Abstract
Introduction
Continuous positive airway pressure (CPAP) therapy improves clinical symptoms in patients with obstructive sleep apnea (OSA); however, the mechanism of this clinical improvement and how it may be associated with the restoration of white matter (WM) structures in the brain is unclear. Therefore, this study investigated the relationship between the structural recovery of brain WM and improvements in cognitive function and emotion after long-term (12 months) CPAP treatment in patients with OSA.
Methods
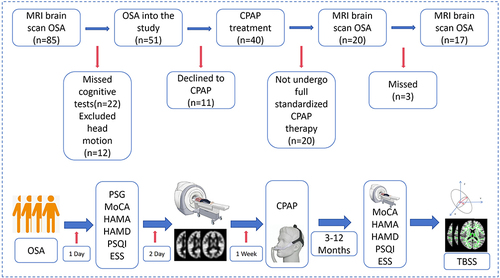
We collected data from 17 patients with OSA before and 12 months after CPAP treatment, including sleep monitoring, clinical assessment, and diffusion tensor imaging (DTI) magnetic resonance imaging.
Results
We observed a partial reversible recovery of brain WM (mean and radial diffusion coefficients) after treatment. This recovery involved the commissural fibers (cingulum, body of corpus callosum), projection fibers (retrolenticular part of the internal capsule, posterior thalamic radiation, posterior limb of the internal capsule, superior corona radiata, posterior corona radiata), association fibers (external capsule, superior longitudinal fasciculus, inferior longitudinal fasciculus), and other regions. In addition, the improvements in WM fibers in one part of the brain significantly were correlated with the Hamilton Anxiety Scale and Hamilton Depression Scale scores.
Discussion
Our results suggest that reversible recovery of reduced brain WM integrity due to OSA may require longer CPAP treatment. Moreover, changes in the integrity of the commissural fibers were associated with emotion regulation. These restored WM areas may explain the cognitive and mood improvements observed after OSA treatment.
Introduction
Obstructive sleep apnea (OSA) is a common sleep disorder with serious health implications. Approximately 936 million people worldwide have OSA, which is a significant medical and social problem.Citation1 OSA manifests as frequent episodes of intermittent apnea during sleep, leading to arousal, sleep fragmentation, and poor sleep quality.Citation2 These clinical signs are associated with the narrowing of the airways, impairment of muscular responses, and respiratory drive, leading to pharyngeal collapse.Citation3 Chronic sleep disorders such as OSA can lead to cognitive and affective dysfunction, including reduced learning ability, poor concentration, memory, executive function, attention, visuospatial skills, anxiety, and depression.Citation4 Sleep fragmentation and intermittent nocturnal hypoxia are major contributors to cognitive and affective impairment.Citation5 Intermittent hypoxia can cause oxidative stress and hypercapnia, which can lead to neurological damage in patients with OSA, including neuronal loss and reduced structural stability of the synapses.Citation6 However, the neuroimaging mechanisms underlying cognitive impairments and mood disorders are not fully understood.
Surgical and non-invasive procedures have been used to treat patients with OSA.Citation7 Currently, continuous positive airway pressure (CPAP) is the gold standard in the treatment of OSA; its mechanism of action is to improve airway collapse in patients by applying airflow pressure during sleep,Citation8 which can effectively improve sleep quality and reduce daytime sleepiness and emotional and neurocognitive abnormalities.Citation9 Recent evidence suggests that CPAP has a positive effect on cognitive and mood improvement,Citation10 but not in all patients.Citation11 Although some of the reasons may be poor patient compliance, resulting in poor CPAP efficacy,Citation12 this does not explain the efficacy response in some patients with significantly improved clinical parameters.Citation13,Citation14 Additionally, variability in the degree of neuronal degeneration in patients with OSA of different severities may explain the heterogeneity in recovery from cognitive impairment with CPAP treatment.Citation15 These differences in cognitive recovery and reversible changes in brain function and structure after CPAP treatment in patients with OSA have become a popular research topic.
Neuroimaging techniques have introduced new ideas to the study of neural mechanisms using structural and functional magnetic resonance imaging (MRI) to explore the relationships between cognitive function, emotion, and the brain.Citation16 Resting-state functional magnetic resonance imaging (fMRI) studies have shown that improvements in cognitive dysfunction in patients with OSA treated with CPAP are associated with functional changes in local brain regions, between brain regions, and between brain networks,Citation17–19 involving the default network,Citation20 sensorimotor networkCitation21 and limbic system.Citation22 While these studies provide evidence of improved brain function after CPAP treatment, improved function does not imply reversible restoration of brain structure. Several morphological studies have provided new evidence for structural brain recovery associated with CPAP treatment. Canessa et al found that increased hippocampal and frontal gray matter volume in patients with OSA after treatment was associated with improved memory and attention and attributed the reversible restoration of brain structure to improved intermittent hypoxia.Citation23 Kim et al reported that structural atrophy in the frontal lobes and hippocampus was partially restored after CPAP treatment, with a compensatory recovery of structures in other brain regions.Citation24 Kumar et al observed that MRI showed significantly higher T2 relaxation values in more brain regions (the amygdala and hippocampus) in a patient with OSA with anxiety symptoms, confirming that the presence of anxiety symptoms may be associated with structural damage to the limbic system.Citation25
Tract-based spatial statistical analysis (TBSS), a technique for analyzing diffusion tensor imaging (DTI) metrics across the white matter (WM) fiber tract skeleton of the brain without a priori assumptions,Citation26 allows for a more reliable alignment of WM fiber tracts, alleviating the problem of obtaining optimal anatomical correspondence between subjects.This technique has been applied to investigate the relationship between changes in WM fiber tracts in the brains of patients with OSA and cognitive function and mood disorders.Citation27,Citation28 However, few treatment-related DTI studies have considered CPAP. Zhang et al observed an association between reversible changes in WM fiber tracts and residual somnolence after one month of CPAP treatment in patients with OSA based on DTI analysis; that is, the poor treatment response in the residual somnolence group was associated with decreased in WM fiber tract integrity in a specific brain region.Citation29 Another DTI-based study found that three months of CPAP treatment only partially restored the reduced integrity of diffuse WM fiber tracts in patients with OSA compared with that before treatment.Citation30 However, we did not observe similar results in our previous study, including no reversible restoration of WM fiber bundle integrity in patients with OSA after short-term (three months) CPAP treatment.Citation31 Moreover, few studies have investigated whether the structure of WM fiber tracts is reversed after long-term CPAP treatment.
Based on these findings, we hypothesized that long-term CPAP treatment reversibly restores the reduced integrity of cerebral WM fibers in patients with moderate-to-severe OSA and that these reversibly restored cerebral WM fibers may be related to cognitive function and emotional disorders. To test this hypothesis, we used the TBSS technique to investigate reversible changes in cerebral WM fiber structure after long-term (12-month) CPAP treatment in patients with moderate-to-severe OSA. We then assessed the correlation between reversible changes in cerebral WM fiber structure and clinical scale scores in these patients.
Materials and Methods
This study adhered to the tenets of the principles of the Declaration of Helsinki and was approved by the Medical Ethics Committee of the First Affiliated Hospital of Nanchang University [2020 (94)]. Written informed consent was obtained from all participants.
shows the recruitment flowchart for this longitudinal study. From August 2020 to August 2023, all patients with OSA were enrolled in the Sleep Monitoring Centre of the Department of Respiratory Medicine, First Affiliated Hospital of Nanchang University, and all patients underwent polysomnography on the same night. The diagnostic criteria were the 2017 American Academy of Sleep Medicine Clinical Practice Guidelines for Obstructive Apnea in Adults. Inclusion criteria were apnea-hypopnea index (AHI) >15, age 20–60 years, right-handed, and standard CPAP treatment for 12 months (4 hours per night, at least 5 days per week). The exclusion criteria were (1) sleep disorders other than OSA, such as insomnia and narcolepsy; (2) respiratory diseases, diabetes mellitus, hypothyroidism, and history of previous CPAP treatment; (3) history of central nervous system disorders, including neurodegenerative disorders, epilepsy, brain tumors, cerebral infarction, and traumatic brain injury; (4) abuse of prohibited drugs or current use of psychotropic medications; (5) psychiatric disorders, including depression and bipolar disorder; (6) MRI contraindications such as internal metal implants and claustrophobia; (7) motion artefacts; and (8) missed examination visits. Finally, this study included 17 patients with OSA who had been treated with CPAP for 12 months.
Neuropsychological Assessment and Polysomnography
The patients completed neuropsychological assessments at baseline and at the 12-month follow-up, including the Montreal Cognitive Assessment (MoCA), Epworth Sleepiness Scale (ESS), Pittsburgh Sleep Quality Index (PSQI), Hamilton Anxiety Scale (HAMA), and Hamilton Depression Scale (HAMD).
The MoCA assesses eight cognitive domains: visuospatial and executive function, naming, memory, attention, language, abstract thinking, calculation, and orientation. The patients are assessed in a quiet environment. The scale has a total score of 30 points, with ≥26 points defined as normal and scores of <26 points suggesting cognitive impairment.Citation32
The ESS contains eight different categories used to assess daytime sleepiness in patients with OSA and has a total score of 24 points (three points per category), with 0, 6, 11, and 16 points corresponding to four different levels of sleepiness. The PSQI is used to assess sleep quality on a scale of 0–21, with higher scores indicating poorer sleep quality.Citation33
The HAMA and HAMD are used to assess patients’ anxiety and depression symptoms, respectively. The HAMA consists of 14 items to assess somatic and psychogenic anxiety. Each item is scored on a five-point scale from 0 to 4, with a total score >29 indicating probable severe anxiety. The HAMD consists of 17 items to assess somatic and psychogenic anxiety. Each item is scored on a five-point scale ranging from 0 to 4, with scores >24, 17–23, 8–16, and 0–7 indicating severe, moderate, mild, and no depression, respectively.Citation34
To exclude sleep disorders other than OSA, all participants underwent polysomnography (PSG) using a physiological monitoring system (Alice 5 LE; Respironics, Orlando, FL, USA), including electroencephalogram (EEG), electrooculogram, electrocardiogram, body position, chest and abdominal respiratory movements and snoring. The PSG parameters assessed included AHI, mean oxygen saturation, minimum blood sample saturation, and percentage of total sleep time with oxygen saturation <90%. All participants avoided hypnotics, alcohol, and coffee the day before polysomnography, and the test was performed from 10 p.m. to 6 a.m. the following morning. According to the American Academy of Sleep Medicine’s Clinical Practice Guidelines for OSA in Adults, obstructive apnea was defined as a reduction in airflow of >90% or no airflow for at least 10s, while hypoventilation was defined as a reduction in airflow of ≥30% during sleep with a ≥4% drop in arterial oxygen saturation. The AHI refers to the total number of apnea and hypoventilation events occurring per hour of sleep in a patient. Patients with AHI values of 5–15/h, 15/h, and ≥30/h were classified as having mild, moderate, and severe OSA, respectively.Citation35
CPAP Treatment
All patients were treated with standard CPAP for at least 12 months at a frequency of at least 4 hours per night, at least 5 days per week. Patients using the device for >4 hours per night were considered compliant and effective.Citation36 CPAP was administered using a standardized ventilator with an auto-tuning mode (YH-480; Yuwell, Jiangsu, China), with the treatment pressure set to 4–20 cmH2O and automatically programmed for nocturnal sleep. The ventilator has a built-in user identity module card that automatically stores and records user data, including the duration of use, AHI, mask leakage, and blood oxygen, which were analyzed by a professional institution after 12 months.
MRI Data Acquisition
All imaging data were acquired using a SIEMENS 3.0-T Trio Tim MRI instrument. A) Conventional structural magnetic resonance (cMRI) data acquisition included axial T1-weighted imaging (T1WI) (repetition time [TR]=600 ms, echo time [TE]=10 ms, matrix=256×256; field of view [FOV]=240×240 mm; layer thickness=5 mm); axial T2-weighted imaging (T2WI) (TR=3000ms, TE=122 ms; matrix=256×256; FOV=240×240 mm; layer thickness=5 mm); axial T2- fluid-attenuated inversion recovery (FLAIR) (TR=3000 ms, TE=122 ms; matrix=256×256; FOV=240×240 mm; layer thickness=5 mm); axis position T2-FLAIR (TR=3000 ms, TE=122 ms; matrix=256×256; FOV=240×240 mm; layer thickness=5 mm). Gross structural lesions of the brain were excluded. B) DTI acquisition included whole-brain acquisition performed using single-shot echo planar imaging (SS-EPI) sequences (TR=5000 ms, TE=100 ms; NEX twice, matrix=256×256; FOV=240 mm×240 mm; layer thickness=30 mm; 30 non-linear diffusion gradients (b values=0 and 1000 s/mm2). 240 mm; layer thickness=3.0 mm, no spacing; 30 non-linear diffusion gradients (b-values=0 and 1000 s/mm2).
Data Preprocessing
All DTI data were preprocessed using FSL V5.0.9 (FMRIB Software Library, http://www.fmri.ox.au.uk/fsl, Centre for Functional Magnetic Resonance Imaging, University of Oxford). Voxel-wise statistical analyses were performed using TBSS.Citation37 The following preprocessing steps were performed. First, the raw DTI data were converted from DICOM to NIFTI format and the quality of the generated files was checked layer-by-layer to assess the basic parameters of the data, including image artefacts, image layers, and data head motion. Second, head eddy current and gradient orientation corrections were performed by aligning the data generated in the previous step to the B=0 image and performing the corresponding gradient orientation correction on the original dispersion gradient table.Citation38 Third, the threshold was set to 0.2 (using the BET tool in FSL) to remove images outside the brain. Finally, the DTI tensor index was calculated and fractional anisotropy (FA), mean diffusivity (MD), axial diffusion coefficient (AD), and radial diffusion coefficient (RD) were generated.
DTI data processing was based on TBSS. (1) All FA values were aligned in standard Montreal Neurological Institute (MNI) space using non-linear alignment parameters and the FMRIB58_FA_1mm template from FSL as the alignment image. (2) An image of the average FA values for all test data was generated to extract and construct the WM fiber skeleton based on the self-generated data. (3) The FA images generated after the previous alignment were projected onto the WM fiber skeleton constructed from the data used in this study to generate separate FA maps for further statistical analysis. The mean FA threshold was 0.2. (4) A design comparison matrix was created and a randomization tool was used to perform permutation tests on the two sets of FA image data. We set the number of permutations to 5000 and used no-threshold clustering enhancement (to control for class I errors) to correct for multiple comparisons. (5) Differential WM areas in the FA maps were extracted (P<0.05). The locations of the differential WM areas were verified using the International Brain Mapping Consortium DTI-81 WM template.Citation39 (6) Similarly, the non-FA DTI index based on the FA skeleton was used to calculate the MD, AD, and RD values for subsequent statistical analyses.
Statistical Analysis
Statistical analyses were performed using IBM SPSS Statistics for Windows, version 23.0, with statistical significance set at P<0.05. Demographic data and clinicopathological scores were expressed as mean ± standard deviation, and the Kolmogorov–Smirnov test was used to determine whether the data followed normal distributions. To compare differences in clinical indicators between the patients before and after CPAP treatment, paired t-test and Mann–Whitney U-test were used for normally and non-normally distributed data, respectively. To assess the relationship between FA, MD, AD, and RD values and clinical assessment scales for DTI after CPAP treatment, we analyzed non-normally distributed continuous data using Spearman correlation analysis.
Result
Characteristics of the demographic and clinical assessment information
Details on the demographic characteristics and clinical assessments of the patients with OSA are shown in . After 12 months of CPAP treatment, the MoCA, HAMA, HAMD, PSQI, and ESS scores differed significantly among patients with OSA (P<0.05).
Table 1 Demographic and Clinical Data of Pre-CPAP and Post-CPAP
TBSS results
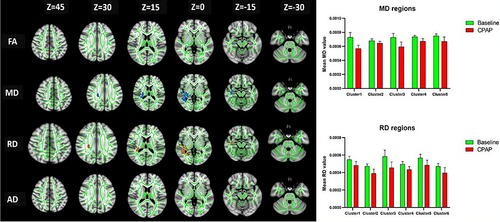
The FA and AD values of the WM fibers did not differ significantly after CPAP treatment compared with baseline in patients with OSA (P>0.05). After long-term treatment, the MD values were significantly increased in the right retrolenticular part of the internal capsule (RLIC), posterior thalamic radiation (PTR), external capsule (EC), superior longitudinal fasciculus (SLF), and the posterior limb of the internal capsule, (PLIC), superior corona radiata (SCR), posterior corona radiata (PCR), cingulum (CG), and body of the corpus callosum (BCC) (), while the RD values were significantly decreased in the RLIC, posterior thalamic radiation (PTC), SLF, EC, SCR, PCR, PTR, PLIC, CG, and inferior longitudinal fasciculus (ILF) (P<0.05) ( and ).
Table 2 Distribution of MD Differences in OSA Patients After 12 Months of CPAP Treatment
Table 3 Distribution of RD Differences in OSA Patients After 12 Months of CPAP Treatment
Relationship in OSA patients between DTI values and clinical assessment
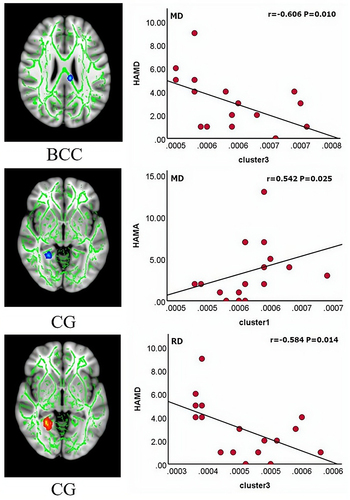
Our results showed a negative correlation between the HAMD score and cluster 3 (r=−606, P=0.010) and a positive correlation between the HAMA score and cluster1 (r=542, P=0.025) in terms of MD values, and a negative correlation between the HAMD score and cluster 3 (r=−584, P=0.014) in terms of RD values ().
Discussion
In recent years, considerable research interest has focused on investigating whether the effects of CPAP treatment on improving mood and cognitive dysfunction in patients with OSA are associated with reversible restoration of cerebral WM fiber integrity. Our previous three-month short-term CPAP treatment studies did not observe reversible recovery of cerebral WM fibers in patients with OSA,Citation31 but did demonstrate partial recovery of brain function.Citation40 In the present study, we observed a partial reversible recovery of WM fibers in patients with OSA after 12 months of CPAP treatment, mainly changes in the MD and RD values of some WM fibers, including the commissural (CG, BCC), projection (RLIC, PTR, PLIC, SCR, and PCR), and association (EC, SLF, and ILF) fibers. Second, we observed significant improvements in cognitive and mood deficits in patients with OSA after long-term treatment, and changes in the fiber integrity of the cingulate and corpus callosum were significantly associated with mood regulation. The results of this study suggest that the reversible recovery of WM structure in patients with OSA may require longer CPAP treatment. In addition, restoring the integrity of the commissural fibers (CG and BCC) is associated with the regulation of emotions.
Reduced cerebral blood flow and prolonged interstitial hypoxia in patients with OSA can cause oxidative stress and ischemic reperfusion, leading to altered cerebral WM fiber integrity.Citation41 Deep WM structures are more susceptible to intermittent hypoxia because of the lack of anastomosis and effective collateral circulation in their supplying and perforating arteries.Citation42 Cross-sectional DTI studies have demonstrated acute or chronic ischemic changes, which may eventually lead to extensive structural changes in the WM.Citation43 However, previous studies have not shown a consistent pattern of brain damage, possibly because of participant selection, such as age,Citation44 and condition severity.Citation45
The main component of the brain WM is the myelin sheath, which is produced by mature oligodendrocytesCitation46 and is vulnerable to hypoxic factors. DTI allows the quantitative observation of microstructural changes in brain tissue. The MD value shows the extent of water diffusion in the brain tissue, which corresponds to increased WM bundles due to intracellular edema and gliosis.Citation15 RD is sensitive to the degree of myelin and axonal degeneration.Citation47 Among previous longitudinal DTI studies, Salsone et al reported a significant increase in FA in the brainstem, upper radial head, and internal capsule and a significant increase in AD in the corpus callosum, upper radial head, and right internal capsule in male patients with OSA after 3 months of CPAP treatment, thus confirming the efficacy of ventilation therapy.Citation48 Castronovo et al observed that long-term CPAP treatment reversed the decline in WM integrity in most pretreatment patients with OSA, which was consistent with the recovery of memory and attention.Citation30 Similarly, the results of the present study showed increased MD and RD values in some brain regions after 12 months of CPAP treatment, confirming the partial restoration of WM integrity in patients with OSA and illustrating the efficacy of CPAP treatment from a neuroimaging perspective.
The MD and RD changes in the WM structure of the brain in the present study occurred mainly in the right cerebral hemisphere, although the mechanism of damage may be bilateral. Similar to the study by Kumar et al,Citation47 preferential recovery of the structural lateralization of the brain was observed. Although the reasons for this are unclear, differences in relative blood flow between the two hemispheres may be one of the causes.Citation49 In addition, right-handedness may have contributed to these results,Citation50 and all patients in the present study were right-handed. In addition, we did not observe changes in FA values, which are important indicators of axon number and density. However, when we relaxed the DTI threshold to 0.09, we observed a significant difference in FA values between the two cerebral hemispheres (Supplementary Material Figure S1). We also observed changes in MD and RD values in the left cerebral hemisphere. We speculate that the fibrous structure of cerebral WM in patients with OSA can be further reversed by prolonged CPAP treatment. We will further investigate the imaging mechanism of WM structure recovery in these patients after 18 months of CPAP treatment.
The WM bundles seen in anatomical studies can be divided into three groups: commissural, projection, and association fibers.Citation51 Commissural fibers connect the cerebral hemispheres, projection fibers are efferent and afferent fibers that connect the cortex with subcortical structures, and association fibers connect the cortical areas within the same hemisphere. After 12 months of CPAP treatment, we observed extensive increases in the MD and RD values of WM fiber bundles involving commissural, projection, and association fibers. Thalamic radiation is a class of fan-shaped bundles of WM fibers that connect the thalamus to the cerebral cortex. Impairment of the integrity of the thalamic radiation may affect the ability of the thalamus to transmit arousing and associative information to the supercortex.Citation52 Koo et al reported reduced WM fiber integrity in the thalamus in patients with untreated OSA.Citation28 Our results are similar to those of previous studies and illustrate the reversible recovery from thalamic radiation from a therapeutic perspective. The retrolenticular portion of the internal capsule is associated with motor function. Previous sleep studies demonstrated that the retrolenticular part of the internal capsule is sensitive to sleep rhythms. We previously reported that increased RD and MD values in the retrolenticular part of the internal capsule may benefit from the partial restoration of sleep rhythms after CPAP treatment in patients with OSA.Citation53 The radial cortex contains ascending and descending fibers that are mainly involved in emotional processing, attention, and cognitive control through the transmission of neural information.Citation54 Our study showed increased MD and RD values in the superior and posterior radial cords, which may be related to improved total sleep time and sleep quality with CPAP treatment.Citation55
The corpus callosum plays an important role in communication between brain hemispheres and is one of the highest-order and late-maturing neural networks in the brain, with high vulnerability to negative experiences. The corpus callosum integrates the prefrontal cortex, cingulate gyrus, and insula, and is associated with emotional, cognitive, and sensory information processing.Citation56 Zhang et al reported that the reduced integrity of the anterior corpus callosum fibers in patients with OSA was associated with reduced prospective memory and sustained attention.Citation57 The cingulate gyrus is involved in the control of autonomic functions, including the regulation of breathing and blood pressure.Citation58 A study of OSA demonstrated that structural changes in the cingulate gyrus alter the state of sympathetic nerve discharge and, thus, participate in central blood pressure and respiratory regulation through sympathetic excitation.Citation59 Based on the results of the present study, we hypothesized that changes in the MD and RD values of the cingulate gyrus may be related to the improvement of intermittent hypoxia after CPAP treatment. In addition, we observed that elevated MD values in the body of the corpus callosum were positively correlated with HAMA, while elevated MD and RD values in the cingulum were negatively correlated with HAMD, suggesting that the structurally reversible restoration of united fibers is associated with improved emotion regulation.
In addition, we observed reversible recovery of the contact fibers, including the EC, SLF, and ILF. The SLF is associated with impaired speech production;Citation60 while damage to ILF structures and the EC are associated with depression;Citation36 and executive impairment,Citation61 respectively. Although these reversibly restored WM fiber regions were more extensive, they were structurally similar to those reported in other sleep-related WM structural studies.Citation61
Limitations
Our study has several limitations. First, the disease duration may have differed among the patients, which may have introduced confounding variables into our DTI observations. Second, the patients in our study were predominantly male with moderate-to-severe OSA; thus, our results cannot be generalized to mild OSA or female patients. Finally, the sample size was small. For longitudinal studies, a larger sample size is usually required to achieve sufficient statistical power. Therefore, we will increase the sample size in future studies.
Conclusion
Based on the TBSS study, this study investigated reversible changes in brain WM fibers before and after long-term CPAP treatment in patients with moderate-to-severe OSA. Our findings confirmed that recovery of the brain WM microstructure may require longer CPAP treatment and that this extensive reversal of WM fibers involves the commissural, projection, and association fibers. Moreover, commissural fibers (CG and BCC) were associated with emotion regulation in patients with OSA, providing new insights into the pathophysiology of WM structures in these patients. In the future, we will continue to investigate changes in WM fibers in patients with OSA after 18 and 24 months of CPAP treatment to identify potential imaging markers for clinical management.
Ethics Statement
The study was approved by the Ethics Committee of the First Affiliated Hospital of Nanchang University. Patients gave written informed consent to participate in this study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
Additional information
Funding
References
- Benjafield AV, Ayas NT, Eastwood PR, et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: a literature-based analysis. Lancet Respir Med. 2019;7(8):687–698. doi:10.1016/S2213-2600(19)30198-5
- Volner K, Chao S, Camacho M. Dynamic sleep MRI in obstructive sleep apnea: a systematic review and meta-analysis. Eur Arch Otorhinolaryngol. 2022;279(2):595–607. doi:10.1007/s00405-021-06942-y
- Iannella G, Magliulo G, Cammaroto G, et al. Effectiveness of drug-induced sleep endoscopy in improving outcomes of barbed pharyngoplasty for obstructive sleep apnea surgery: a prospective randomized trial. Sleep Breath. 2022;26(4):1621–1632. doi:10.1007/s11325-021-02528-4
- Vanek J, Prasko J, Genzor S, et al. Obstructive sleep apnea, depression and cognitive impairment. Sleep Med. 2020;72:50–58. doi:10.1016/j.sleep.2020.03.017
- Lim DC, Pack AI. Obstructive sleep apnea and cognitive impairment: addressing the blood-brain barrier. Sleep Med Rev. 2014;18(1):35–48. doi:10.1016/j.smrv.2012.12.003
- Lavie L. Oxidative stress in obstructive sleep apnea and intermittent hypoxia--revisited--The bad ugly and good: implications to the heart and brain. Sleep Med Rev. 2015;20:27–45.
- Maniaci A, Di Luca M, Lechien JR, et al. Lateral pharyngoplasty vs. traditional uvulopalatopharyngoplasty for patients with OSA: systematic review and meta-analysis. Sleep Breath. 2022;26(4):1539–1550. doi:10.1007/s11325-021-02520-y
- Oliver C, Li H, Biswas B, et al. A systematic review on adherence to continuous positive airway pressure (CPAP) treatment for obstructive sleep apnoea (OSA) in individuals with mild cognitive impairment and Alzheimer’s disease dementia. Sleep Med Rev. 2023;73:101869. doi:10.1016/j.smrv.2023.101869
- Aurora RN, Collop NA, Jacobowitz O, et al. Quality measures for the care of adult patients with obstructive sleep apnea. J Clin Sleep Med. 2015;11(3):357–383. doi:10.5664/jcsm.4556
- Pattison E, Tolson J, Barnes M, et al. Improved depressive symptoms, and emotional regulation and reactivity, in individuals with obstructive sleep apnea after short- and long-term CPAP therapy use. Sleep Med. 2023;111:13–20. doi:10.1016/j.sleep.2023.08.024
- Mok Y, Melehan KL, Phillips CL, et al. Does CPAP treat depressive symptoms in individuals with OSA? An analysis of two 12-week randomized sham CPAP-controlled trials. Sleep Med. 2020;73:11–14. doi:10.1016/j.sleep.2020.04.021
- Gasa M, Tamisier R, Launois SH, et al. Residual sleepiness in sleep apnea patients treated by continuous positive airway pressure. J Sleep Res. 2013;22(4):389–397. doi:10.1111/jsr.12039
- McMillan A, Bratton DJ, Faria R, et al. Continuous positive airway pressure in older people with obstructive sleep apnoea syndrome (PREDICT): a 12-month, multicentre, randomised trial. Lancet Respir Med. 2014;2(10):804–812.
- Dostalova V, Kolečkárová S, Kuška M, et al. Effects of continuous positive airway pressure on neurocognitive and neuropsychiatric function in obstructive sleep apnea. J Sleep Res. 2019;28(5):e12761.
- Baril AA, Martineau-Dussault M-È, Sanchez E, et al. Obstructive sleep apnea and the brain: a focus on gray and white matter structure. Curr Neurol Neurosci Rep. 2021;21(3):11. doi:10.1007/s11910-021-01094-2
- Liu X, Wang Z, Liu S, et al. Activation network improves spatiotemporal modelling of human brain communication processes. Neuroimage. 2023;285:120472. doi:10.1016/j.neuroimage.2023.120472
- Dalmases M, Solé-Padullés C, Torres M, et al. Effect of CPAP on cognition, brain function, and structure among elderly patients with OSA: a randomized pilot study. Chest. 2015;148(5):1214–1223. doi:10.1378/chest.15-0171
- Li H, Li L, Shao Y, et al. Abnormal intrinsic functional hubs in severe male obstructive sleep apnea: evidence from a voxel-wise degree centrality analysis. PLoS One. 2016;11(10):e0164031. doi:10.1371/journal.pone.0164031
- Peng DC, Dai X-J, Gong -H-H, et al. Altered intrinsic regional brain activity in male patients with severe obstructive sleep apnea: a resting-state functional magnetic resonance imaging study. Neuropsychiatr Dis Treat. 2014;10:1819–1826. doi:10.2147/NDT.S67805
- Prilipko O, Huynh N, Schwartz S, Prilipko O, et al. The effects of CPAP treatment on task positive and default mode networks in obstructive sleep apnea patients: an fMRI study. PLoS One. 2012;7(12):e47433.
- Huang L, Li H, Shu Y, et al. Changes in functional connectivity of hippocampal subregions in patients with obstructive sleep apnea after six months of continuous positive airway pressure treatment. Brain Sci. 2023;13(5):838. doi:10.3390/brainsci13050838
- Fatouleh RH, Lundblad LC, Macey PM, et al. Reversal of functional changes in the brain associated with obstructive sleep apnoea following 6 months of CPAP. Neuroimage Clin. 2015;7:799–806. doi:10.1016/j.nicl.2015.02.010
- Canessa N, Castronovo V, Cappa SF, et al. Obstructive sleep apnea: brain structural changes and neurocognitive function before and after treatment. Am J Respir Crit Care Med. 2011;183(10):1419–1426. doi:10.1164/rccm.201005-0693OC
- Kim H, Joo E, Suh S, et al. Effects of long-term treatment on brain volume in patients with obstructive sleep apnea syndrome. Hum Brain Mapp. 2016;37(1):395–409. doi:10.1002/hbm.23038
- Kumar R, Macey PM, Cross RL, et al. Neural alterations associated with anxiety symptoms in obstructive sleep apnea syndrome. Depress Anxiety. 2009;26(5):480–491. doi:10.1002/da.20531
- Smith SM, Jenkinson M, Johansen-Berg H, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31(4):1487–1505. doi:10.1016/j.neuroimage.2006.02.024
- Baril AA, Gagnon K, Descoteaux M, et al. Cerebral white matter diffusion properties and free-water with obstructive sleep apnea severity in older adults. Hum Brain Mapp. 2020;41(10):2686–2701. doi:10.1002/hbm.24971
- Koo DL, Kim HR, Kim H, et al. White matter tract-specific alterations in male patients with untreated obstructive sleep apnea are associated with worse cognitive function. Sleep. 2020;43(3). doi:10.1093/sleep/zsz247
- Zhang J, Weaver TE, Zhong Z, et al. White matter structural differences in OSA patients experiencing residual daytime sleepiness with high CPAP use: a non-Gaussian diffusion MRI study. Sleep Med. 2019;53:51–59. doi:10.1016/j.sleep.2018.09.011
- Castronovo V, Scifo P, Castellano A, et al. White matter integrity in obstructive sleep apnea before and after treatment. Sleep. 2014;37(9):1465–1475. doi:10.5665/sleep.3994
- Liu X, Wei Z, Chen L, et al. Effects of 3-month CPAP therapy on brain structure in obstructive sleep apnea: a diffusion tensor imaging study. Front Neurol. 2022;13:913193. doi:10.3389/fneur.2022.913193
- Chen KL, Xu Y, Chu A-Q, et al. Validation of the Chinese version of Montreal cognitive assessment basic for screening mild cognitive impairment. J Am Geriatr Soc. 2016;64(12):e285–e290. doi:10.1111/jgs.14530
- Backhaus J, Junghanns K, Broocks A, et al. Test-retest reliability and validity of the Pittsburgh Sleep Quality Index in primary insomnia. J Psychosom Res. 2002;53(3):737–740. doi:10.1016/S0022-3999(02)00330-6
- Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23(1):56–62. doi:10.1136/jnnp.23.1.56
- Berry RB, Brooks R, Gamaldo C, et al. AASM Scoring Manual Updates for 2017 (Version 2.4). J Clin Sleep Med. 2017;13(5):665–666. doi:10.5664/jcsm.6576
- Olvet DM, Delaparte L, Yeh F-C, et al. A comprehensive examination of white matter tracts and connectometry in major depressive disorder. Depress Anxiety. 2016;33(1):56–65. doi:10.1002/da.22445
- Jenkinson M, Beckmann CF, Behrens TEJ, et al. FSL. Neuroimage. 2012;62(2):782–790. doi:10.1016/j.neuroimage.2011.09.015
- Van Dijk KR, Sabuncu MR, Buckner RL. The influence of head motion on intrinsic functional connectivity MRI. Neuroimage. 2012;59(1):431–438. doi:10.1016/j.neuroimage.2011.07.044
- Mori S, Oishi K, Jiang H, et al. Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template. Neuroimage. 2008;40(2):570–582. doi:10.1016/j.neuroimage.2007.12.035
- Li P, Shu Y, Liu X, et al. The effects of CPAP treatment on resting-state network centrality in obstructive sleep apnea patients. Front Neurol. 2022;13:801121. doi:10.3389/fneur.2022.801121
- Jelic S, Padeletti M, Kawut SM, et al. Inflammation, oxidative stress, and repair capacity of the vascular endothelium in obstructive sleep apnea. Circulation. 2008;117(17):2270–2278. doi:10.1161/CIRCULATIONAHA.107.741512
- Brown WR, Thore CR. Review: cerebral microvascular pathology in ageing and neurodegeneration. Neuropathol Appl Neurobiol. 2011;37(1):56–74. doi:10.1111/j.1365-2990.2010.01139.x
- Kumar R, Chavez AS, Macey PM, et al. Altered global and regional brain mean diffusivity in patients with obstructive sleep apnea. J Neurosci Res. 2012;90(10):2043–2052. doi:10.1002/jnr.23083
- Li Y, Wen H, Li H, et al. Characterisation of brain microstructural alterations in children with obstructive sleep apnea syndrome using diffusion kurtosis imaging. J Sleep Res. 2023;32(2):e13710. doi:10.1111/jsr.13710
- Girolami S, Tardio M, Loredana S, et al. Sleep body position correlates with cognitive performance in middle-old obstructive sleep apnea subjects. Sleep Med X. 2022;4:100050. doi:10.1016/j.sleepx.2022.100050
- Chang KJ, Redmond SA, Chan JR. Remodeling myelination: implications for mechanisms of neural plasticity. Nat Neurosci. 2016;19(2):190–197. doi:10.1038/nn.4200
- Kumar R, Pham TT, Macey PM, et al. Abnormal myelin and axonal integrity in recently diagnosed patients with obstructive sleep apnea. Sleep. 2014;37(4):723–732. doi:10.5665/sleep.3578
- Salsone M, Caligiuri ME, Castronovo V, et al. Microstructural changes in normal-appearing white matter in male sleep apnea patients are reversible after treatment: a pilot study. J Neurosci Res. 2021;99(10):2646–2656. doi:10.1002/jnr.24858
- Minkner K, Lovblad KO, Yilmaz H, et al. White matter lesions in watershed territories studied with MRI and parenchymography: a comparative study. Neuroradiology. 2005;47(6):425–430. doi:10.1007/s00234-005-1358-8
- Xiong Y, Zhou XJ, Nisi RA, et al. Brain white matter changes in CPAP-treated obstructive sleep apnea patients with residual sleepiness. J Magn Reson Imaging. 2017;45(5):1371–1378. doi:10.1002/jmri.25463
- Duarte JA, de Araújo e Silva JQ, Goldani AA, et al. Neurobiological underpinnings of bipolar disorder focusing on findings of diffusion tensor imaging: a systematic review. Braz J Psychiatry. 2016;38(2):167–175. doi:10.1590/1516-4446-2015-1793
- Guillery RW, Sherman SM. Thalamic relay functions and their role in corticocortical communication: generalizations from the visual system. Neuron. 2002;33(2):163–175. doi:10.1016/S0896-6273(01)00582-7
- Garcia-Marin LM, Alcauter S, Campos AI, et al. Evidence of genetic overlap between circadian preference and brain white matter microstructure. Twin research and Human Genetics. 2021;24(1):1–6. doi:10.1017/thg.2021.4
- Yang Y, Zhu D-M, Zhang C, et al. Brain structural and functional alterations specific to low sleep efficiency in major depressive disorder. Front Neurosci. 2020;14:50. doi:10.3389/fnins.2020.00050
- Reyes S, Rimkus CDM, Lozoff B, et al. Nighttime sleep characteristics and white matter integrity in young adults. Nat Sci Sleep. 2022;14:1363–1373. doi:10.2147/NSS.S360311
- Ambrosi E, Rossi-Espagnet MC, Kotzalidis GD, et al. Structural brain alterations in bipolar disorder II: a combined voxel-based morphometry (VBM) and diffusion tensor imaging (DTI) study. J Affect Disord. 2013;150(2):610–615. doi:10.1016/j.jad.2013.02.023
- Zhang B, Zhu D-M, Zhao W, et al. Selective microstructural integrity impairments of the anterior corpus callosum are associated with cognitive deficits in obstructive sleep apnea. Brain Behav. 2019;9(12):e01482. doi:10.1002/brb3.1482
- Joo EY, Tae WS, Lee MJ, et al. Reduced brain gray matter concentration in patients with obstructive sleep apnea syndrome. Sleep. 2010;33(2):235–241. doi:10.1093/sleep/33.2.235
- Taylor KS, Millar PJ, Murai H, et al. Cortical autonomic network gray matter and sympathetic nerve activity in obstructive sleep apnea. Sleep. 2018;41(2). doi:10.1093/sleep/zsx208
- Cipolotti L, Molenberghs P, Dominguez J, et al. Fluency and rule breaking behaviour in the frontal cortex. Neuropsychologia. 2020;137:107308. doi:10.1016/j.neuropsychologia.2019.107308
- Grumbach P, Opel N, Martin S, et al. Sleep duration is associated with white matter microstructure and cognitive performance in healthy adults. Hum Brain Mapp. 2020;41(15):4397–4405. doi:10.1002/hbm.25132