sleep Center (Burlingame, CA, USA). Patients between the ages of 18 and 80 years with an untreated apnea–hypopnea index (AHI) of five events or more per hour were included. Subjects with the following conditions were excluded: oral or dental infection or a condition that would limit the use of the mouthpiece, night-shift work schedule, abnormal nasal patency, pregnancy or intention to become pregnant during the study, unstable medical conditions other than uncomplicated OSA, or clinically significant disease which might pose additional risk to the subject or confound study results.
The OPT system (ApniCure, Inc, Redwood City, CA, USA) has three components: a mouthpiece, a console containing a pump, and tubing connecting the mouthpiece to the console. The mouthpiece is available in ten different sizes. The pump in the console applies continuous negative pressure to the mouthpiece and the console controls the pump to maintain constant negative pressure in the mouth. The system provides negative pressure to the oral cavity moving the soft palate anteriorly into contact with the tongue to reduce obstruction and improve airflow during sleep (). With the device in operation, the patient breathes normally through the nose. The negative pressure produced in the oral cavity is isolated from the nasal–pharyngeal airway by a natural seal that occurs between the soft palate and tongue. The lightweight mouthpiece is not affixed to the teeth and can be easily removed or inserted by the patient at any time.
Figure 2 © ApniCure, Inc. With the mouthpiece in place, gentle oral vacuum creates a pressure gradient intended to move the soft palate against the tongue to relieve airway obstruction during sleep.
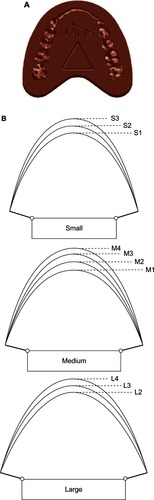
The Western Institutional Review Board (Olympia, WA, USA) approved this study. For each participant, written informed consent was obtained, inclusion and exclusion criteria were confirmed, and a brief oral cavity examination was performed. An imprint of the teeth was obtained on a piece of wax and compared to a visual template to identify which one of the ten available mouthpiece sizes was most appropriate (). After mouthpiece selection, the initial mouthpiece insertion provided an opportunity for the participant and technician to consider fit and comfort. Full-night polysomnography (PSG) without the device was performed to establish a baseline level of OSA severity without treatment and then another full-night PSG was performed with the device.
Figure 3 © ApniCure, Inc. An imprint of the teeth is obtained on a piece of wax (A) and compared to a visual template (B) to identify which one of the ten available mouthpiece sizes is most appropriate.
PSG data were collected using Sandman SD32 equipment and Sandman Elite software (Natus Medical, San Carlos, CA, USA). In accordance with the American Academy of Sleep Medicine 2007 protocol,Citation11 the following signals were collected on each night: electroencephalogram, electrooculogram, electromyogram (EMG), electrocardiogram, respiratory inductance plethysmograph, oxygen saturation, leg movement (EMG), nasal airflow (cannula), nasal and oral airflow (thermocouple), and body position (video). Subjects were encouraged to sleep in the supine position.
PSG data were scored manually according to American Academy of Sleep Medicine standard “recommended” criteriaCitation11 by a single blinded professional scorer. In accordance with this scoring scheme, hypopneas were required to have at least a 30% airflow reduction and a 4% oxygen desaturation. The oxygen desaturation index (ODI) was calculated as the total number of events with 4% or more decrease in oxygen saturation divided by the total sleep time (TST). Minimum oxygen saturation was determined. The percent of the night with oxygen saturation greater than or equal to 90% was calculated. AHI was calculated as the sum of the number of apnea events and hypopnea events divided by TST in hours. Measures of sleep architecture were scored.
Subjects were categorized into three OSA severity groups (mild OSA: AHI ≥ 5 events/hour and AHI < 15 events/hour; moderate OSA: AHI ≥ 15 events/hour and AHI < 30 events/hour; severe OSA: AHI ≥ 30 events/hour) based on the AHI measurement obtained from the baseline PSG. Measurements obtained at baseline and while on treatment were summarized by the baseline OSA severity. The change from baseline to treatment and percent change from baseline to treatment for all these measurements were calculated and summarized descriptively by the baseline OSA severity. A paired t-test was used for the test of change or percent change from baseline to treatment within each group with P < 0.05 considered significant. SAS (version 9.1; SAS Institute Inc, Cary, NC, USA) and Prism 6.0 (GraphPad Software, La Jolla, CA, USA) software were used for statistical analysis.
Results
Of the 76 subjects who were studied, 56 were male and 20 were female. The age ranged from 25 years to 75 years, averaging 50.8 ± 12.0 years (mean ± standard deviation [SD]) with ten subjects aged 65 years or older. Body weight averaged 98.0 ± 18.2 kg (mean ± SD), body mass index ranged from 22.6 kg/m2 to 57.9 kg/m2 and averaged 32.5 ± 5.8 kg/m2 (mean ± SD), and neck circumference was 41.8 ± 3.9 cm (mean ± SD).
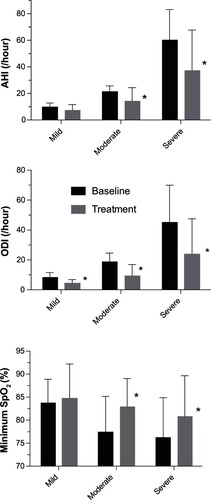
AHI, ODI, and minimum oxygen saturation were significantly reduced by treatment. Baseline AHI was 38.7 ± 27.5 events/hour (mean ± SD) and was reduced with treatment to 24.6 ± 25.7 events/hour (P < 0.001, Cohen’s d 0.53). Individual responses are shown in . ODI was 30.1 ± 23.7 events/hour at baseline versus 15.8 ± 19.1 events/hour with treatment (P < 0.001, Cohen’s d 0.66). The minimum oxygen saturation increased with treatment from 77.9 ± 8.3 to 82.2 ± 7.9 (P < 0.001, Cohen’s d 0.53). The percentage of the night with oxygen saturation of 90% or greater was not significantly different between baseline (90.8 ± 12.5) and treatment (93.2 ± 12.2; P = 0.11). shows the extent to which treatment reduced the degree of OSA severity in the 76 subjects. Treatment produced AHI less than or equal to ten events/hour in 38% of the subjects. AHI was reduced by at least 50% with treatment as compared to baseline in 47% of the subjects. Treatment produced the combined effect of AHI less than ten events per hour and reduction in AHI of at least 50% from baseline in 25% of the subjects. The alternate combined criteria of treatment AHI less than 20 events per hour and reduction in AHI of at least 50% from baseline has been suggested by a panel of advisors to the United States Food and Drug AdministrationCitation12 and was observed in 41% of subjects as follows: 5 of 14 subjects (36%) with mild OSA, 10 of 24 (42%) subjects with moderate OSA, and 16 of 38 (42%) subjects with severe OSA. AHI, ODI, and minimum oxygen saturation data are shown by baseline OSA severity in .
Figure 4 Individual apnea–hypopnea index (AHI) measures are shown with the horizontal lines denoting median and quartile values.
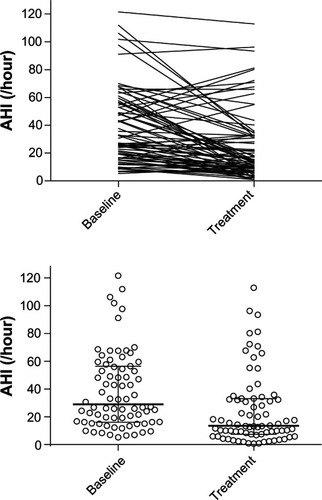
Figure 5 Based on AHI measurements at baseline and with treatment, OSA severity was identified for each subject and the portion of the 76-subject population at each severity level is shown.
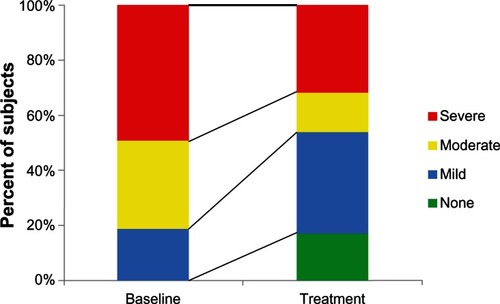
Figure 6 AHI, ODI, and minimum oxygen saturation for baseline and treatment conditions are shown as mean ± SD.
Abbreviations: AHI, apnea–hypopnea index; ODI, oxygen desaturation index; SD, standard deviation.
TST was significantly reduced on the initial night of treatment from 346.4 ± 63.4 minutes to 303.0 ± 81.5 minutes. Sleep onset latency was not significantly altered with treatment. Total stage N1 shifts were significantly reduced with treatment from 76.9 ± 35.0 to 60.1 ± 25.9 (P < 0.001, Cohen’s d 0.55). Similarly, total shifts of all sleep stages were reduced with treatment from 198.7 ± 74.3 to 158.5 ± 56.4 (P < 0.001, Cohen’s d 0.61). Total awakenings were significantly reduced with treatment from 48.4 ± 25.7 to 39.0 ± 16.5 (P = 0.001, Cohen’s d 0.44). Wakefulness after sleep onset was significantly increased with treatment from 83.6 ± 53.9 minutes to 103.1 ± 57.8 (P = 0.012, Cohen’s d 0.35).
Device-related adverse events were reported by 30% of subjects and all were mild. The most common adverse events were oral tissue discomfort (12 subjects), oral tissue irritation (11 subjects), and dental discomfort (5 subjects).
Discussion
In this acute treatment-feasibility evaluation, OPT improved sleep-related breathing disturbance in subjects with mild-to-severe OSA. Mean AHI was reduced from 38.7 ± 27.5 events/hour (mean ± SD) without treatment to 24.6 ± 25.7 events/hour with treatment (P < 0.001). Some subjects had a vigorous response to OPT while others did not respond. AHI reduction with OPT exceeded 72% in the upper quartile in this study. Since clinically relevant improvements were observed in some subjects, this study suggests that treatment with this noninvasive therapy could potentially be worthwhile for individuals who are evaluated and identified as having a clinically useful response. Improvements with OPT were notable even in some subjects with severe OSA. No significant safety issues were identified in this study. These results suggest that while OPT would not be appropriate for treating all patients, a subset of strong responders can be identified with PSG. Individual evaluation of OPT can be performed safely and rapidly without the need to customize equipment to patients, and without limiting the future use of other therapeutic options for those with an insufficient response to OPT.
As the present work was a feasibility study, there are important limitations that should be recognized in interpreting these results and in planning additional studies. With data from only a single night of treatment per subject, durability of treatment is not evaluated and chronic-treatment studies are needed. OPT was associated with significant reductions in stage shifts and awakenings, consistent with the expectation that relief of airway obstruction during sleep should lead to improvements in sleep measures. However, as is expected with the first night of treatment with a new device, total sleep duration was reduced and wakefulness after sleep onset was increased. With chronic therapy, improvements in these measures and further improvements in stage-shifts and awakenings and in daytime symptoms such as excessive daytime sleepiness can be anticipated but should be confirmed directly by future studies. In each subject, the sequence of PSG studies was not randomized and the baseline study was completed before the PSG with OPT; however, order of PSG evaluation is unlikely to have any significant effect on respiratory parameters.Citation13 Moving beyond this feasibility study, in future studies, a randomized sequence of treatment conditions on PSG nights may be employed. Study designs with control conditions and blinding are desirable. In the present study, each subject served as his or her own control and the subjects were not blinded with regard to treatment condition. Blinding of subjects to treatment versus baseline condition or use of a sham condition are problematic to achieve due to the recognizable oral sensation that OPT produces. The mouthpiece also feels very differently in the presence and absence of the applied vacuum. Comparisons to other treatment methods using multiple treatment arms or parallel treatment groups might be considered in future study designs.
The response to therapy varied across subjects, raising the possibility that future studies might explore how the response could be predicted in individuals. Such insight would further guide the potential clinical application of OPT. Considerations for the etiology of lack of response in individuals may include inadequate lip seal on the mouthpiece, opening of the mouth during sleep, or inadequate tissue movement with the tested device configuration. These factors, along with the night-to-night variability inherent in PSG measurement of AHI and the potential for unfavorable positioning of the mouthpiece in oral cavity relative to the tongue may explain the observation that some subjects had treatment AHI in excess of the baseline measurement. Design improvements might lead to enhanced performance allowing OPT to become relevant for a broader portion of patients. OSA has multiple phenotypes and understanding the multiple anatomic and physiologic factors that are important in OSA is complex.Citation14 Evaluation of OPT using magnetic resonance imaging may provide insight into the mechanism of action and facilitate identifying clinical predictors. Such work may also stimulate new thinking about how the implementation of OPT can evolve to be suitable for more patients.Citation15 Ongoing research may also lead to refinement of the technology and to consideration of combinations of therapeutic methods.
Mild oral tissue discomfort was the most frequent adverse event noted. Evolution of mouthpiece shape and the materials used might help increase comfort. By way of comparison, the pressure change associated with OPT is less than that with denture sealsCitation16 or breast-feeding.Citation17 Sleep architecture results may improve if comfort during OPT use can be increased. An acclimation period as typically occurs with other devices used to treat sleep apnea might enhance tolerability. The present safety results suggest that OPT is feasible for treatment of OSA. Following the demonstration of feasibility in this study, design improvements may be considered to make OPT easily applied in clinical practice. This study used mouthpieces in premanufactured sizes, which provided the potential for easier clinical application than custom production; however, optimizing the fitting procedure and identifying the most appropriate mouthpiece size for an individual patient may merit further consideration.
The results of this study confirm the feasibility of OPT for treatment of OSA as reported previously in a study of OPT in a different cohort of 71 subjects with OSA using individually customized mouthpieces.Citation10 The magnitude of AHI reduction in the present study was similar to the reduction from 34.4 ± 28.9 (mean ± SD) events per hour to 20.7 ± 23.3 events per hour found in the prior study. OPT produced significant improvements in ODI in both studies. In addition, stage-N1 sleep shifts, total sleep-stage shifts, and awakenings were significantly reduced with treatment in both studies. There were no serious device-related adverse events in either study.
Patients who reject therapy with PAP or mandibular advancement devices might be candidates for treatment with OPT and should be studied to further define how their OSA can be managed successfully. Adherence to therapy is an important topic in the treatment of OSA and was not evaluated in this single-night comparison study.Citation18 Effectiveness of response to OPT can be evaluated with PSG and the console can electronically record usage data to provide objective information regarding compliance with therapy. Trial usage of OPT may be pursued without limiting the future selection of alternative treatment methods. OPT offers promise as a noninvasive treatment approach for selected patients.
Conclusion
This study demonstrates that OPT can improve OSA in certain subjects identifiable by PSG during systematic usage. In appropriately responsive patients, OPT shows potential as a clinically useful new alternative for treatment of OSA without the need for custom manufacture of an oral device component. Additional research evaluating sustained nightly treatment is needed to confirm and extend the findings of this study.
Acknowledgments
Financial support for the conduct of this study was provided by ApniCure, Inc.
Disclosure
Dr Farid-Moayer was the principal investigator. He has no conflicts of interest to disclose. Dr Siegel is employed by ApniCure and the USA Department of Veterans Affairs and is Clinical Associate Professor of Anesthesia (Affiliated), Stanford University School of Medicine. Dr Black is a consultant to ApniCure and is Consulting Associate Professor, Stanford Center for Sleep Research and Medicine, Stanford University School of Medicine.
References
- YoungTFinnLPeppardPESleep disordered breathing and mortality: eighteen-year follow-up of the Wisconsin sleep cohortSleep200831610711078s18714778
- LevyPPepinJLArnaudCBaguetJPDematteisMMachFObstructive sleep apnea and atherosclerosisProg Cardiovas Dis2009515400410
- GharibehTMehraRObstructive sleep apnea syndrome: natural history, diagnosis, and emerging treatment optionsNat Sci Sleep2010223325523616712
- ShapiroGKShapiroCMFactors that influence CPAP adherence: an overviewSleep Breath201014432333520661654
- ChanASCistulliPAOral appliance treatment of obstructive sleep apnea: an updateCurr Opin Pulm Med200915659159619710614
- MarklundMVerbraeckenJRanderathWNon-CPAP therapies in obstructive sleep apnoea: mandibular advancement device therapyEur Respir J20113951241124722075487
- van MaanenJPWietsheRVan KesterenEREvaluation of a new simple treatment for positional sleep apnoea patientsJ Sleep Res201121332232922017727
- HoltyJEGuilleminaultCSurgical options for the treatment of obstructive sleep apneaMed Clin North Am201094347951520451028
- KezerianEJBoudewynsAEiseleDWElectrical stimulation of the hypoglossal nerve in the treatment of obstructive sleep apneaSleep Med Rev201014529930520116305
- Farid-MoayerMSiegelLCBlackJA feasibility evaluation of oral pressure therapy for the treatment of obstructive sleep apneaTher Adv Respir Dis20137131223184709
- IberCAncoli-IsraelSChessonAQuanSFThe AASM Manual for the Scoring of Sleep and Associated EventsWestchester, NYAmerican Academy of Sleep Medicine2007
- FDA Ear, Nose, and Throat Devices and Dental Products Joint Advisory Panel Meeting Open Session October 6, 2004 [Internet] Available from: http://www.fda.gov/ohrms/dockets/ac/04/minutes/2004-4071m1_summary%20minutes.pdfAccessed February 26, 2013
- GouverisHSelivanovaOBausmerUGoepelBMannWFirst-night-effect on polysomnographic respiratory sleep parameters in patients with sleep-disordered breathing and upper airway pathologyEur Arch Otorhinolaryngol201026791449145320127101
- RihaRLGislassonTDiefenbachKThe phenotype and genotype of adult obstructive sleep apnoea/hypopnoea syndromeEur Respir J200933364665519251800
- SchwabRKimCSiegelLExamining the mechanism of action of a new device using oral pressure therapy for treatment of obstructive sleep apnea [abstract]J Sleep Res201221Suppl 1O310
- GrossmanESForbesMEStudies related to reaction of supporting soft tissue to denture wear: the histological response of vervet monkey oral epithelium to a −80 mmHg vacuumJ Oral Rehabil19901765875972283554
- GeddesDTKentJCMitoulasLRHartmannPETongue movement and intra-oral vacuum in breastfeeding infantsEarly Hum Dev200884747147718262736
- WeaverTEGrunsteinRRAdherence to continuous positive airway pressure therapy: the challenge to effective treatmentProc Am Thorac Soc20085217317818250209
