Abstract
Purpose
This study aimed to evaluate nocturnal sleep structure and anxiety, depression, and fatigue in patients with narcolepsy type 1 (NT1).
Methods
Thirty NT1 patients and thirty-five healthy controls were enrolled and evaluated using the Epworth sleepiness scale (ESS), Generalized Anxiety Disorder-7, Patient Health Questionnaire-9, Fatigue Severity Scale (FSS), polysomnography, multiple sleep latency test, and brain function state monitoring. Statistical analyses were performed using SPSS Statistics for Windows, version 23.0. Benjamini-Hochberg correction was performed to control the false discovery rate.
Results
Apart from typical clinical manifestations, patients with NT1 are prone to comorbidities such as nocturnal sleep disorders, anxiety, depression, and fatigue. Compared with the control group, patients with NT1 exhibited abnormal sleep structure, including increased total sleep time (Padj=0.007), decreased sleep efficiency (Padj=0.002), shortening of sleep onset latency (Padj<0.001), elevated wake after sleep onset (Padj=0.002), increased N1% (Padj=0.006), and reduced N2%, N3%, and REM% (Padj=0.007, Padj<0.001, Padj=0.013). Thirty-seven percent of patients had moderate to severe obstructive sleep apnea-hypopnea syndrome. And sixty percent of patients were complicated with REM sleep without atonia. Patients with NT1 displayed increased anxiety propensity (Padj<0.001), and increased brain fatigue (Padj=0.020) in brain function state monitoring. FSS scores were positively correlated with brain fatigue (Padj<0.001) and mean sleep latency was inversely correlated with FSS scores and brain fatigue (Padj=0.013, Padj=0.029). Additionally, ESS scores and brain fatigue decreased after 3 months of therapy (P=0.012, P=0.030).
Conclusion
NT1 patients had abnormal nocturnal sleep structures, who showed increased anxiety, depression, and fatigue. Excessive daytime sleepiness and fatigue improved after 3 months of treatment with methylphenidate hydrochloride prolonged-release tablets in combination with venlafaxine.
Introduction
Narcolepsy is a rare lifelong sleep disorder characterized by excessive daytime sleepiness (EDS), cataplexy, sleep hallucinations, and sleep paralysis. The prevalence of narcolepsy is estimated to be between 0.00023% and 0.05% in various nations worldwide, with a higher prevalence observed in males.Citation1–4 It can occur at any age, with two peaks at ages 15 and 35.Citation1,Citation4,Citation5 Early onset narcolepsy, with an onset age before 15 years, accounts for 86% of all cases.Citation6 EDS is frequently the initial symptom in younger individuals, whereas cataplexy is more common in individuals over 60 years of age.Citation7 In addition, patients with narcolepsy have disrupted sleep structure and are prone to comorbidities with other sleep disorders.Citation8 Based on the presence of cataplexy or low or absent hypocretin-1 (Hcrt-1) levels in cerebrospinal fluid (CSF), the International Classification of Sleep Disorders, Third Edition (ICSD-3) classifies narcolepsy into Narcolepsy Type 1 (NT1) and Narcolepsy Type 2. Selective loss of hypocretin (Hcrt) neurons in the lateral hypothalamus is the characteristic pathological change in NT1.Citation9 Current evidence favors NT1 is an autoimmune condition mediated by interactions between CD4+ T cells and CD8+ T cells in genetically susceptible individuals carrying HLA-DQB1* 06:02 or HLA-DQB1* 03:01 alleles, which affects Hcrt neurons and interferes with sleep-wakefulness neural circuits.Citation2,Citation10,Citation11 A study of 9312 narcolepsy patients conducted by Ruoff et al reported that 25.1% of the patients had anxiety disorder, and younger narcolepsy patients (aged 18–24 years) had a higher incidence of anxiety disorder and used more anti-anxiety medications.Citation12 Depression, especially suicidal tendencies, is common in NT1.Citation13–16 Based on previous research, narcolepsy patients reported experiencing depressive emotions at a frequency ranging from 18%-57%, with a prevalence of depression disorder of 32.7%.Citation14,Citation17,Citation18 Some studies have reported the presence of fatigue, with up to 60% of patients with narcolepsy reporting fatigue.Citation4 However, the prevalence of anxiety, depression, and fatigue in Chinese patients with narcolepsy is still unclear. We hypothesized that anxiety, depression, and brain fatigue may be abnormal in patients with NT1. The aim of this study was to evaluate nocturnal sleep structure, anxiety, depression, and fatigue in patients with NT1 using questionnaires, such as the Epworth sleepiness scale (ESS), Generalized Anxiety Disorder-7 (GAD-7), Patient Health Questionnaire-9 (PHQ-9), and Fatigue Severity Scale (FSS), polysomnography (PSG), multiple sleep latency test (MSLT), and brain function state monitoring.
Materials and Methods
Participants
Thirty patients with NT1 were enrolled from the sleep center of the First Hospital of Jilin University as the study group. All patients had persistent uncontrollable sleepiness lasting more than 3 months and met one or two of the ICSD-3 diagnostic criteria: (1) cataplexy, with a mean sleep latency ≤8 min, and the occurrence of two or more sleep onset rapid eye movement periods (SOREMPs) in MSLT; (2) CSF concentration of Hcrt-1 ≤110 pg/mL or less than 1/3 of the mean value for the normal population. Thirty-five healthy controls were enrolled from the health examination center of the same hospital. All participants in both groups were evaluated using PSG, ESS, GAD-7, PHQ-9, FSS, and brain function state monitoring at the time of initial diagnosis. NT1 patients also underwent MSLT at initial diagnosis, and the ESS and brain function state monitoring were re-evaluated after one and three months of treatment with methylphenidate hydrochloride prolonged-release tablets in combination with venlafaxine, respectively. However, the control group did not receive MSLT and re-evaluation. These examinations were performed by well-trained professional doctors who were unaware of the diagnosis of the participants. The study complies with the Ethics Committee of the First Hospital of Jilin University (Approval No: 2019–363) and followed the guidelines of the Declaration of Helsinki (1964).Citation19 And all participants provided informed consent prior to being enrolled in this study.
Scales Evaluation
The validated Chinese version of ESS was developed to assess the degree of daytime sleepiness through the performance of eight scenes.Citation20,Citation21 A total score >6 indicates the presence of sleepiness, a score >11 indicates excessive sleepiness, and a score >16 indicates dangerous sleepiness.Citation20,Citation22
The Chinese version of the GAD-7 is a commonly used scale for evaluating generalized anxiety disorders.Citation23,Citation24 It consists of seven questions that assess the frequency and severity of anxiety symptoms in the past two weeks, with a total score of 0–4 indicating no anxiety, a score of 5–9 indicating mild anxiety, a score of 10–14 indicating moderate anxiety, and a score of 15–21 indicating severe anxiety.Citation25
The Chinese version of the PHQ-9, derived from the depression module of the Patient Health Questionnaire edited by Spitzer et al, is an internationally recognized depression detection scale.Citation26,Citation27 The scale is a 9-item self-assessment of feelings over the preceding two weeks. A total score of 0–4 indicates no depression, a score of 5–9 indicates mild depression, a score of 10–14 indicates moderate depression, a score of 15–19 indicates moderate to severe depression, and a score of 20–27 indicates severe depression.Citation28
The Chinese version of the FSS was used to measure the level of fatigue and its impact on activities of daily living.Citation29,Citation30 The scale consists of nine questions, each rated from 1 to 7 (1 = strongly disagree and 7 = strongly agree). The higher the score, the greater the degree of fatigue.Citation30
Nocturnal PSG
All subjects underwent at least 8 h of PSG (Compumedics, Abbotsford, Australia) monitoring at the sleep center of the First Hospital of Jilin University, which had passed clinilabs certification. PSG results were analyzed by two registered polysomnographic technologists with reference to the revised interpretation criteria for sleep stages and related events published by the American Academy of Sleep Medicine, version 2.6.Citation31 The sleep structure parameters we analyzed included total sleep time (TST), sleep efficiency (SE), sleep onset latency (SOL), wake after sleep onset (WASO), and percentage of each sleep stage (N1%, N2%, N3%, and REM%). We also analyzed other sleep disorders comorbidities, such as obstructive sleep apnea-hypopnea syndrome, REM sleep without atonia, and restless legs syndrome.
MSLT
The MSLT was performed on the day after nocturnal PSG according American Academy of Sleep Medicine manual, provided that the TST was >6 h.Citation32 The MSLT consisted of five nap tests with a two-hour interval. The first nap test was conducted within 1.5 to 3 h after the end of PSG. If a test lasted for 20 min without falling asleep after turning off the lights, the test ended and the sleep latency was recorded as 20 min. And the test would continue for 15 min after the first epoch of sleep to determine whether SOREMP (REM period occurring within 15 min of sleep onset) occurred, if patients fell asleep within 20 min.Citation33
Brain Function State Monitoring
EEG recording electrodes were placed at Fpz, Fp1, Fp2, A1, and A2 according to the international 10/20 system for EEG recording. Prefrontal cortex EEG activity of subjects with normal body temperatures was recorded in a quiet, relaxed state for 6 min. During EEG signal acquisition, patients were required to complete actions such as closing, opening, and staring according to system prompts. Two channels of EEG data were recorded and analyzed using an HXD-I monitor (Beijing Easymonitor Technology Co., Ltd., Beijing, China), and specific EEG data vectors were computed using algorithms such as wavelet analysis, spectral analysis, multiple regression, and pattern recognition to obtain a series of intermediate variables (metadata). At the same time, a “two compartment” model between the left and right cerebral cortex and subcortical region was established, dynamic and static coordination relationships between the left cortex, left subcortex, right cortex and right subcortex were calculated, and a set of feature indices to reflect emotions, sleep, and cognitive functions were obtained. We analyzed anxiety propensity and brain fatigue indices. The computational formulae are as follows:
,
.
Where the calculation window is n, i_10, i_22, i_23, i_24, i_35, i_45, i_48, i_52, i_66, i_77 are the metadata calculated through a specific algorithm combination, and a1, a2, ······, an are multiple regression weighted coefficients.
Statistical Analysis
Statistical analyses were performed using SPSS Statistics for Windows, version 23.0. Group differences were analyzed using chi-square test and multivariable logistic regression analysis to correct sex, age, and body mass index (BMI). Spearman’s rank correlation was performed for correlation analysis and paired Wilcoxon signed rank test was performed to compare changes before and after treatment. To control the false discovery rate, P values were adjusted using the Benjamini-Hochberg correction for multiple testing. Statistical significance was set at P-values <0.05.
Results
General Data and Clinical Symptoms in Patients with NT1
There was no significant difference in age or sex between the study and control groups (). The NT1 group consisted of 21 males (70%) and 9 females (30%), with a median age of 24.5 (17.0, 36.0) years and a male-to-female ratio of 2.3:1. The median age of onset was 16.5 (13.0, 34.3) years, with half of the cases occurring between the ages of 10 and 19. The time from disease onset to NT1 diagnosis in these patients range from 8 months to 7 years. The BMI of the patients did not differ from that of the controls (). Thirty-seven percent of the patients were overweight (24.9 kg/m2<BMI<30 kg/m2), and one-tenth were obese (BMI≥30 kg/m2).
Table 1 Comparison of General Data Between Patients with NT1 and the Control Group
All patients in the study group underwent cerebral magnetic resonance imaging, and none showed any significant abnormalities. Among the 30 patients, 29 (96.7%) experienced EDS, 26 (86.7%) experienced cataplexy, 19 (63.3%) experienced sleep paralysis, and 12 (40.0%) experienced sleep hallucinations. Seven patients (23.3%) had all four clinical manifestations.
Nocturnal PSG in Patients with NT1
Compared with the control group, patients with NT1 had an increased TST (Padj=0.007), decreased SE (Padj=0.002), a reduction in SOL (Padj <0.001), an increase in WASO (Padj=0.002), an increase in N1% (Padj=0.006), and a decrease in N2% (Padj=0.007), N3% (Padj <0.001), and REM% (Padj=0.013) (). In NT1 group, eleven patients (36.7%) had moderate to severe obstructive sleep apnea-hypopnea syndrome. In addition, 18 cases (60.0%) were complicated with REM sleep without atonia, eight cases (26.7%) had REM sleep behavior disorder, and seven cases (23.3%) exhibited sleep talking. Restless legs syndrome occurred in one patient (3.3%), and five individuals (16.7%) had periodic limb movement disorder.
Table 2 Comparison of PSG Parameters Between Patients with NT1 and the Control Group
Evaluation of Sleepiness in Patients with NT1
Patients with NT1 scored higher on the ESS than those in the control group (17.00 [11.75, 19.00] vs 3.00 [1.00, 4.00], Waldχ2=9.707, Padj=0.003) (). The mean sleep latency in the MSLT was 2.70 (1.48, 5.45) minutes. Seven cases (23.3%) exhibited SOREMPs in PSG, and the average number of SOREMPs was 3.00 (2.00, 4.00) in the MSLT.
Table 3 Comparison of ESS, GAD-7, PHQ-9, and FSS Scores Between Patients with NT1 and the Control Group
Evaluation of Anxiety and Depression in Patients with NT1
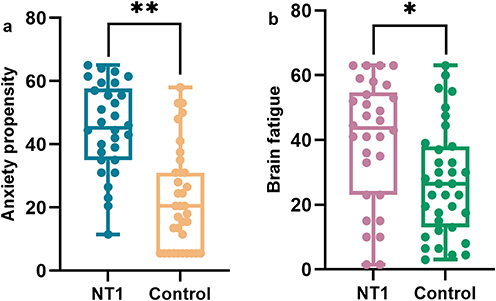
Patients with NT1 scored significantly higher than controls on the GAD-7 (7.00 [4.00, 8.25] vs 2.00 [1.00, 3.00], Waldχ2=16.951, Padj<0.001) and PHQ-9 (11.50 [8.00, 15.25] vs 2.00 [1.00, 4.00], Waldχ2=11.818, Padj=0.002) (). They also demonstrated an increase in anxiety propensity (27.50 [15.00, 45.50] vs 7.00 [0.00, 14.00], Waldχ2=14.637, Padj<0.001) ().
Figure 1 Comparison of anxiety propensity and brain fatigue between patients with NT1 and the control group (Ranks plot). (a) Comparison of anxiety propensity between NT1 patients and the control group; (b) Comparison of brain fatigue between NT1 patients and the control group.
Evaluation of Fatigue in Patients with NT1
According to statistical analysis, patients with NT1 had higher FSS scores than the control group (32.00 [29.00, 40.25] vs 28.00 [26.00, 29.00], Waldχ2=9.426, Padj=0.003) (). Brain fatigue was also higher than that of the control group (29.00 [15.00, 55.00] vs 16.00 [10.00, 23.00], Waldχ2=5.425, Padj =0.020) ().
Correlation Between PSG Parameters, ESS, GAD-7, PHQ-9, and FSS Scores, MSLT, and Brain Function State Monitoring Indices in Patients with NT1
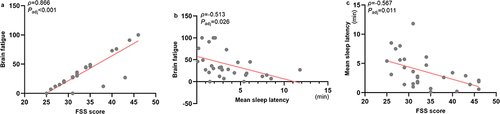
We analyzed the correlation between PSG parameters (TST, SE, SOL, and WASO) and brain function state monitoring indices (anxiety propensity and brain fatigue) in patients with NT1 (Supplementary Table 1). There was no significant correlation after Benjamini-Hochberg correction. According to the correlation analysis between ESS, GAD-7, PHQ-9, and FSS scores, and brain function state monitoring indices (Supplementary Table 1), there was a positive correlation between FSS scores and brain fatigue (ρ=0.866, Padj<0.001) (). GAD-7 scores were positively correlated with anxiety propensity in NT1 group before correction (ρ=0.456, P=0.011), but the correlation became less precise after Benjamini-Hochberg (Padj=0.059). In the correlation analysis between MSLT parameters (mean sleep latency and SOREMP) and brain function state monitoring indices (Supplementary Table 1), we found that the mean sleep latency was negatively correlated with brain fatigue (ρ=−0.513, Padj=0.026) (). Based on the positive correlation of brain fatigue with FSS scores, and the negative correlation of brain fatigue with mean sleep latency, further analysis revealed that FSS scores were negatively correlated with mean sleep latency (ρ=−0.567, Padj=0.011) ().
Figure 2 Correlation between FSS scores, mean sleep latency, and brain fatigue in patients with NT1. (a) Correlation between FSS scores and brain fatigue in patients with NT1; (b) Correlation between mean sleep latency and brain fatigue in patients with NT1; (c) Correlation between FSS scores and mean sleep latency in patients with NT1.
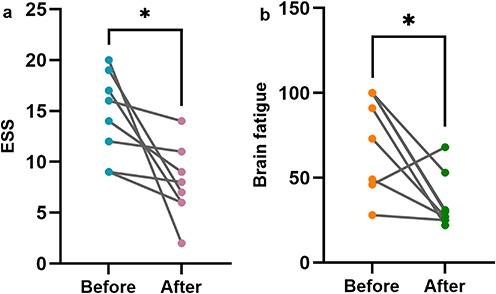
Changes in ESS Scores, Anxiety Propensity, and Brain Fatigue Before and After Treatment
After one month of treatment, 11 patients actively cooperated with the follow-up and were re-examined with the ESS and brain function state monitoring, while the remaining 19 patients were lost to follow-up for various reasons. There were no significant changes in ESS scores, anxiety propensity, and brain fatigue (−2.00 [−5.00, 2.00], Z=−0.939, P=0.348; −4.00 [−16.00, 19.00], Z=−0.089, P=0.929; −16.00 [−40.00, 29.00], Z=−0.357, P=0.721).
After 3 months of treatment, only 8 patients cooperated with follow-up and were re-examined. ESS scores decreased (−4.00 [−11.75, −1.25], Z=−2.524, P=0.012), and brain fatigue decreased (−46.50 [−69.00, −7.75], Z=−2.176, P=0.030) compared to baseline (). There were no significant changes in anxiety propensity (11.00 [−15.25, 20.50], Z=−0.491, P=0.623).
Discussion
Narcolepsy is a rare, lifelong, sleep-increasing disease characterized by EDS, cataplexy, sleep hallucinations, and sleep paralysis. We found that NT1 occurs in males mostly (70%), with the age of onset concentrated between 10 and 19 years old. The time from disease onset to NT1 diagnosis in these patients range from 8 months to 7 years, which was significantly shorter than 8 years previously reported for narcolepsy.Citation3 We considered that this may be due to the fact that our research subject was NT1. And they were in their most stressful secondary school years, receiving more attention from teachers and parents and being diagnosed earlier. In addition, it may benefit from the popular science propaganda and diagnostic level of our sleep center. Almost all of our patients experienced EDS and cataplexy, approximately two-thirds had sleep paralysis, two-fifths had sleep hallucinations, and one-quarter had all four of these clinical manifestations. The absence of Hcrt neurons in patients with narcolepsy may mean that the activity of these wakefulness-promoting neurons is less sustained, leading to EDS and a rapid transition to sleep. Hcrt neurons inhibit REM sleep, and the reduction of Hcrt signals in narcolepsy exhibit REM sleep dysregulation characterized by poor circadian rhythm of REM sleep, rapid transition to REM sleep, and physiological disturbances of REM sleep.Citation2 In healthy individuals, the activation of Hcrt neurons on REM sleep-suppressing regions (such as the ventral lateral periaqueductal grey and lateral pontine tegmentum, dorsal raphe, and locus coeruleus) is balanced by the inhibition of the amygdala on these regions. However, in patients with narcolepsy, the excitatory drive of Hcrt neurons is absent and signals from the amygdala inhibit these REM sleep-suppressing regions, thereby inhibiting the sublaterodorsal nucleus and activating GABAergic premotor neurons that inhibit motor neurons, resulting in cataplexy.Citation2
Studies have found that narcolepsy patients exhibit abnormal sleep structures: fragmented sleep, prolonged WASO, increased conversion frequency between NREM and REM sleep, reduced SE, reduced SOL, increased N1%, and reduced N2%.Citation34–36 A recent meta-analysis of narcolepsy concluded that the TST increased, the N3% decreased considerably, and the REM% did not change significantly.Citation37 However, further analysis revealed that the REM% was significantly decreased in NT1.Citation37 Our results align with those of previous studies, confirming characteristic sleep structures in NT1 patients, including increased TST, decreased SE, reduced SOL, increased WASO, increased N1%, decreased N2%, reduced N3%, and diminished REM%. The mean sleep latency in the MSLT was significantly shorter than normal level. Approximately a quarter of the patients exhibited SOREMP in PSG, and the average number of SOREMPs was three in the MSLT. Therefore, some studies have suggested that narcolepsy is a disorder of state boundaries, including but not limited to REM sleep and wakefulness.Citation38 This may be a consequence of disrupted sleep/wake-stabilizing mechanisms owing to the loss of Hcrt neurons in the hypothalamus. In addition to an abnormal sleep structure, patients with narcolepsy often comorbided other sleep disorders, including REM sleep without atonia, REM sleep behavior disorder, obstructive sleep apnea-hypopnea syndrome, and periodic limb movement disorder. According to previous research, NT1 patients are more likely to have REM sleep without atonia and a greater symptom severity.Citation39 The incidence of REM sleep behavior disorder in the population with narcolepsy ranges from 36.4% to 63.5%. Patients with comorbid REM sleep behavior disorder are more likely to experience cataplexy and severe EDS.Citation8,Citation40,Citation41 Sleep apnea affects 24%–33.8% of patients, which is significantly higher than the prevalence in the general population, and is linked to advancing age and an elevated BMI.Citation13,Citation42–45 Dauvilliers et al found that patients with narcolepsy have a higher risk of developing periodic limb movement disorder than the general public.Citation46 In our study, a greater variety of other sleep disorders was showed. More than half of the patients had complications of obstructive sleep apnea-hypopnea syndrome or REM sleep without atonia, and more than a quarter of the patients had REM sleep behavior disorder. Additionally, restless legs syndrome, periodic limb movement disorder, and sleep talking were identified. However, except for obstructive sleep apnea-hypopnea syndrome, the prevalence of REM sleep behavior disorder, REM sleep without atonia, restless legs syndrome, and periodic limb movement disorder was slightly lower than that previously reported.Citation8,Citation39,Citation41,Citation47 The differences may be due to the fact that the younger age of the patients with narcolepsy in our study resulted in a lower prevalence of sleep disorders related to advanced age.Citation48
Anxiety and depression are associated with a decrease in Hcrt-1 levels. Hcrt stimulates noradrenergic neurons in the locus coeruleus, and serotonergic neurons in the dorsal raphe nucleus. In addition, the activation of GABA neurons in the basolateral amygdala and intercalated region of the amygdala by Hcrt can inhibit anxiety and depression.Citation49 Furthermore, dopamine, serotonin (5-HT), and norepinephrine (NE) are structurally connected to the amygdala, which regulates mood.Citation50,Citation51 The decrease in Hcrt levels in NT1 would result in decreased amounts and activities of dopamine, 5-HT, and NE, decreased binding of Hcrt to GABA neurons, and susceptibility to anxiety and depression.Citation52 In addition, corticotropin-releasing hormone (CRH) may be associated with negative emotions. Recent studies had found a reduced number of neurons expressing and hyperactivity of the remaining CRH neurons in the paraventricular nucleus (PVN) of the hypothalamus in patients with NT1.Citation53,Citation54 CRH increased the rate-limiting enzymes of biosynthesis for NE and 5-HT, which in turn inhibited the activation of NE and 5-HT.Citation55 Antagonists of CRH1 receptors showed antidepressant-like effects, confirming the relationship between CRH and anxiety and depression.Citation56,Citation57 We found NT1 patients had higher GAD-7 and PHQ-9 scores and anxiety propensity than the control group. Their scores showed that approximately 73% (22/30) of the NT1 patients had varying degrees of anxiety: 53.3% (16/30) had mild anxiety, 16.7% (5/30) had moderate anxiety, and 3.3% (1/30) had severe anxiety. In addition, 60% (18/30) of patients might have experienced moderate or severe depression. We have previously reported higher rates of anxiety and depression in NT1 patients.Citation47 We speculated that patients with NT1 may have experienced more severe mood disorders and received anti-anxiety and antidepressant treatments prior to the initial diagnosis of narcolepsy. However, the correlation between anxiety propensity and GAD-7 scores became less precise after Benjamini-Hochberg correction. We believe the correlation may become more reliable in larger sample sizes.
A lack of Hcrt reduces excitatory signaling to neurons involved in the synthesis of the wake-promoting neurotransmitters NE, dopamine, 5-HT, and histamine, and may subsequently result in reduced activation of the cortex, basal forebrain, hypothalamus, and brainstem.Citation58 Additionally, the PVN and CRH have been found to be associated with arousal and consciousness.Citation59,Citation60 PVN is the major site of CRH-containing cell bodies, and cases with lesions of it showed EDS, which decreased when the PVN area recovered after treatment.Citation61 Intraventricular injection of Hcrt-1 stimulated the pituitary-adrenal axis through activation of CRH neurons and stimulated arousal in rats.Citation60 This explains the significantly higher ESS scores in patients with NT1 compared to controls. In our study, we found that NT1 patients were more fatigued than healthy controls, as evidenced by higher FSS scores and brain fatigue. Moreover, there was a positive correlation between FSS scores and brain fatigue, which increased the credibility of the results. Fatigue should be distinguished from EDS, as it is more difficult to treat and can lead to severe functional impairment.Citation62 Some studies concluded that fatigue was unrelated to sleepiness, depression, and obesity.Citation63,Citation64 We speculated that shortened mean sleep latency, coupled with shortened SOL, decreased SE, and prolonged WASO could indicate more serious sleep disturbances and prevented adequate rest and recovery, leading to increased fatigue. This is probably why mean sleep latency was inversely related to FSS scores and brain fatigue.
The choice of medication depends on the patient’s symptoms, financial condition and the type of medications available. After one month of treatment, ESS scores, anxiety propensity, and brain fatigue did not change significantly. However, ESS scores and brain fatigue decreased significantly after 3 months of treatment, reflecting improvements in EDS and nocturnal sleep disturbances. Narcolepsy is a chronic sleep disorder. It is not easy to achieve significant improvement and better control in the short term. Therefore, long-term adherence to medication is necessary. With the development of medication, NT1 patients have more choices. Pitolisant (a reverse agonist of histamine) has been approved by the European Medicines Agency, the US Food and Drug Administration (FDA), and Chinese National Medical Products Administration as first-line treatment for EDS and cataplexy of adult narcolepsy; Solriamfetol is a selective dopamine and norepinephrine reuptake inhibitor approved by the FDA as first-line treatment for EDS in patients with narcolepsy.Citation4 Both of them may cause adverse reactions of anxiety.Citation65,Citation66 The dosage of selective serotonin reuptake inhibitors or serotonin norepinephrine reuptake inhibitors used to treat cataplexy is insufficient to control anxiety and depression. For NT1 patients with anxiety or depression, higher doses or more types of anxiolytics or antidepressants are needed.
This study has several limitations. It was an observational study without in-depth investigation into underlying mechanisms. We did not examine PSG+MSLT and the concentration of Hcrt-1 after treatment, which will be refined in subsequent studies. In addition, our study was limited by insufficient sample size. Though the rating scales were used widely all over the world, which were really subjective and self-influenced. In future research, maintenance of wakefulness test and immunology research would be included with larger samples to provide stronger evidence for the clinical function assessment of NT1.
Conclusion
NT1 patients exhibit an abnormal sleep structure, including an extended TST, decreased SE, decreased SOL, prolonged WASO, increased N1%, and decreased N2%, N3%, and REM%. Patients with NT1 typically comorbid obstructive sleep apnea-hypopnea syndrome, REM sleep behavior disorder, and other sleep disorders. They demonstrated higher anxiety, depression, and fatigue compared to the healthy controls. ESS scores and brain fatigue decreased simultaneously after 3 months of treatment.
Disclosure
The authors report no conflicts of interest in this work.
Acknowledgments
We would like to thank Editage (www.editage.cn) for English language editing.
Additional information
Funding
References
- Tiseo C, Vacca A, Felbush A, et al. Migraine and sleep disorders: a systematic review. J Headache Pain. 2020;21(1):126. doi:10.1186/s10194-020-01192-5
- Mahoney CE, Cogswell A, Koralnik IJ, Scammell TE. The neurobiological basis of narcolepsy. Nat Rev Neurosci. 2019;20(2):83–93. doi:10.1038/s41583-018-0097-x
- Disorders CSoS. Chinese guidelines for diagnosis and treatment of narcolepsy (2022). Chin J Neurol. 2022;55(5):406–420.
- Bassetti CLA, Adamantidis A, Burdakov D, et al. Narcolepsy - clinical spectrum, aetiopathophysiology, diagnosis and treatment. Nat Rev Neurol. 2019;15(9):519–539. doi:10.1038/s41582-019-0226-9
- Kornum BR, Knudsen S, Ollila HM, et al. Narcolepsy. Nat Rev Dis Primers. 2017;3:16100. doi:10.1038/nrdp.2016.100
- Ouyang H, Han F, Zhou ZC, Zhang J. Differences in clinical and genetic characteristics between early- and late-onset narcolepsy in a Han Chinese cohort. Neural Regen Res. 2020;15(10):1887–1893. doi:10.4103/1673-5374.280322
- Wu M, Li X, Li SX, et al. Early- and late-onset narcolepsy: possibly two distinct clinical phenotypes. Sleep Breath. 2023;27(6):2443–2452. doi:10.1007/s11325-023-02820-5
- Nevsimalova S, Pisko J, Buskova J, et al. Narcolepsy: clinical differences and association with other sleep disorders in different age groups. J Neurol. 2013;260(3):767–775. doi:10.1007/s00415-012-6702-4
- Thannickal TC, Moore RY, Nienhuis R, et al. Reduced number of hypocretin neurons in human narcolepsy. Neuron. 2000;27(3):469–474. doi:10.1016/S0896-6273(00)00058-1
- Iranzo A. Sleep and neurological autoimmune diseases. Neuropsychopharmacology. 2020;45(1):129–140.
- Latorre D, Kallweit U, Armentani E, et al. T cells in patients with narcolepsy target self-antigens of hypocretin neurons. Nature. 2018;562(7725):63–68. doi:10.1038/s41586-018-0540-1
- Ruoff CM, Reaven NL, Funk SE, et al. High rates of psychiatric comorbidity in narcolepsy: findings From the Burden of Narcolepsy Disease (BOND) study of 9312 patients in the United States. J Clin Psychiatry. 2017;78(2):171–176. doi:10.4088/JCP.15m10262
- Cohen A, Mandrekar J, St Louis EK, Silber MH, Kotagal S. Comorbidities in a community sample of narcolepsy. Sleep Med. 2018;43:14–18. doi:10.1016/j.sleep.2017.11.1125
- Lee MJ, Lee SY, Yuan SS, et al. Comorbidity of narcolepsy and depressive disorders: a nationwide population-based study in Taiwan. Sleep Med. 2017;39:95–100. doi:10.1016/j.sleep.2017.07.022
- Inocente CO, Gustin MP, Lavault S, et al. Depressive feelings in children with narcolepsy. Sleep Med. 2014;15(3):309–314. doi:10.1016/j.sleep.2013.08.798
- Lopez R, Barateau L, Evangelista E, Dauvilliers Y. Depression and hypersomnia: a complex association. Sleep Med Clin. 2017;12(3):395–405. doi:10.1016/j.jsmc.2017.03.016
- Sateia MJ. International classification of sleep disorders-third edition: highlights and modifications. Chest. 2014;146(5):1387–1394. doi:10.1378/chest.14-0970
- Vignatelli L, Plazzi G, Peschechera F, Delaj L, D’Alessandro R. A 5-year prospective cohort study on health-related quality of life in patients with narcolepsy. Sleep Med. 2011;12(1):19–23. doi:10.1016/j.sleep.2010.07.008
- World Medical A. World medical association declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191–2194. doi:10.1001/jama.2013.281053
- Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540–545. doi:10.1093/sleep/14.6.540
- Peng LL, Li JR, Sun JJ, et al. Epworth 嗜睡量表简体中文版信度和效度评价 [Reliability and validity of the simplified Chinese version of Epworth sleepiness scale]. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2011;46(1):44–49. Chinese.
- Alqurashi YD, Dawidziuk A, Alqarni A, et al. A visual analog scale for the assessment of mild sleepiness in patients with obstructive sleep apnea and healthy participants. Ann Thorac Med. 2021;16(2):141–147. doi:10.4103/atm.ATM_437_20
- Tong X, An DM, McGonigal A, Park SP, Zhou D. Validation of the Generalized Anxiety Disorder-7 (GAD-7) among Chinese people with epilepsy. Epilepsy Res. 2016;120:31–36. doi:10.1016/j.eplepsyres.2015.11.019
- Del Bianco C, Ulivi M, Liguori C, et al. Alexithymia, impulsiveness, emotion, and eating dyscontrol: similarities and differences between narcolepsy type 1 and type 2. Sleep Biol Rhythms. 2023;21(1):39–50. doi:10.1007/s41105-022-00414-4
- Xi Y, Elkana O, Jiao WE, Li D, Tao ZZ. Associations between social support and anxiety during the COVID-19 lockdown in young and middle-aged Israelis: a cross-sectional study. World J Psychiatr. 2022;12(9):1194–1203. doi:10.5498/wjp.v12.i9.1194
- Sun XY, Li YX, Yu CQ, Li LM. 中文版抑郁量表信效度研究的系统综述 [Reliability and validity of depression scales of Chinese version: a systematic review]. Zhonghua Liu Xing Bing Xue Za Zhi. 2017;38(1):110–116. Chinese.
- Wasling HB, Bornstein A, Wasling P. Quality of life and procrastination in post-H1N1 narcolepsy, sporadic narcolepsy and idiopathic hypersomnia, a Swedish cross-sectional study. Sleep Med. 2020;76:104–112. doi:10.1016/j.sleep.2020.10.014
- Zhao LP, Huang G, Duan YX, Wang Y, Chen GM, Zhang WW. 结直肠癌抑郁患者的静息态脑功能低频振幅研究 [Amplitude of low-frequency fluctuations of resting-state functional MRI in colorectal cancer patients with depression]. Zhonghua Zhong Liu Za Zhi. 2019;41(11):844–848. Chinese.
- Wang MY, Liu IC, Chiu CH, Tsai PS. Cultural adaptation and validation of the Chinese version of the Fatigue Severity Scale in patients with major depressive disorder and nondepressive people. Qual Life Res. 2016;25(1):89–99.
- Valko PO, Bassetti CL, Bloch KE, Held U, Baumann CR. Validation of the fatigue severity scale in a Swiss cohort. Sleep. 2008;31(11):1601–1607. doi:10.1093/sleep/31.11.1601
- Berry RBQS, Abreu AR. for the American Academy of Sleep Medicine. Am Acad Sleep Med. 2020;2020:1419–1425.
- Berry RB, Brooks R, Gamaldo C, et al. AASM scoring manual updates for 2017 (Version 2.4). J Clin Sleep Med. 2017;13(5):665–666. doi:10.5664/jcsm.6576
- Siegenthaler P, Valko PO, Renzel R, et al. How reliable is a simplified MSLT nap termination protocol? Sleep Med. 2023;109:285–292. doi:10.1016/j.sleep.2023.07.016
- Inocente CO, Lavault S, Lecendreux M, et al. Impact of obesity in children with narcolepsy. CNS Neurosci Ther. 2013;19(7):521–528. doi:10.1111/cns.12105
- Xu X, Wu HJ, Zhuang JH, et al. Sleep-wake patterns, non-rapid eye movement, and rapid eye movement sleep cycles in teenage narcolepsy. Sleep Med. 2017;33:47–56. doi:10.1016/j.sleep.2016.08.012
- Nevsimalova S. The diagnosis and treatment of pediatric narcolepsy. Curr Neurol Neurosci. 2014;14(8):469.
- Zhang Y, Ren R, Yang LH, et al. Polysomnographic nighttime features of narcolepsy: a systematic review and meta-analysis. Sleep Med Rev. 2021;58:101488. doi:10.1016/j.smrv.2021.101488
- Schoch SF, Werth E, Poryazova R, Scammell TE, Baumann CR, Imbach LL. Dysregulation of sleep behavioral states in narcolepsy. Sleep. 2017;40(12). doi:10.1093/sleep/zsx170
- Dauvilliers Y, Jennum P, Plazzi G. Rapid eye movement sleep behavior disorder and rapid eye movement sleep without atonia in narcolepsy. Sleep Med. 2013;14(8):775–781. doi:10.1016/j.sleep.2012.10.006
- Knudsen S, Gammeltoft S, Jennum PJ. Rapid eye movement sleep behaviour disorder in patients with narcolepsy is associated with hypocretin-1 deficiency. Brain. 2010;133(2):568–579. doi:10.1093/brain/awp320
- Antelmi E, Pizza F, Franceschini C, Ferri R, Plazzi G. REM sleep behavior disorder in narcolepsy: a secondary form or an intrinsic feature? Sleep Med Rev. 2020;50:101254. doi:10.1016/j.smrv.2019.101254
- Frauscher B, Ehrmann L, Mitterling T, et al. Delayed diagnosis, range of severity, and multiple sleep comorbidities: a clinical and polysomnographic analysis of 100 patients of the Innsbruck narcolepsy cohort. J Clin Sleep Med. 2013;9(8):805–812. doi:10.5664/jcsm.2926
- Pataka AD, Frangulyan RR, Mackay TW, Douglas NJ, Riha RL. Narcolepsy and sleep-disordered breathing. Eur J Neurol. 2012;19(5):696–702. doi:10.1111/j.1468-1331.2011.03610.x
- Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328(17):1230–1235. doi:10.1056/NEJM199304293281704
- Pizza F, Tartarotti S, Poryazova R, Baumann CR, Bassetti CL. Sleep-disordered breathing and periodic limb movements in narcolepsy with cataplexy: a systematic analysis of 35 consecutive patients. Eur Neurol. 2013;70(1–2):22–26. doi:10.1159/000348719
- Dauvilliers Y, Pennestri MH, Petit D, Dang-Vu T, Lavigne G, Montplaisir J. Periodic leg movements during sleep and wakefulness in narcolepsy. J Sleep Res. 2007;16(3):333–339. doi:10.1111/j.1365-2869.2007.00601.x
- Szabo ST, Thorpy MJ, Mayer G, Peever JH, Kilduff TS. Neurobiological and immunogenetic aspects of narcolepsy: implications for pharmacotherapy. Sleep Med Rev. 2019;43:23–36. doi:10.1016/j.smrv.2018.09.006
- Iranzo A. Parasomnias and sleep-related movement disorders in older adults. Sleep Med Clin. 2022;17(2):295–305. doi:10.1016/j.jsmc.2022.02.005
- Staton CD, Yaeger JDW, Khalid D, et al. Orexin 2 receptor stimulation enhances resilience, while orexin 2 inhibition promotes susceptibility, to social stress, anxiety and depression. Neuropharmacology. 2018;143:79–94. doi:10.1016/j.neuropharm.2018.09.016
- Del Sette P, Veneruso M, Cordani R, et al. Narcolepsy and emotions: is there a place for a theory of mind approach? Sleep Med. 2023;102:84–89. doi:10.1016/j.sleep.2022.12.013
- Chellappa SL, Aeschbach D. Sleep and anxiety: from mechanisms to interventions. Sleep Med Rev. 2022;61:101583. doi:10.1016/j.smrv.2021.101583
- Jacobson LH, Hoyer D, de Lecea L. Hypocretins (orexins): the ultimate translational neuropeptides. J Intern Med. 2022;291(5):533–556. doi:10.1111/joim.13406
- Shan L, Linssen S, Harteman Z, et al. Activated wake systems in narcolepsy type 1. Ann Neurol. 2023;94(4):762–771. doi:10.1002/ana.26736
- Shan L, Balesar R, Swaab DF, Lammers GJ, Fronczek R. Reduced numbers of corticotropin-releasing hormone neurons in narcolepsy type 1. Ann Neurol. 2022;91(2):282–288. doi:10.1002/ana.26300
- Holsboer F. The rationale for corticotropin-releasing hormone receptor (CRH-R) antagonists to treat depression and anxiety. J Psychiatr Res. 1999;33(3):181–214. doi:10.1016/S0022-3956(98)90056-5
- Rana T, Behl T, Sehgal A, et al. Exploring the role of neuropeptides in depression and anxiety. Prog Neuropsychopharmacol Biol Psychiatry. 2022;114:110478. doi:10.1016/j.pnpbp.2021.110478
- Held K, Kunzel H, Ising M, et al. Treatment with the CRH1-receptor-antagonist R121919 improves sleep-EEG in patients with depression. J Psychiatr Res. 2004;38(2):129–136. doi:10.1016/S0022-3956(03)00076-1
- Thorpy MJ. Recently approved and upcoming treatments for narcolepsy. CNS Drugs. 2020;34(1):9–27. doi:10.1007/s40263-019-00689-1
- Chen CR, Zhong YH, Jiang S, et al. Dysfunctions of the paraventricular hypothalamic nucleus induce hypersomnia in mice. Elife. 2021;10. doi:10.7554/eLife.69909
- Kok SW, Roelfsema F, Overeem S, et al. Dynamics of the pituitary-adrenal ensemble in hypocretin-deficient narcoleptic humans: blunted basal adrenocorticotropin release and evidence for normal time-keeping by the master pacemaker. J Clin Endocrinol Metab. 2002;87(11):5085–5091. doi:10.1210/jc.2002-020638
- Wang Z, Zhong YH, Jiang S, Qu WM, Huang ZL, Chen CR. Case report: dysfunction of the paraventricular hypothalamic nucleus area induces hypersomnia in patients. Front Neurosci. 2022;16:830474. doi:10.3389/fnins.2022.830474
- Droogleever Fortuyn HA, Fronczek R, Smitshoek M, et al. Severe fatigue in narcolepsy with cataplexy. J Sleep Res. 2012;21(2):163–169. doi:10.1111/j.1365-2869.2011.00943.x
- Cremaschi RC, Hirotsu C, Tufik S, Coelho FM. High fatigue frequency in narcolepsy type 1 and type 2 in a Brazilian sleep center. Sleep Med. 2018;52:234. doi:10.1016/j.sleep.2018.08.013
- Johansson K, Wasling P, Axelsson M. Fatigue, insomnia and daytime sleepiness in multiple sclerosis versus narcolepsy. Acta Neurol Scand. 2021;144(5):566–575. doi:10.1111/ane.13497
- Malhotra A, Shapiro C, Pepin JL, et al. Long-term study of the safety and maintenance of efficacy of solriamfetol (JZP-110) in the treatment of excessive sleepiness in participants with narcolepsy or obstructive sleep apnea. Sleep. 2020;43(2). doi:10.1093/sleep/zsz220
- Dauvilliers Y, Arnulf I, Szakacs Z, et al. Long-term use of pitolisant to treat patients with narcolepsy: harmony III Study. Sleep. 2019;42(11). doi:10.1093/sleep/zsz174