Abstract
Objective
Compared to low arousal threshold (AT), high AT is an easily overlooked characteristic for obstructive sleep apnea (OSA) severity estimation. This study aims to evaluate the relationship between high AT, hypertension and diabetes in OSA, compared to those with apnea–hypopnea index (AHI).
Methods
A total of 3400 adults diagnosed with OSA were retrospectively recruited. Propensity score matching (PSM) was conducted to further categorize these patients into the low and high AT groups based on the strategy established by previous literature. The different degrees of AHI and quantified AT (AT score) were subsequently measured. The correlation of AT and AHI with the occurrence of various comorbidities in OSA was estimated by logistic regression analysis with odds ratio (OR).
Results
After PSM, 938 pairs of patients arose. The median AT score of high and low AT group was 21.7 and 12.2 scores, and the adjusted OR of high AT for hypertension and diabetes was 1.31 (95% CI = 1.07–1.62, P < 0.01) and 1.45 (95% CI = 1.01–2.08, P < 0.05), respectively. Compared to low AT score group, the OR significantly increased in patients with very high AT score (30 ≤ AT score), especially for diabetes (OR = 1.79, 95% CI = 1.02–3.13, P < 0.05). The significant association was not observed in AHI with increasing prevalent diabetes.
Conclusion
Higher AT is significantly associated with increased prevalence of hypertension and diabetes in patients with OSA. Compared with AHI, AT score is a potentially comprehensive indicator for better evaluating the relationship between OSA and related comorbidities.
Introduction
Obstructive sleep apnea (OSA) affects up to one billion individuals globally,Citation1 which is an independent risk factor for a number of disorders, especially cardiovascular and metabolic diseases such as hypertension and diabetes.Citation2,Citation3 Patients with untreated severe OSA had a higher risk of fatal cardiovascular events.Citation4 The apnea–hypopnea index (AHI) is currently considered the gold standard for diagnosing OSA, as it measures the frequency of apneas and hypopneas during sleep.Citation5 A prospective cohort study, which categorized OSA severity by AHI, showed that patients with moderate and severe OSA had a higher 10-year risk of fatal cardiovascular disease.Citation6 Nevertheless, AHI fails to provide information about the duration and severity of blood gas changes and comprehensively explain the characteristics of OSA.Citation7 Some studies have found that patients sharing the same AHI displayed considerable variation in pathogenesis, comorbidity rates, and prognosis since OSA is a heterogeneous disease, suggesting that AHI may have limitation to examine the relationship between OSA and relevant comorbidities.Citation8 Recently, researchers have proposed some new indicator including hypoxic burden (HB), ventilation burden, sleep arousal burden, and sleep breathing impairment index, which are more closely associated with the risk of comorbidities than AHI.Citation9,Citation10 In a large cohort study, Trzepizur et al found that HB and T90 were the better indicators associated with cardiovascular morbidity than AHI.Citation11 Moreover, the studies of endotype have revealed that in addition to anatomical factors, non-anatomical factors such as high loop gain, low arousal threshold, critical closing pressure, and upper airway muscle reactiveness play significant roles in OSA.Citation12
Arousal threshold (AT) is characterized by the amount of ventilatory drive in response to a respiratory stimulus that ends in excitation.Citation13 Frequent arousals due to low AT can lead to sleep fragmentation and ventilation instability.Citation14 As previous studies shown, approximately one-third to half of patients with OSA exhibit low AT.Citation12 Cortical arousal induces rapid physiological changes, facilitating prompt alleviation of acute respiratory episodes and their associated consequences. Previous research indicated that a higher prevalence of low AT in patients with OSA and asthma or COPD compared to those with OSA alone.Citation15,Citation16 A higher AT, more frequently observed in patients with more severe OSA, may result in more severe partial airway obstruction, lower oxygen saturation and hypercapnia during sleep.Citation17,Citation18 Heinzer RC et al demonstrated that in patients with a severely compromised pharyngeal airway, an elevated arousal threshold would likely prolong the duration of the apnea as an arousal which will probably be needed to reopen the collapsed upper airway.Citation19 These pathophysiological mechanisms are related to adverse consequences such as endothelial dysfunction, sympathetic excitation, inflammation, oxidative stress, and metabolic dysfunction,Citation20 which eventually develop to multiple comorbidities.Citation21 Zinchuk et al reported that patients with OSA and high AT were more likely to comorbid with hypertension and other comorbidities, while the results were not statistically significant after adjusting for BMI values.Citation22 It remains unclear with less evidence that whether high AT is associated with increased prevalent comorbidities such as hypertension and diabetes in patients with OSA. Another important issue is that whether there exists a cut-off point to distinguish between normal threshold and pathological high threshold. We hypothesized that high AT would be a greater risk factor for hypertension and diabetes than high AHI.
Methods
Participants
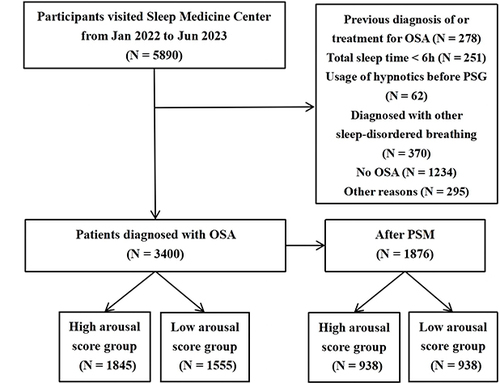
All study subjects were enrolled from Jan 2022 to Jun 2023, at the Sleep Medicine Center of the First Affiliated Hospital of Guangzhou Medical University, including adults referred for polysomnography (PSG) evaluation because of snoring or daytime sleepiness and the suspicion of sleep-disordered breathing by the primary care physician. The procedures followed in this study were in accordance with the Declaration of Helsinki, approved by the Ethics Committee of the First Affiliated Hospital of Guangzhou Medical University, Ethics Approval No. 05, 2017, and all patients have signed informed consent forms. The primary inclusion criteria were adults diagnosed with OSA, with completed overnight PSG data, completely independent behavioral and cognitive functioning, and electronic or paper records of past medical history for diagnosing other comorbidities such as hypertension and diabetes. The exclusion criteria were (1) previous diagnosis of or treatment for OSA; (2) respiratory events dominated by central or mixed sleep apnea; (3) total sleep time less than 6 hours; (4) usage of hypnotics before PSG; and (5) patients with restless leg syndrome, narcolepsy and other sleep disorders. All participants met the inclusion and exclusion criteria. The flow chart of this study is shown in .
Data Extraction
We retrospectively collected the variables associated with OSA: demographic characteristics and lifestyles, including age, gender, weight, body mass index (BMI), smoking history, and alcohol consumption. After checking the medical history, we confirmed the comorbidities of hypertension, diabetes, coronary heart disease (CHD) and cerebrovascular disease (CVD). A typical definition of hypertension relies on an average systolic BP (SBP) of ≥140 mm Hg, diastolic BP (DBP) of ≥90 mm Hg, or self-reported usage of antihypertensive medication.Citation23 The diagnostic criteria for diabetes mellitus are as follows: Fasting Plasma Glucose (FPG): Fasting blood glucose level ≥7.0 mmol/L (126 mg/dL). Oral Glucose Tolerance Test (OGTT): 2-hour postprandial blood glucose level ≥11.1 mmol/L (200 mg/dL). Random Plasma Glucose: Random blood glucose level ≥11.1 mmol/L (200 mg/dL) in the presence of classic symptoms of diabetes like polyuria, polydipsia, unexplained weight loss, etc. Meeting any one of the above criteria may lead to a preliminary diagnosis of diabetes mellitus.Citation24 CHD is defined as a cardiovascular disease caused by reduced blood flow to the heart muscle due to narrowed or blocked coronary arteries, and CVD as the diseases of the blood vessels supplying the brain, including stroke.Citation25 Data extraction from PSG included AHI, oxygen desaturation index (ODI), total sleep time spent with arterial oxygen saturation <90% (T90%), wakeup after sleep onset (WASO), the number of microarousal events per hour of recording (MAI), the percentage of stage 1, 2, 3 and rapid eye movement sleep in total sleep time (N1%, N2%, N3%, REM%), mean pulse oxygen saturation (MSpO2), lowest pulse oxygen saturation (LSpO2) and the Fhypopnea, which is defined as the fraction of the respiratory events that were hypopnea.
PSG Parameters
Sleep studies were performed at sleep center using Alice 6 PSG (Philips Wellcome, USA) for at least 7 hours, and the use of alcohol, coffee, sedatives and hypnotics was prohibited on the same day. The monitoring indicator included electroencephalogram, electromyography, blood oxygen saturation, electrooculogram, electrocardiogram, snoring, mouth airflow, nasal airflow, chest breathing and body position.Citation26 All data were separately manually analyzed by two trained sleep professionals, according to the American Academy of Sleep Medicine Manual for Scoring Sleep and Associated events in 2017.Citation27 The minimum duration of respiratory events was 10s. Apneas were defined as a cessation of airflow for 10s, while hypopneas were defined as a reduction of the airflow signal amplitude ≥30%, associated with a 3% oxygen desaturation. The AHI was calculated as the number of apneas and hypopneas divided by the recording duration in hours. OSA was defined as an AHI ≥ 5 events/h. AHI ≥5 and <15 events/h, AHI ≥15 and <30 events/h and AHI ≥30 events/h were classified as low, moderate and high, respectively.
Arousal Threshold
To assess the AT, we used a simple and novel method based on PSG data and clinical characteristics which has been widely used in previous studies.Citation28,Citation29 Low AT was determined in a paitent with OSA if two or more than two of the following three criterion were met: AHI < 30 events/h, a nadir SpO2 >82.5%, and the proportion of hypopneas >58.3%. Otherwise, the rest were considered to have high AT.Citation30 In addition, as the multiple logistic regression shown in the study of Edwards et al, the formula for quantifying AT score is as followed (where male sex = 1 and female sex = 0):Citation31,Citation32
As a higher arousal threshold indicates a larger absolute value, in order to better clarify and understand the relationship between the AT score and different variables, we converted the AT score to a positive absolute number. We defined low AT as less than 15 scores, high AT as 15 to 30 scores, and very high AT as larger than 30 scores, respectively.
Statistical Analysis
In order to minimize the confounding effect of demographic characteristics and hypoxia-related PSG parameters on the relationship between high AT and various comorbidities, we performed a 1:1 propensity score matching (PSM) with a caliper value of 0.01. The selected matching factor were based on risk factor affecting the occurrence of hypertension and diabetes derived from previous studies,Citation33,Citation34 including age, gender, BMI, smoking, alcohol consumption, ODI and T90%. All analyses were conducted using SPSS version 26.0 software. The continuous data showed non-normal distributions and thus were presented as median (interquartile range, IQR: P25, P75). The non-parametric tests and Mann–Whitney U-test were employed to compare group difference. Categorical variables were expressed as counts (%) and compared between groups using the chi-square test for valuating unadjusted odds ratio (OR). The multiple logistic regression models were used to examine the adjusted OR of high AT (reference as low AT), very high and high AT score (reference as low AT score), high AHI (reference as low AHI) for different comorbidities. ODI and T90% were selected as confounding factors to adjusted for the indicator of hypoxia. Model 1 was adjusted for age, BMI, male%, smoking, alcohol consumption; Model 2 was adjusted for ODI and T90% plus all covariates in Model 1. The P value of less than 0.05 was determined to be statistically significant.
Results
Participants
The characteristics of participants categorized by the levels of AT are shown in . A total of 3400 participants were recruited, with 1845 classified in the high AT group and 1555 in the low AT group. After performing PSM, both groups included 938 subjects and their age, ODI, BMI, the proportion of male, smoking history and alcohol consumption history were comparable and did not show statistical significance. Statistically significant differences (P < 0.001) were still observed in AHI (39.1 vs 14.9 events/h), LSpO2 (74.5 vs 84.0%), and Fhypopnea (38.6 vs 68.6%) between high and low AT group, since the assessment of arousal threshold was based on these three features. The AT score of high and low AT group was 21.7 (17.2, 28.7) and 12.2 (9.4, 15.1), respectively. In high AT group, 299 (31.9%) and 79 (8.4%) patients had the coexistence of hypertension and diabetes, while 250 (26.7%) and 55 (5.9%) patients were complicated with hypertension and diabetes, respectively, in low AT group. The occurrence of hypertension and diabetes were statistically different between the two groups (P = 0.013, P = 0.031), and there were no statistical significance in CHD and CVD after PSM.
Table 1 Baseline Characteristics of Participants with OSA Stratified by AT Before PSM (n=3400) and After PSM (n=1876)
The Association Between High AT and the Occurrence of Hypertension, Diabetes
The results of multivariate logistic regression adjusted for age, BMI, male%, smoking, alcohol consumption, ODI, and T90% are displayed in . Before PSM, patients with high AT had an increased occurrence of hypertension with an unadjusted OR of 1.32 (95% CI = 1.14–1.54) (P < 0.01) and adjusted OR of 1.36 (95% CI = 1.14–1.62) (P < 0.01). After PSM, the results were still statistically significant, although the unadjusted and adjusted OR decreased to 1.29 (95% CI = 1.06–1.55) (P < 0.05) and 1.31 (95% CI = 1.07–1.62) (P < 0.01), respectively. On the other hand, high AT was not associated with the higher prevalent diabetes before PSM, while the results after PSM indicated statistically significant relationships with the unadjusted OR of 1.48 (95% CI = 1.03–2.11) (P < 0.05) and adjusted OR of 1.45 (95% CI = 1.01–2.08) (P < 0.05), respectively.
Table 2 The Relationship Between High at and the Occurrence of Hypertension, Diabetes, CHD and CVD in Patients with OSA Before and After PSM, Compared with Low AT
Subgroup analyses were subsequently conducted, the result of male subgroup showed that high AT was a risk factor for hypertension and diabetes (Table S1). In subgroup analyses according to BMI and age, only high AT was associated with the more frequency of hypertension in BMI < 27 kg/m2 and Age <60 years subgroups (Tables S2 and S3).
The Association of AT Score and AHI with the Occurrence of Hypertension and Diabetes
Compared with low AT score (AT score ≤ 15) after PSM, high- (15 ≤ AT score < 30) and very high AT score (30 ≤ AT score) were both significantly associated with hypertension, and the association was strengthened with the increase of AT score (). In parallel, only high AHI (AHI ≥ 30) had a higher odds of hypertension than low AHI (5 ≤ AT score < 15) (). The results of subgroups by gender, BMI and age for AT score were similar to those for high AT (compared with low AT), but the stepwise increase in the odds of hypertension were more remarkable, especially in BMI < 27 kg/m2 subgroup (Tables S4-S6). All ORs were similar in both Model 1 and Model 2.
Table 3 The Relationship Between the Different Threshold of AT Score, AHI and the Occurrence of Hypertension in Patients with OSA (After PSM)
For another, the stratification of AHI did not effectively identify the prevalent diabetes (). Compared with low AT score, patients with OSA and very high AT score were more likely to comorbid with diabetes, with the OR of 1.79 (Model 2, 95% CI = 1.02–3.13, P < 0.05). The OR dramatically increased to 3.58 (Model 2, 95% CI = 1.06–12.09, P < 0.05) in the subgroup of Age >60 years (Table S7). There were no significant differences between the subgroups by gender and BMI (Tables S8 and S9).
Table 4 The Relationship Between the Different Threshold of AT Score, AHI and the Occurrence of Diabetes in Patients with OSA (After PSM)
Discussion
This retrospective study evaluated the relationship between AT and the comorbidities including hypertension and diabetes in patients with OSA. Additionally, AT score was served as a potential predictor for exploring the deeper association. In the study, we found a stronger correlation between higher AT and the occurrence of hypertension and diabetes in OSA patients than that of AHI. When classified all patients according to AT score, the result suggested that more frequency of hypertension and diabetes were observed in those with very high AT score, compared to low AT score group. Previous study indicated that patients with OSA and high AT combined with a higher prevalence of hypertension and diabetes, but there were no statistically significant after adjustment for BMI.Citation22 PSM was performed to minimize the selection bias and confounding effect from demographic characteristics and hypoxia indicators in our study, in order to better evaluate the potential relationship between AT and comorbidities.
OSA is an independent risk factor for a number of comorbidities, strongly associated with higher rates of cardiovascular mortality.Citation2 However, there are few clinical indicators for identifying the presence of comorbidities in OSA. Arousal threshold is a new indicator to assess the endotype of OSA, which contributes to increased duration of nocturnal apnea, and the potential mechanisms may involve altered central chemosensitivity and upper airway collapse, dysfunction of peripheral mechanoreceptors, or altered mechanisms of arousal.Citation30,Citation35 According to the gold standard for measuring AT, patients with OSA and high AT need increased stimuli (ie, respiratory drive and negative chest pressure) for achieving arousal, requiring an increase in the recruitment of upper airway dilators, which subsequently result in prolonged duration of respiratory events, lower oxygen saturation, hypercapnia and sympathetic activation.Citation36 Although AT means the average negative chest pressure before cortical awakenings, which indicates that it would be more difficult to wake up during a respiratory event, there is no absolute dose effect between AT and the number of arousals. For one thing, short-term arousals may be frequently occurred, served as a protective factor for alleviating severe respiratory events and restoring ventilation, especially in those with high AHI (or more obstructive events) and obesity hypoventilation syndrome.Citation13,Citation19 For another, pharyngeal vibration or the loud noise before airway opening can contribute to the arousals in some patients with severe obstructive events.Citation36 Therefore, patients with high AT may suffer from high AHI due to more severe upper airway collapse, with louder snoring and greater intensity of upper airway muscle vibration, leading to more arousals and sleep fragmentation.Citation37 In addition, chronic intermittent hypoxia induced by OSA leads to hyperactivation of the carotid body (CB), which reflexively causes sympathetic excitation and makes a contribution to the maintenance of glucose homeostasis.Citation38 CB chemoreceptor dysregulation may be one of the mechanisms mediating the co-occurrence of OSA with diabetes and hypertension.Citation38 These mechanisms are possible mediators of the association between high AT and the higher occurrence of comorbidities. Meanwhile, whether the intensity of arousal has an additional effect on the occurrence of comorbidities, and whether the distribution of arousal types is different between patients with low and high AT is still unknown, which needs more studies to demonstrate.
This study showed that high AT was significantly and positively associated with the occurrence of hypertension in patients with OSA. Previous studies have reported that sleep-related hypoxia indicators such as T90% and hypoxic burden have the similar association.Citation6,Citation39 Hypoxia causes an increase in nocturnal blood pressure by promoting cortex arousals and inducing sympathetic nervous activity, which may eventually lead to resistant hypertension.Citation40 In addition to prolonged and deeper hypoxia, increased negative intrathoracic pressure and respiratory effort due to elevated AT not only produce sympathetic activation and hypercapnia, but also significant shear stress and vascular wall remodeling, which are also potential mechanisms favoring the development of hypertension.Citation41,Citation42 On the other side, our subgroup analyses indicated that the positive association between high AT and hypertension was only observed in male patients with less obese and young age. A possible explanation could be that progesterone and estrogen in women augment the arousal response to transient hyperventilation and hypercapnia associated with apneic episodes.Citation43 However, the interaction between high AT and low obesity (or young age) in patients with OSA and hypertension is unclear.
There were a number of longitudinal cohort studies indicating a significant association between OSA and increasing risk of diabetes.Citation44 The precise pathophysiological and causative correlation between dysregulated glucose metabolism and obstructive sleep apnea (OSA) remain uncertain. Several potential mechanisms, including OSA-induced intermittent hypoxia, hypercapnia, sympathetic arousal, sleep fragmentation, and inflammation, make contributions to insulin resistance and β-cell dysfunction.Citation45 The study by Oltmanns KM et al demonstrated that the severity of hypoxia and the duration of exposure may have an adverse impact on insulin sensitivity. Muraki et al reported that nocturnal intermittent hypoxia in OSA (ODI ≥ 15) was positively associated with diabetes.Citation46 For another, the CB chemoreceptor dysregulation due to OSA-induced hypercapnia and hypoxia mediates insulin resistance and dysglycemia.Citation47 It has been shown that sympathetic activation is observed in patients with type 2 diabetes, and that high plasma insulin levels and insulin resistance are closely related and interact with each other.Citation48 Sympathetic excitation causes adrenaline release, which decreases glucose metabolism and decreases insulin-mediated glucose uptake in skeletal muscle cells, causing insulin resistance.Citation49 In theory, patients with OSA and higher AT have lower oxygen saturation, more arousals for ventilation restore and more severe hypercapnia, with prolonged respiratory events. Thus, AT may be a more comprehensive indicator for better assessing the relationship between OSA and diabetes than AHI, which as our study showed.
Low AT is considered to be one of the most important endotypes in OSA.Citation12 Nevertheless, there is no clear definition for the remaining OSA population with non-low AT. In our study, based on AT scores, we found that compared to low or high AT, very high AT (AT scores ≥ 30) seemed to be an another pathophysiological feature, closely associated with hypertension and diabetes. As low and very high AT are related to various complications in OSA, it is possible that the high AT (15 ≤ AT score < 30) is the normal AT level range, similar to blood pressure and glucose, which can be classified into three levels. This hypothesis requires more prospective studies to compare the prognosis of patients with different AT levels by means of measuring intrathoracic negative pressure.
Previous research indicated AT was found to drop during continuous positive airway pressure (CPAP) therapy, supporting the theory that more severe OSA is associated with higher AT.Citation36 By lowering the AT to normal levels, we hypothesize that the duration of airway obstruction becomes shorter and carotid body modulation is more stable, with reduced hypoxia, carbon dioxide retention and sympathetic activation, which needs to be proven by more research. According to our study, the coexistence of diabetes and hypertension were more observed in patients with higher AT, which highlights the necessity for them to be evaluated as suitable candidates for receiving CPAP therapy. It has been suggested that CPAP treatment could significantly reduce blood pressure, plasma norepinephrine and sympathetic activity.Citation50,Citation51 Above all, the classification of participants based on AHI may not effectively identify individuals at the higher prevalence of comorbid hypertension and diabetes. Prospectively accessing the AT of patients with OSA may help determine whether individuals with a higher AT benefit more from CPAP than those with a lower AT.
There are some limitations in our study. Firstly, it was a retrospective study and could not determine the causation between high AT and other comorbidities. For another, some patients in retrospective study may be missed for hypertension and other complications, which can lead to an underestimation of true effect. Secondly, the definition of high AT and the measurement of AT score were based on previous studies, instead of the gold standard. Moreover, we did not record the medication history of included patients for hypertension and diabetes, which may influence the results to some extent.
Conclusions
High AT is significantly associated with increased prevalent hypertension and diabetes in patients with OSA. Compared with AHI, AT score is a potentially comprehensive indicator for better evaluating the relationship between OSA and related comorbidities. More large-scale studies are needed to verify the application value of AT score and the correlation between AT and comorbidities in OSA.
Disclosure
The authors have no conflicts of interest to report for this work.
Acknowledgments
We would like to thank Professor Nanshan Zhong from State Key Laboratory of Respiratory Disease for the constructive advice he gave. We thank Home for Researchers editorial team for language editing service.
Data Sharing Statement
The data used to support the findings of this study are available from the corresponding author upon request.
Additional information
Funding
References
- Benjafield AV, Ayas NT, Eastwood PR, et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: a literature-based analysis. Lancet Respir Med. 2019;7(8):687–698. doi:10.1016/S2213-2600(19)30198-5
- Gottlieb DJ, Yenokyan G, Newman AB, et al. Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: the sleep heart health study. Circulation. 2010;122(4):352–360. doi:10.1161/CIRCULATIONAHA.109.901801
- Xu PH, Hui CKM, Lui MMS, Lam DCL, Fong DYT, Ip MSM. Incident type 2 diabetes in OSA and effect of CPAP treatment: a retrospective clinic cohort study. Chest. 2019;156(4):743–753. doi:10.1016/j.chest.2019.04.130
- Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365(9464):1046–1053. doi:10.1016/S0140-6736(05)71141-7
- Qaseem A, Dallas P, Owens DK, Starkey M, Holty JEC, Shekelle P. Diagnosis of obstructive sleep apnea in adults: a clinical practice guideline from theAmerican college of physicians. Ann Intern Med. 2014;161(3):210. doi:10.7326/M12-3187
- Højager A, Schoos MM, Tingsgaard PK, Bock TG, Homøe P. Estimates of 10-year risk of cardiovascular death and adherence to cardiovascular risk factor management in Danish patients investigated for obstructive sleep apnea. Sleep Med. 2023;104:22–28. doi:10.1016/j.sleep.2023.02.009
- Azarbarzin A, Sands SA, Stone KL, et al. The hypoxic burden of sleep apnoea predicts cardiovascular disease-related mortality: the osteoporotic fractures in men study and the sleep heart health study. Eur Heart J. 2019;40(14):1149–1157. doi:10.1093/eurheartj/ehy624
- Guo J, Xiao Y. New metrics from polysomnography: precision medicine for OSA interventions. Nat Sci Sleep. 2023;Volume 15:69–77. doi:10.2147/NSS.S400048
- Labarca G, Vena D, Hu WH, et al. Sleep apnea physiological burdens and cardiovascular morbidity and mortality. Am J Respir Crit Care Med. 2023;208(7):802–813. doi:10.1164/rccm.202209-1808OC
- Cao W, Luo J, Huang R, Xiao Y. The association between sleep breathing impairment index and cardiovascular risk in male patients with obstructive sleep apnea. Nat Sci Sleep. 2022;Volume 14:53–60. doi:10.2147/NSS.S343661
- Trzepizur W, Blanchard M, Ganem T, et al. Sleep apnea-specific hypoxic burden, symptom subtypes, and risk of cardiovascular events and all-cause mortality. Am J Respir Crit Care Med. 2022;205(1):108–117. doi:10.1164/rccm.202105-1274OC
- Eckert DJ. Phenotypic approaches to obstructive sleep apnoea - new pathways for targeted therapy. Sleep Med Rev. 2018;37:45–59. doi:10.1016/j.smrv.2016.12.003
- Eckert DJ, White DP, Jordan AS, Malhotra A, Wellman A. Defining phenotypic causes of obstructive sleep apnea. Identification of novel therapeutic targets. Am J Respir Crit Care Med. 2013;188(8):996–1004. doi:10.1164/rccm.201303-0448OC
- Zavodny J, Roth C, Bassetti CL, Mathis J, Douglas NJ, Gugger M. Effects of sleep fragmentation on the arousability to resistive loading in NREM and REM sleep in normal men. Sleep. 2006;29(4):525–532. doi:10.1093/sleep/29.4.525
- Antonaglia C, Passuti G, Giudici F, et al. Low arousal threshold: a common pathophysiological trait in patients with obstructive sleep apnea syndrome and asthma. Sleep Breath. 2023;27(3):933–941. doi:10.1007/s11325-022-02665-4
- Yamaguchi Y, Shiota S, Kusunoki Y, et al. Polysomnographic features of low arousal threshold in overlap syndrome involving obstructive sleep apnea and chronic obstructive pulmonary disease. Sleep Breath. 2019;23(4):1095–1100. doi:10.1007/s11325-019-01786-7
- Hang LW, Huang CS, Cheng WJ. Clinical characteristics of Asian patients with sleep apnea with low arousal threshold and sleep structure change with continuous positive airway pressure. Sleep Breath. 2021;25(3):1309–1317. doi:10.1007/s11325-020-02235-6
- Ayas NT, Brown R, Shea SA. Hypercapnia can induce arousal from sleep in the absence of altered respiratory mechanoreception. Am J Respir Crit Care Med. 2000;162(3):1004–1008. doi:10.1164/ajrccm.162.3.9908040
- Heinzer RC, White DP, Jordan AS, et al. Trazodone increases arousal threshold in obstructive sleep apnoea. Eur Respir J. 2008;31(6):1308–1312. doi:10.1183/09031936.00067607
- Eckert DJ, Younes MK. Arousal from sleep: implications for obstructive sleep apnea pathogenesis and treatment. J Appl Physiol. 2014;116(3):302–313. doi:10.1152/japplphysiol.00649.2013
- Kario K, Hettrick DA, Prejbisz A, Januszewicz A. Obstructive sleep apnea–induced neurogenic nocturnal hypertension: a potential role of renal denervation? Hypertension. 2021;77(4):1047–1060. doi:10.1161/HYPERTENSIONAHA.120.16378
- Zinchuk A, Edwards BA, Jeon S, et al. Prevalence, associated clinical features, and impact on continuous positive airway pressure use of a low respiratory arousal threshold among male United States veterans with obstructive sleep apnea. J Clin Sleep Med. 2018;14(05):809–817. doi:10.5664/jcsm.7112
- Bakris G, Ali W, Parati G. ACC/AHA versus ESC/ESH on hypertension guidelines: JACC guideline comparison. J Am Coll Cardiol. 2019;73(23):3018–3026. doi:10.1016/j.jacc.2019.03.507
- American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes—2018. Diabetes Care. 2018;41(Supplement_1):S13–S27. doi:10.2337/dc18-S002.
- D’Agostino RB, Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: the Framingham heart study. Circulation. 2008;117(6):743–753. doi:10.1161/CIRCULATIONAHA.107.699579
- Berry RB, Budhiraja R, Gottlieb DJ, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM manual for the scoring of sleep and associated events. deliberations of the sleep apnea definitions task force of the American academy of sleep medicine. J Clin Sleep Med. 2012;08(05):597–619. doi:10.5664/jcsm.2172
- Berry RB, Brooks R, Gamaldo C, et al. AASM scoring manual updates for 2017 (Version 2.4). J Clin Sleep Med. 2017;13(5):665–666. doi:10.5664/jcsm.6576
- Huang W, Wang X, Xu C, et al. Prevalence, characteristics, and respiratory arousal threshold of positional obstructive sleep apnea in China: a large scale study from shanghai sleep health study cohort. Respir Res. 2022;23(1):240. doi:10.1186/s12931-022-02141-3
- Fu X, Li J, Wu JJ, et al. Reduced cortical arousability to nocturnal apneic episodes in patients with wake-up ischemic stroke. Sleep Med. 2020;66:252–258. doi:10.1016/j.sleep.2019.09.007
- Edwards BA, Eckert DJ, McSharry DG, et al. Clinical predictors of the respiratory arousal threshold in patients with obstructive sleep apnea. Am J Respir Crit Care Med. 2014;190(11):1293–1300. doi:10.1164/rccm.201404-0718OC
- Thomson LDJ, Landry SA, Singleton R, et al. The effect of hypopnea scoring on the arousal threshold in patients with obstructive sleep apnea. Am J Respir Crit Care Med. 2020;202(9):1308–1311. doi:10.1164/rccm.202003-0589LE
- Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. the report of an American academy of sleep medicine task force. Sleep. 1999;22(5):667–689. doi:10.1093/sleep/22.5.667
- Shen Y, Chang C, Zhang J, Jiang Y, Ni B, Wang Y. Prevalence and risk factors associated with hypertension and prehypertension in a working population at high altitude in China: a cross-sectional study. Environ Health Preventative Med. 2017;22(1):19. doi:10.1186/s12199-017-0634-7
- Labarca G, Campos J, Thibaut K, Dreyse J, Jorquera J. Do T90 and SaO2 nadir identify a different phenotype in obstructive sleep apnea? Sleep Breath. 2019;23(3):1007–1010. doi:10.1007/s11325-019-01860-0
- Sforza E, Krieger J, Petiau C. Nocturnal evolution of respiratory effort in obstructive sleep apnoea syndrome: influence on arousal threshold. Eur Respir J. 1998;12(6):1257–1263. doi:10.1183/09031936.98.12061257
- Younes M. Role of arousals in the pathogenesis of obstructive sleep apnea. Am J Respir Crit Care Med. 2004;169(5):623–633. doi:10.1164/rccm.200307-1023OC
- Kohler M, Stradling JR. Mechanisms of vascular damage in obstructive sleep apnea. Nat Rev Cardiol. 2010;7(12):677–685. doi:10.1038/nrcardio.2010.145
- Conde SV, Sacramento JF, Guarino MP, et al. Carotid body, insulin, and metabolic diseases: unraveling the links. Front Physiol. 2014;5:418. doi:10.3389/fphys.2014.00418
- Wang L, hui WD, Zhang J, Cao J. Time under 90% oxygen saturation and systemic hypertension in patients with obstructive sleep apnea syndrome. Nat Sci Sleep. 2022;Volume 14:2123–2132. doi:10.2147/NSS.S388238
- Brown J, Yazdi F, Jodari-Karimi M, Owen JG, Reisin E. Obstructive sleep apnea and hypertension: updates to a critical relationship. Curr Hypertens Rep. 2022;24(6):173–184. doi:10.1007/s11906-022-01181-w
- Eskandari D, Zou D, Grote L, Schneider H, Penzel T, Hedner J. Independent associations between arterial bicarbonate, apnea severity and hypertension in obstructive sleep apnea. Respir Res. 2017;18(1):130. doi:10.1186/s12931-017-0607-9
- Martinot JB, Le-Dong NN, Malhotra A, Pépin JL. Respiratory effort during sleep and prevalent hypertension in obstructive sleep apnoea. Eur Respir J. 2023;61(3):2201486. doi:10.1183/13993003.01486-2022
- Behan M, Wenninger JM. Sex steroidal hormones and respiratory control. Respir Physiol Neurobiol. 2008;164(1–2):213–221. doi:10.1016/j.resp.2008.06.006
- Marshall NS, Wong KKH, Phillips CL, Liu PY, Knuiman MW, Grunstein RR. Is sleep apnea an independent risk factor for prevalent and incident diabetes in the Busselton Health Study? J Clin Sleep Med JCSM off Publ Am Acad Sleep Med. 2009;5(1):15–20.
- Reutrakul S, Mokhlesi B. Obstructive sleep apnea and diabetes: a state of the art review. Chest. 2017;152(5):1070–1086. doi:10.1016/j.chest.2017.05.009
- Muraki I, Tanigawa T, Yamagishi K, et al. Nocturnal intermittent hypoxia and metabolic syndrome; the effect of being overweight: the CIRCS study. J Atheroscler Thromb. 2010;17(4):369–377. doi:10.5551/jat.3319
- Joon Yun A, Lee PY, Bazar KA. Autonomic dysregulation as a basis of cardiovascular, endocrine, and inflammatory disturbances associated with obstructive sleep apnea and other conditions of chronic hypoxia, hypercapnia, and acidosis. Med Hypotheses. 2004;62(6):852–856. doi:10.1016/S0306-9877(03)00322-0
- Grassi G, Biffi A, Dell’Oro R, et al. Sympathetic neural abnormalities in type 1 and type 2 diabetes: a systematic review and meta-analysis. J Hypertens. 2020;38(8):1436–1442. doi:10.1097/HJH.0000000000002431
- Jamerson KA, Julius S, Gudbrandsson T, Andersson O, Brant DO. Reflex sympathetic activation induces acute insulin resistance in the human forearm. Hypertension. 1993;21(5):618–623. doi:10.1161/01.hyp.21.5.618
- Hedner J, Darpö B, Ejnell H, Carlson J, Caidahl K. Reduction in sympathetic activity after long-term CPAP treatment in sleep apnoea: cardiovascular implications. European Respiratory Journal. 1995;8(2):222–229. doi:10.1183/09031936.95.08020222
- Mills PJ, Kennedy BP, Loredo JS, Dimsdale JE, Ziegler MG. Effects of nasal continuous positive airway pressure and oxygen supplementation on norepinephrine kinetics and cardiovascular responses in obstructive sleep apnea. J Appl Physiol. 2006;100(1):343–348. doi:10.1152/japplphysiol.00494.2005