Abstract
Sleep and circadian disorders in Alzheimer’s disease (AD) are more frequent than in the general population and appear early in the course of the disease. Quality of sleep and quality of life are parallel in these patients, and such disorders also represent a heavy burden for caregivers. Although alterations in melatonin and hypocretins (orexins) seem to play a key role in the origin of these disturbances, the etiology of these disorders is multifactorial, including many factors such as environment, behavior, treatments, and comorbidities, among others. A comprehensive evaluation of sleep in each patient is essential in the design of the treatment that includes nonpharmacological and pharmacological approaches. One particularly interesting point is the possibility of a role of sleep disorders in the pathogenesis of AD, raising the possibility that treating the sleep disorder may alter the course of the disease. In this review, we present an update on the role of sleep disorders in AD, the bidirectional influence of sleep problems and AD, and treatment options. Behavioral measures, bright light therapy (BLT), melatonin, and other drugs are likely well known and correctly managed by the physicians in charge of these patients. In spite of the multiple treatments used, evidence of efficacy is scarce and more randomized double-blind placebo-controlled studies are needed. Future directions for treatment are the establishment of BLT protocols and the development of drugs with new mechanisms of action, especially hypocretin receptor antagonists, melatonin receptor agonists, and molecules that modulate the circadian clock.
Introduction
Alzheimer’s disease (AD) is the most frequent cause of dementia in the elderly. It has been estimated that in 2013, AD affected 4.7 million individuals aged 65 years or older in the United States, a number that is projected to increase to approximately 14 million by 2050.Citation1 The classic hallmarks are progressive deterioration of memory, language, and intellect. Sleep and circadian rhythm disorders are very frequent in AD, and it has been reported that up to 45% of patients may have sleep problems.Citation2–Citation4 The most frequent disturbances are excessive awakenings (23%), early morning awakening (11%), excessive daytime sleepiness (10%), and napping for more than 1 hour during the day (14%).Citation5 Such disturbances can appear early in the course of the disease, although they tend to be correlated with the severity of the cognitive decline.Citation3 Sleep-related breathing disorders (SRBDs) are also very frequent in AD patients and in this group are clearly more prevalent than in the general population.Citation6,Citation7
At least three issues highlight the relevance of the treatment of sleep disorders in patients with AD:
Sleep disturbances are associated with increased memory and cognitive impairment.Citation8
Sleep and nighttime behavioral disturbances such as wandering, day/night confusion, getting up repeatedly during the night, and nightmares or hallucinations cause significant caregiver burden and are a primary cause of patient institutionalization.Citation5,Citation9
There is increasing evidence of the role of sleep disturbances in the pathophysiology of AD and a bidirectional relationship has been proposed.Citation10–Citation12
This article reviews the main sleep problems in these patients and the interactions between sleep disorders and AD. The clinical evaluation of sleep disturbances, the current treatments for sleep disturbances, and the new perspectives are also addressed.
Architectural disturbances of sleep in AD patients
Normal aging is accompanied by sleep architecture changes, such as increased sleep latency, difficulty in sleep maintenance, decrease in slow-wave sleep (SWS), early morning awakenings, and increased daytime somnolence.Citation13
The sleep disturbances present in patients with AD are similar, but more severe than would be expected by the patient’s age.Citation14 Sometimes sleep disturbances in AD are so prominent that should be classified as a primary comorbid sleep disorder, such as chronic insomnia. The change that seems most specific to AD is a quantitative decrease in the rapid eye movement (REM) stage.Citation15,Citation16 In particular, electroencephalogram (EEG) slowing during REM sleep has been proposed as a biological marker of AD.Citation16
The architectural changes present in AD patients are probably related to cognition impairment.Citation17,Citation18 The cognitive impairment could be different depending on the sleep stage that is altered. For example, Rauchs et alCitation19,Citation20 found that the mean intensity of fast spindles was positively correlated, in AD patients, with immediate recall performance, while the amount of SWS was positively correlated with the ability to retrieve recent autobiographical memories.
Circadian disturbances in AD patients
Abnormalities in sleep–wake patterns and circadian-related disorders are also common in AD patients.Citation21 In extreme cases, a complete day/night sleep pattern reversal can be observed.Citation22 Some authors have proposed that the sundowning phenomenon could be also due to a disorder of the circadian rhythm.Citation23–Citation25 This phenomenon corresponds to an exacerbation of behavioral symptoms of dementia in the late afternoon.Citation26 The abnormalities in the circadian timing system in AD patients are also manifested in other circadian systems such as body temperature and hormone concentrations.Citation24,Citation27–Citation29 StranahanCitation30 found that disturbances in the circadian timing system also affect the activity of the hippocampus, worsening learning capacities.
SRBDs in AD patients
SRBDs are also more frequent in patients with AD than in the general population and are present in 40%–70% of these patients.Citation6,Citation7 In a longitudinal cohort study of sleep disorders using polysomnography (PSG), it was found that the probability of moderate-to-severe sleep-disordered breathing was significantly higher in healthy participants with the APOE E4 allele, independent of age, sex, body mass index, or race.Citation31 It has been suggested that SRBD could cause AD.Citation32 Recently, it has been reported that the presence of SRBD was associated with cognitive decline at an earlier age.Citation33 Once the dementia is established, the severity of the sleep disturbances seems to be correlated with the severity of the dementia.Citation34 Apneas alter sleep architecture and lead to a decreased amount of REM sleep and SWS, which causes more frequent awakenings than in patients without apneas.Citation35 However, Yaffe et alCitation36 found that the oxygen desaturation index and the percentage of time in apnea or hypopnea were associated with cognitive decline but not with sleep fragmentation or sleep duration. These disturbances and the daytime sleepiness could be responsible for additional cognitive symptoms in AD patients that would be reversible.Citation37,Citation38
Interactions between sleep disturbances and AD
A bidirectional relationship between sleep disturbances and AD has been proposed (). A poor quality of sleep and daytime somnolence seems to increase the risk of developing AD. Citation11,Citation39–Citation44 However, as pointed out by Ju et al,Citation10 since the pathological changes occur 10–15 years before the clinical onset of AD, some patients in these studies could already have preclinical AD and could not be considered as incidental cases. In fact, some authors have suggested the change of sleep pattern may predict AD.Citation45 The physiopathological mechanism is not completely understood, but an association between sleep disturbances and amyloid-β accumulation has been demonstrated in miceCitation10,Citation11,Citation40 and humans.Citation46,Citation47 In healthy older adults, Spira et alCitation46 found a correlation between self-reported shorter sleep duration or poorer sleep quality and larger amyloid-β burden as assessed by positron emission tomography. Conversely, in a prospective longitudinal cohort study, Lim et alCitation47 found that a better sleep consolidation, measured by actigraphy, reduced the incidence of AD, cognitive decline, and neurofibrillary tangle density (determined by autopsy) in those subjects with the APOE E4 allele. The amount of SWS could explain, at least in part, these findings because the level of cerebrospinal fluid (CSF) amyloid-β is lower during this sleep stage and much higher during wakefulness and the REM stage.Citation48 Therefore, patients with sleep fragmentation and decreased SWS would have higher CSF amyloid-β levels, leading to the formation of amyloid plaques.
Figure 1 Bidirectional relationship between sleep and AD pathology.
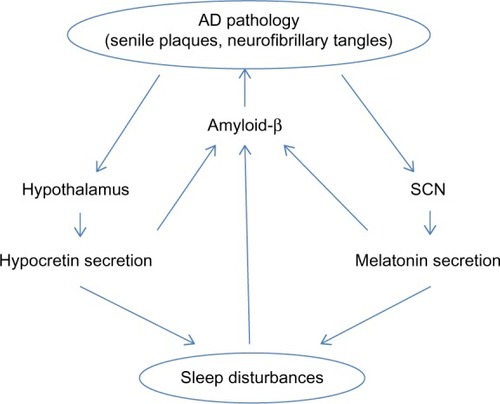
On the other hand, AD also influences sleep, especially the sleep–wake cycle.Citation49 Patients with AD suffer some disturbances in the secretion of neurotransmitters related to sleep–wake systems, mainly hypocretins (orexins) and melatonin secretion.Citation29,Citation50 Hypocretin-1 and -2 are produced by a small cluster of neurons in the posterior hypothalamus.Citation51,Citation52 The hypocretin system acts a stabilizing factor in the sleep–wake flip-flop, keeping it in the waking state.Citation53,Citation54 The disturbances in the secretion of neurotransmitters not only influence the quality of sleep, but also play a role in the pathogenesis of the AD itself through changes in amyloid-β, originating a complex circle. In fact, it has been reported in mice that physiologic circadian fluctuations of CSF amyloid-β levels are related to the hypocretin system.Citation55 In AD patients Fronczek et alCitation56 found low levels of hypocretin-1 levels in ventricular CSF portmortem. However, in humans the relationships between specific AD biomarkers and hypocretin-1 remain unclear. Most studies have found normal levels of CSF hypocretins.Citation57–Citation61 However, a continuous CSF sampling study via indwelling intrathecal catheter collecting hourly CSF samples found that lower mean amyloid-β42 was related to lower levels of hypocretin-1,Citation62 even though these were within the normal range. In 2013, after a review of the literature, Slats et alCitation50 concluded that, although in AD patients hypocretin neurons are decreased, the levels and the circadian rhythm of CSF hypocretins do not differ from those of healthy volunteers. More recently, Dauvilliers et al,Citation63 in a study of patients with cognitive impairment of different origins, surprisingly found that CSF hypocretin-1 concentrations were significantly higher in early stage AD, suggesting that this finding could contribute to AD diagnosis. Therefore, further studies are needed to clarify the complete role of the hypocretin system in the pathophysiology of AD.
Melatonin plays a key role not only in sleep disturbances but also in the pathogenesis of AD. Melatonin is a tryptophan metabolite that is synthesized in the pineal gland and has several physiological functions including the regulation of circadian rhythms, clearance of free radicals, improvement of immunity, and inhibition of the oxidation of biomolecules.Citation64 CSF melatonin levels are already decreased in the preclinical stages of ADCitation65,Citation66 and continue decreasing further as AD progresses.Citation67 Some irregularities in the pattern of the melatonin rhythm also occur.Citation65 The defect in the secretion of melatonin is not due to the lesion of the pineal gland but to the involvement of the suprachiasmatic nucleus (SCN), the master clock of the circadian rhythm, which would lead to a decrease in the expression of clock genes and to a loss of the noradrenergic control in the pineal gland.Citation24,Citation68 The reduced optical transmission in elderly people also influences the activity of SCN. The presence of the ApoE4 allele, a known genetic risk factor for AD, has been linked to a lower level of CSF melatoninCitation69 and to the presence of sleep alterations (eg, REM sleep reduction and obstructive sleep apnea syndrome).Citation69–Citation71 On the other hand, melatonin has several antiamyloidogenic and antioxidant effectsCitation72 and its deficit could influence the progression of the disease. It has the ability to regulate APP metabolism, reducing amyloid-β levels and preventing its aggregation. It also influences hyperphosphorylation, but has no effect once deposition has started.Citation72
There is some evidence that other neurotransmitters related to sleep, such as melanin-concentrating hormone, are also altered in AD, but more studies are needed to clarify their role.Citation60
Clinical evaluation of sleep disturbances in AD
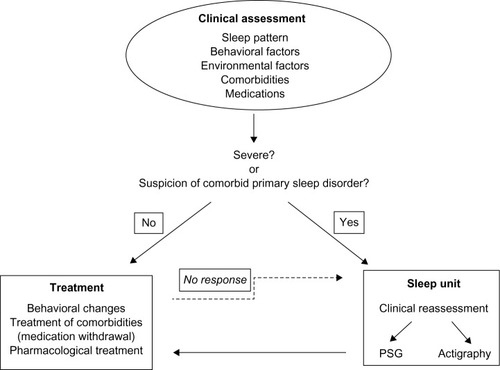
The evaluation of a patient with sleep complaints must begin with the characterization of the pattern of the sleep disruption and the identification of possible factors that affect or worsen the quality of sleep. As shown in , these may include behavioral and environmental factors, comorbidities, and medications.Citation4 The identification of primary sleep disorders in demented patients may be difficult because the manifestations of the sleep disorders can be atypical. For example, restless legs syndrome (RLS) may be expressed only by nocturnal agitation.Citation73 A specific guideline to establish the diagnosis of probable RLS has been developed.Citation74 It emphasizes the behavioral indicators and supportive features. Studies assessing the prevalence of RLS with the usual criteria report a prevalence of 4%–6%.Citation75,Citation76 However, Rose et al,Citation73 using the new criteria, reported a prevalence of 24%.
Figure 2 Diagram with the clinical evaluation of the sleep disorders in AD patients.
Bedtime ritual and habits, time spent in bed, amount of day-time activity, ambient light, and nocturnal noise may affect sleep quality.Citation5 Physicians must always bear in mind that they are dealing with an elderly patient, who is at risk for many common sleep disorders typical in this age group, especially congestive heart failure with nocturnal respiratory distress and nocturia, chronic obstructive pulmonary disease, gastroesophageal reflux, arthritis, and nocturia.Citation57–Citation82 Psychiatric diseases cannot be forgotten since about 50% of AD patients have symptoms of dysthymia or depression.Citation83 In relation to medications, it is important to evaluate both prescription and over-the-counter medications, and the use of social drugs such as caffeine, nicotine, and alcohol.Citation84
The information about the sleep and other health problems obtained from demented patients is not always reliable because they are not aware of the presence or severity of their problems. Sleep questionnaires are also of limited value because AD patients tend to underscore sleep disturbances.Citation85 Therefore, the clinical evaluation usually requires information from the caregivers who suffer the burden of the behavioral alterations, both the nighttime and daytime consequences. When the origin of the sleep problems is not identified during the clinical interview, a structured evaluation must be used. In 2003 Tractenberg et alCitation86 proposed an instrument to assess symptoms of sleep disturbances and disorders in AD patients – the Sleep Disorders Inventory. Yesavage et al proposed new criteria to identify sleep disturbances related to AD to facilitate future research.Citation87
When the clinical involvement of sleep disturbances is severe, when there is no good response to the usual treatments or when there is a reason to suspect that a patient has a comorbid primary sleep disorder, the most appropriate course of action is to refer the patient to a sleep unit for further investigations. The gold standard to record sleep is PSG. However, it can be difficult or impossible to perform this procedure due to the need for minimum patient cooperation. Moreover, the stage scoring may be complicated due to the diffuse slowing of the EEG.Citation14 Wrist actigraphy has been used as a more feasible alternative.Citation88 Ancoli-Israel et al,Citation89 using EEG recordings and actigraphy, found a significant correlation between total sleep and wake time, and a high sensitivity and specificity for actigraphy as compared to behavioral observations.
Treatment of sleep disturbances in AD
The aim of the treatment is to improve the quality of life of patients and caregivers. Because of the impact of sleep disorders on cognition, it seems logical to think that the treatment would also improve some cognitive domains. Some authors have even suggested a possible preventive effect for the progression of the AD.Citation10,Citation41,Citation50,Citation72
As mentioned in the previous section, a thorough evaluation, including habits, comorbidities, and treatments, is critical to choosing the most appropriate treatment for each patient. The treatment of comorbidities or the withdrawal of a medication might be the first step when we are faced with sleep disturbances in a patient with AD. In the following section, we discuss treatment differentiating between nonpharmacological and pharmacological approaches (). The treatment of primary sleep disorders is discussed separately.
Table 1 Treatments for sleep disturbances in AD patients
Nonpharmacological treatment
The nonpharmacological approach tends to be considered the first-line treatment in AD patients, although scientific evidence of its effectiveness is limited. In 2013, after a structured critical literature review, Brown et alCitation90 concluded that there was a paucity of conclusive research for nonpharmacological sleep interventions in people with dementia, with the evidence being conclusive only for the use of light therapy (LT).
During daytime, AD patients should be encouraged to exercise regularly for at least 30 minutes and walk outdoors. Intake of stimulants such as caffeine or tea should be limited, and naps longer than half an hour or after 1 pm should be avoided. Time in bed should be reduced. The schedule for going to sleep and getting up must be regular and the bedroom should be reserved only for sleeping. Nighttime noise and light exposure and sleep disruptions should be reduced. The last point is especially difficult but important in nursing homes.Citation91 The efficacy of these measures is well established in demented elderly and in AD.Citation92–Citation95
Bright light therapy (BLT) is a chronotherapeutic intervention used to treat circadian disturbances in AD patients.Citation93 Several classic studies have shown that BLT improves night-time sleep, decreases nocturnal awakenings, increases daytime wakefulness, reduces evening agitational behavior, consolidates rest/activity patterns,Citation96–Citation99 and also improves cognition.Citation98–Citation101 Ancoli-Israel et alCitation102 compared bright light during morning versus dim light during morning and bright light during evening for 10 days. They found a lengthening of the maximum sleep bouts during night only in the first condition, with no significant changes in total sleep time or awakenings. Dowling et alCitation103 found improvement in the sleep parameters exclusively in the group with more severe involvement of the sleep–wake rhythm. They used a light intensity of 2,500 lux during only 1 hour, Monday through Friday during 10 weeks. The same group in 2008 published the results in 50 patients using the same method of luminotherapy combined or not with melatonin, and they found no changes in sleep parameters using bright therapy alone, but there were changes when it was combined with melatonin.Citation104 The season could have an effect on the response to BLT. Burns et al,Citation105 applying bright light at 1,000 lux from 10 am to 12 noon, found that the benefit was higher during winter than during summer. A limitation for BLT in the demented population is that the patient must be calm during that time. Riemersma-van der Lek et al,Citation101 in a placebo-controlled double-blind study, found that unattended exposure to bright light during daytime (from 9 am to 6 pm) slightly improved sleep and slowed down cognitive decline (follow-up: 6 months–5 years). The improvement was greater when the therapy was given in combination with melatonin. Figueiro et alCitation106 also obtained an improvement in sleep parameters using a tailored lighting with bright light from 6 or 8 am to 6 pm. As Hanford et alCitation107 underlined, it is important to note that the short-term history of light exposure affects the sensitivity of the circadian system to light. The higher the exposure to light during the day, the lower the sensitivity of the circadian system to light, as measured by nocturnal melatonin suppression and phase shifting. In spite of these positive reports, the Cochrane systematic review carried out by Forbes et alCitation108 concluded that there is insufficient evidence of its effectiveness. They found only ten studies that fulfilled their search criteriaCitation93,Citation101–Citation105,Citation109–Citation112 and the authors concluded that the lack of evidence could well have been due to the heterogeneity of the studies (light intensity, duration of the exposition per day, treatment duration, etc).
Pharmacological treatment
The most commonly used drugs are melatonin, z-hypnotics such as zolpidem, sedating antidepressants, and antipsychotics. Usually benzodiazepines are avoided because they may worsen cognitive function. Cholinesterase inhibitors, the first-line treatment for AD, can also improve sleep quality. However, there are few studies assessing the efficacy of these drugs. In the critical review of the evidence for current treatments, McCleery et alCitation113 found eligible randomized controlled trials for only three drugs: melatonin, trazodone, and ramelteon.
Melatonin
Melatonin is considered not only a chronobiotic treatment, but is also used to treat insomnia.Citation114 The relationship between alterations in the melatonin system and the pathogenesis of AD makes melatonin a particularly interesting target. Furthermore, it has cytoprotective, antioxidant, and antiamyloidogenic effects.Citation72,Citation115–Citation117 Research has focused not only on the improvement of sleep domains but also on the effect on cognition and AD progression.
We found eight randomized placebo-controlled studies.Citation101,Citation104,Citation118–Citation123 All but oneCitation123 used actigraphy to objectively measure the changes in sleep parameters. There were no relevant side effects. The results in efficacy were equivocal. Five studies found improvement in nocturnal sleep.Citation101,Citation104,Citation118,Citation122,Citation123 Two of these studies combined melatonin and BLT.Citation101,Citation104 In a multicenter, double-blind, randomized placebo-controlled trial that has been previously cited in our discussion of BLT, the researchers found that melatonin by itself improved sleep onset latency and total sleep time, but that it had adverse effects on mood and aggravated withdrawn behavior.Citation101 The authors evaluated the effects of daily supplementation of light and/or melatonin in a diverse population with dementia and reported that the combination of BLT and melatonin avoided these adverse effects. Dowling et alCitation104 studied a group treated with BLT and melatonin and a group treated with BLT and placebo and found that only the group with melatonin showed improved sleep parameters. The authors had no group on melatonin without BLT and they concluded that further studies were needed to determine whether the effect was due to the melatonin itself or the two zeitgebers. The largest multicenter, randomized, placebo-controlled trial included 157 subjects.Citation120 In this study, melatonin facilitated sleep in some subjects but collectively there was only a trend toward increased nocturnal sleep time and decreased awakenings after sleep onset as determined by actigraphy. However, the improvement in subjective measures (caregiver ratings of quality of sleep) was significant. Finally, there are two trials that failed to prove efficacy.Citation119,Citation121 The discrepancy could be due to several facts such as the dosage, the release form, or the study duration. Interindividual differences, not uncommon in patients suffering neurodegenerative diseases, could also explain part of the inconsistent results. Neuropsychological assessment was performed in only two of these trialsCitation118,Citation123 and both found improvement in cognitive functions.
The effect of melatonin on AD progression has been tested in patients with mild cognitive impairment (MCI). Approximately 12% of MCI patients convert to AD or another dementia every year, and it has been suggested that MCI is a prodromal AD.Citation124 In a retrospective analysis of 25 MCI patients, Furio et alCitation125 found that melatonin significantly improved cognitive and emotional performance and daily sleep–wake cycles. In a follow-up study, the same group obtained similar results in a larger sample of patients.Citation126
Hypnotics
Hypnotics are classified into benzodiazepines and nonbenzodiazepines. The side effects of benzodiazepines include daytime sedation, anterograde amnesia, daytime sleepiness, confusion, and risk of falls. Given these risks, they are not recommended for AD patients. They also have a deleterious effect on cognition.Citation127 Furthermore, several studies suggest that the long-term use of benzodiazepines increases the risk of AD.Citation128–Citation131 However, other studies have found no such association.Citation132
The side effects profile of nonbenzodiazepines such as zolpidem and zaleplon makes them more suitable for short-term use in patients with AD, but they must be used with caution.Citation133,Citation134 In fact, an increased risk of reversible dementia has been described associated with the use of zolpidem.Citation135
Antidepressants
Sedating antidepressants are used when there is concomitant depression. However, tricyclic antidepressants have anticholinergic activity and may exacerbate the cholinergic disturbances inherent in AD, and should be avoided.Citation5 They also have other side effects such as somnolence, sedation, and dizziness, which are of great concern in the demented population.Citation14
Serotonin reuptake inhibitors with a sedating profile, especially mirtazapine, are also used to treat insomnia. It has been reported that mirtazapine was useful in the treatment of insomnia in three depressed patients with AD.Citation136 However, the risk of undesirable side effects is also high.Citation137
Trazodone is a triazolopyridine antidepressant that offers a dual action on serotonergic receptors by blocking the 2A receptor and inhibiting serotonin reuptake. It improves sleep in patients with depression, but there is insufficient evidence for its use in patients with insomnia without depression.Citation138 However, its usefulness in treating sleep disturbances in patients with AD has been demonstrated. In a double-blind, randomized, controlled trial in 30 patients with AD, trazodone (50 mg during 2 weeks) increased total nocturnal sleep time without significant daytime somnolence or negative effects on cognition or functionality.Citation139 There were no serious adverse effects.
Antipsychotics
Antipsychotics are frequently administered to control behavioral and neuropsychiatric manifestations of AD. Sometimes, when the first-line treatments have failed, they are also used to treat insomnia. However, they are associated with sedation, increased risk of falls, and might also have serious cardiac side effects.Citation140,Citation141 Furthermore, they can aggravate sleep–wake cycle disturbances.Citation142
Antihistaminic drugs
Antihistaminic drugs are included as an option to treat insomnia.Citation143 They have a wide range of side effects including sedation, cognitive impairment, increased daytime somnolence, and anticholinergic responses. Therefore, they do not seem appropriate to be used in AD patients.
Treatment of AD: effect on sleep
Acetylcholinesterase inhibitors are a common treatment for AD. Acetylcholine not only plays a key role in memory functions but also is related to vigilance states. Levels increase during wakefulness, decrease in non-REM sleep, and rise again in REM sleep. Polysomnographic studies in patients taking acetylcholinesterase inhibitors have shown an increase in the percentage of REM sleep, reduced REM latency, and a decrease in REM sleep slow band power.Citation144–Citation146 However, disagreement exists surrounding the effects of acetylcholinesterase inhibitors on sleep, although such discrepancies could be due to the time of administration.Citation147 For example, insomnia and nightmares are frequent side effects of donezepilCitation148 when it is administered at night but produces a slight improvement in sleep quality when administered in the morning.Citation149 Galantamine has the best profile regarding sleep and may be the first choice of cholinesterase inhibitor in mild-to-moderate dementia patients in terms of improving sleep quality.Citation149–Citation151 One case of rivastigmine induced REM behavior disorder (RBD) has been reported.Citation152 However, it seems that donezepil and rivastigmine might be effective to treat RBD.Citation153,Citation154
Treatment of primary sleep disorders
Sleep-related breathing disorders
As mentioned earlier, SRBDsCitation6 have been identified as an independent risk factor for cognitive decline,Citation36 although the mechanism remains unknown. Obstructive sleep apnea syndrome (OSAS) produces intermittent hypoxia, sleep disturbances, and daytime sleepiness that lead to cognitive impairment that could be reversible with the treatment of the breathing disorders.Citation38
Nocturnal continuous pressure airway pressure (CPAP) is the most effective treatment for OSAS. It restores respiratory function and consolidated sleep, increasing SWS and REM sleep.Citation155 Despite inconsistencies, there is some evidence for the effectiveness of CPAP in improving cognition in patients with OSAS.Citation37 In a CPAP treatment versus placebo-CPAP randomized study, CPAP decreased subjective sleepiness and improved a composite neuropsychological score in mild-to-moderate AD patients with an apnea–hypopnea index (AHI) >10.Citation156,Citation157 Osorio et alCitation33 analyzing data from patients of the AD Neuroimaging Initiative cohort on CPAP treatment suggested that CPAP treatment might delay progression of cognitive impairment. However, the tolerance to CPAP is a limiting factor in patients with dementia.
Interestingly, there are some data supporting a possible positive effect of donezepil on OSAS. In 2008, Moraes et alCitation158 published the results of a randomized, double-blind, placebo-controlled trial including 23 patients with mild-moderate AD patients with an AHI >5/h. They found that donepezil improved AHI and oxygen saturation in patients with AD. This treatment also increased REM sleep duration and reduced ADAS-cog scores. In 2012 another randomized, double-blind, placebo-controlled trial found similar results with improvement on obstructive sleep apnea index, oxygen saturation, and sleepiness.Citation159
RLS/periodic limb movement disorders
Nocturnal agitation could be the clinical manifestation of the RLS in some patients with AD. RLS may also cause an inability to fall asleep or to remain asleep. These sleep disturbances might exacerbate cognitive symptoms or even accelerate neurocognitive degeneration.Citation160,Citation161 Thus, a correct diagnosis will probably lead to appropriate treatment. However, the impact of the treatment of RLS, especially dopaminergic agents, on nocturnal agitation and cognitive function in AD patients is not known.Citation14 On the other hand, according to Peter-Derex et al, the pharmacological treatment of periodic limb movements in sleep not associated with RLS is not recommended.
Future directions
There is increasing research interest in the treatment of insomnia and circadian disturbances to modulate the receptors of the neurotransmitters directly involved in the control of sleep and the sleep–wake cycle. The most widely studied molecules are the agonists of melatonin receptors and the antagonists of orexin receptors. Both mechanisms are of special interest in AD due to the role that these neurotransmitters seem to play in sleep disturbances and in the pathogenesis of the disease. Interestingly, currently several groups are designing agents that directly target the circadian clock itself.
Melatonin receptor agonists
Ramelteon is a melatonin receptor agonist with high affinity for the melatonin receptors MT1 and MT2 used to treat insomnia.Citation162 It is well tolerated and appears to lack significant adverse effects.Citation163 The subjective efficacy of ramelteon was evaluated in clinical trials that included 829 elderly outpatients with chronic insomnia. Significant reductions in sleep onset latency and increases in total sleep time were obtained over 5 weeks of treatment.Citation164
Agomelatine is a melatonin MT1 and MT2 receptor agonist and a weak 5-HT2C antagonist.Citation165 It has been approved for the treatment of depression.Citation166 Agomelatine appears to improve sleep quality and reduce wakefulness after sleep onset in depressive patients without causing daytime sedation.Citation167–Citation169 Although it seems well tolerated, there are concerns over its risks because it appears to have the potential to cause severe hepatotoxicity.Citation170,Citation171
Tasimelteon is also a specific MT1 and MT2 agonist, and is the only drug approved by the US Food and Drug Administration (FDA) for the treatment of non-24-hour sleep–wake disorder. Physiologic monitoring revealed that tasimelteon resulted in a higher proportion of individuals becoming entrained to the 24-hour cycle compared with placebo.Citation172 Safety assessments indicated that tasimelteon is well tolerated, with the most common adverse events being headache, elevated alanine aminotransferase levels, nightmares or unusual dreams, and upper respiratory or urinary tract infections.
To our knowledge, no study on the efficacy of ramelteon, agomelatine, and tasimelteon in the treatment of comorbid insomnia in patients suffering from AD has been published to date.
Orexin receptor antagonists
Currently a new generation of hypnotics is emerging. The orexin receptor antagonists include the single orexin receptor antagonists and the dual orexin receptor antagonists (DORAs).Citation173–Citation179
Suvorexant is the first orexin receptor antagonist (DORA) that has been shown to be effective in treating insomnia. It appears to be suitable as a chronic therapy for insomnia given the minimal risk of physical dependence.Citation180 Suvorexant has fewer adverse effects than the classical hypnotics, and it has been found to be generally safe and well tolerated.Citation181 At the recommended therapeutic dose of less than 20 mg, the most common adverse effect reported was somnolence. However, the FDA and the sponsor disagreed over the effective versus safe doses (November 2012). The FDA considered that 5–15 mg were efficient and probably safe, whereas the sponsors had proposed 15–40 mg. The final approved doses are 5, 10, 15, and 20 mg. The major issues are next-morning somnolence and safety, as seen in driving tests. However, signs of muscle weakness, weird dreams, sleep walking, other nighttime behaviors, and suicidal ideation were also reported. On the other hand, it has been found that suvorexant does not aggravate apneas or oxygen desaturations in patients with mild-to-moderate obstructive sleep apnea using twice the approved dose.Citation182 However, all these data were obtained in studies with healthy volunteers and the efficacy and side effects in patients with AD remain unknown.
Modulators of the circadian clock
At present several researchers are focusing on the identification of molecules and receptors that can alter the expression of clock genes.Citation183–Citation189 Although such research is in a preliminary phase, these molecules could be the target to develop drugs that modulate the circadian clock.
Conclusion
Sleep and circadian disturbances are very frequent in AD patients and appear early in the course of the disease. They include a wide range of problems that severely affect the quality of life of the patient, family, and caregivers. In recent years, increasing evidence for the role of melatonin and hypocretins in the cause and mechanism of these disturbances has been found. There is a bidirectional relationship between these disorders and AD pathophysiology, a fact that raises the possibility of modifying the course of AD itself by treating the sleep disorders. The current treatments include nonpharmacological and pharmacological approaches. However, overall these are still unsatisfactory. Evidence of efficacy is scarce and contradictory results are common, with the only exceptions being the use of BLT and melatonin. Future directions for treatment include the establishment of protocols for effective BLT, the development of melatonin receptor agonists, hypocretin (orexin) receptor antagonists, and, although as yet in a very preliminary phase, the modulation of the circadian clock.
Disclosure
The authors report no conflicts of interest in this work.
References
- HebertLEWeuveJScherrPAEvansDAAlzheimer disease in the United States (2010–2050) estimated using the 2010 censusNeurology201380191778178323390181
- PistacchiMGioulisMContinFSansonFMarsalaSZSleep disturbance and cognitive disorder: epidemiological analysis in a cohort of 263 patientsNeurol Sci201435121955196225034185
- MoranMLynchCAWalshCCoenRCoakleyDLawlorBASleep disturbance in mild to moderate Alzheimer’s diseaseSleep Med20056434735215978517
- CiprianiGLucettiCDantiSNutiASleep disturbances and dementiaPsychogeriatrics2015151657425515641
- VitielloMVBorsonSSleep disturbances in patients with Alzheimer’s disease: epidemiology, pathophysiology and treatmentCNS Drugs2001151077779611602004
- HochCCReynoldsCF3rdKupferDJHouckPRBermanSRStackJASleep-disordered breathing in normal and pathologic agingJ Clin Psychiatry198647104995033759914
- Ancoli-IsraelSKlauberMRButtersNParkerLKripkeDFDementia in institutionalized elderly: relation to sleep apneaJ Am Geriatr Soc19913932582632005339
- ShinHYHanHJShinDJParkHMLeeYBParkKHSleep problems associated with behavioral and psychological symptoms as well as cognitive functions in Alzheimer’s diseaseJ Clin Neurol201410320320925045372
- GauglerJEEdwardsABFemiaEEPredictors of institutionalization of cognitively impaired elders: family help and the timing of placementJ Gerontol B Psychol Sci Soc Sci2000554P247P25511584881
- JuYELuceyBPHoltzmanDMSleep and Alzheimer disease pathology – a bidirectional relationshipNat Rev Neurol201410211511924366271
- GuarnieriBSorbiSSleep and cognitive decline: a strong bidirectional relationship. It is time for specific recommendations on routine assessment and the management of sleep disorders in patients with mild cognitive impairment and dementiaEur Neurol2015741–2434826159605
- VillaCFerini-StrambiLCombiRThe synergistic relationship between Alzheimer’s disease and sleep disorders: an updateJ Alzheimers Dis201546357158025835421
- CookeJRAncoli-IsraelSNormal and abnormal sleep in the elderlyHandb Clin Neurol20119865366521056216
- Peter-DerexLYamminePBastujiHCroisileBSleep and Alzheimer’s diseaseSleep Med Rev201519293824846773
- PrinzPNPeskindERVitalianoPPChanges in the sleep and waking EEGs of nondemented and demented elderly subjectsJ Am Geriatr Soc198230286937199061
- PetitDGagnonJFFantiniMLFerini-StrambiLMontplaisirJSleep and quantitative EEG in neurodegenerative disordersJ Psychosom Res200456548749615172204
- BornJRaschBGaisSSleep to rememberNeuroscientist200612541042416957003
- DiekelmannSBornJThe memory function of sleepNat Rev Neurosci201011211412620046194
- RauchsGSchabusMParapaticsSIs there a link between sleep changes and memory in Alzheimer’s disease?Neuroreport200819111159116218596620
- RauchsGPiolinoPBertranFRetrieval of recent autobiographical memories is associated with slow-wave sleep in early ADFront Behav Neurosci2013711424065896
- SongYDowlingGAWallhagenMILeeKAStrawbridgeWJSleep in older adults with Alzheimer’s diseaseJ Neurosci Nurs2010424190198 quiz 199–20020804113
- BliwiseDLSleep disorders in Alzheimer’s disease and other dementiasClin Cornerstone20046Suppl 1AS16S2815259536
- KhachiyantsNTrinkleDSonSJKimKYSundown syndrome in persons with dementia: an updatePsychiatry Investig201184275287
- CooganANSchutovaBHusungSThe circadian system in Alzheimer’s disease: disturbances, mechanisms, and opportunitiesBiol Psychiatry201374533333923273723
- GnanasekaranG“Sundowning” as a biological phenomenon: current understandings and future directions: an updateAging Clin Exp Res Epub201585
- FerrazzoliDSicaFSancesarioGSundowning syndrome: a possible marker of frailty in Alzheimer’s disease?CNS Neurol Disord Drug Targets201312452552823574165
- GiubileiFPatacchioliFRAntoniniGAltered circadian cortisol secretion in Alzheimer’s disease: clinical and neuroradiological aspectsJ Neurosci Res200166226226511592122
- HarperDGStopaEGMcKeeACDifferential circadian rhythm disturbances in men with Alzheimer disease and frontotemporal degenerationArch Gen Psychiatry200158435336011296096
- VidenovicALazarASBarkerRAOvereemS“The clocks that time us” – circadian rhythms in neurodegenerative disordersNat Rev Neurol2014101268369325385339
- StranahanAMChronobiological approaches to Alzheimer’s diseaseCurr Alzheimer Res201291939822329654
- KadotaniHKadotaniTYoungTAssociation between apolipoprotein E epsilon4 and sleep-disordered breathing in adultsJAMA2001285222888289011401610
- PanWKastinAJCan sleep apnea cause Alzheimer’s disease?Neurosci Biobehav Rev20144765666925451764
- OsorioRSGumbTPirragliaESleep-disordered breathing advances cognitive decline in the elderlyNeurology201584191964197125878183
- ReynoldsCF3rdKupferDJTaskaLSSleep apnea in Alzheimer’s dementia: correlation with mental deteriorationJ Clin Psychiatry19854672572614008448
- CookeJRLiuLNatarajanLThe effect of sleep-disordered breathing on stages of sleep in patients with Alzheimer’s diseaseBehav Sleep Med20064421922717083302
- YaffeKLaffanAMHarrisonSLSleep-disordered breathing, hypoxia, and risk of mild cognitive impairment and dementia in older womenJAMA2011306661361921828324
- KielbSAAncoli-IsraelSRebokGWSpiraAPCognition in obstructive sleep apnea-hypopnea syndrome (OSAS): current clinical knowledge and the impact of treatmentNeuromolecular Med201214318019322569877
- LalCStrangeCBachmanDNeurocognitive impairment in obstructive sleep apneaChest201214161601161022670023
- BenedictCBybergLCedernaesJSelf-reported sleep disturbance is associated with Alzheimer’s disease risk in menAlzheimers Dement20151191090109725438949
- JuYEMcLelandJSToedebuschCDSleep quality and preclinical Alzheimer diseaseJAMA Neurol201370558759323479184
- LimASKowgierMYuLBuchmanASBennettDASleep fragmentation and the risk of incident Alzheimer’s disease and cognitive decline in older personsSleep20133671027103223814339
- TranahGJBlackwellTStoneKLCircadian activity rhythms and risk of incident dementia and mild cognitive impairment in older womenAnn Neurol201170572273222162057
- TsapanouAGuYO’SheaDDaytime somnolence as an early sign of cognitive decline in a community-based study of older peopleInt J Geriatr Psychiatry Epub2015615
- SpiraAPChen-EdinboroLPWuMNYaffeKImpact of sleep on the risk of cognitive decline and dementiaCurr Opin Psychiatry201427647848325188896
- HahnEAWangHXAndelRFratiglioniLA change in sleep pattern may predict Alzheimer diseaseAm J Geriatr Psychiatry201422111262127123954041
- SpiraAPGamaldoAAAnYSelf-reported sleep and beta-amyloid deposition in community-dwelling older adultsJAMA Neurol201370121537154324145859
- LimASYuLKowgierMSchneiderJABuchmanASBennettDAModification of the relationship of the apolipoprotein E epsilon4 allele to the risk of Alzheimer disease and neurofibrillary tangle density by sleepJAMA Neurol201370121544155124145819
- HuangYPotterRSigurdsonWBeta-amyloid dynamics in human plasmaArch Neurol201269121591159723229043
- MusiekESXiongDDHoltzmanDMSleep, circadian rhythms, and the pathogenesis of Alzheimer diseaseExp Mol Med201547e14825766617
- SlatsDClaassenJAVerbeekMMOvereemSReciprocal interactions between sleep, circadian rhythms and Alzheimer’s disease: focus on the role of hypocretin and melatoninAgeing Res Rev201312118820022575905
- de LeceaLKilduffTSPeyronCThe hypocretins: hypothalamus-specific peptides with neuroexcitatory activityProc Natl Acad Sci U S A19989513223279419374
- PeyronCTigheDKvan den PolANNeurons containing hypocretin (orexin) project to multiple neuronal systemsJ Neurosci199818239996100159822755
- MiedaMTsujinoNSakuraiTDifferential roles of orexin receptors in the regulation of sleep/wakefulnessFront Endocrinol (Lausanne)201345723730297
- TsujinoNSakuraiTRole of orexin in modulating arousal, feeding, and motivationFront Behav Neurosci201372823616752
- KangJELimMMBatemanRJAmyloid-beta dynamics are regulated by orexin and the sleep-wake cycleScience200932659551005100719779148
- FronczekRvan GeestSFrolichMHypocretin (orexin) loss in Alzheimer’s diseaseNeurobiol Aging20123381642165021546124
- RipleyBOvereemSFujikiNCSF hypocretin/orexin levels in narcolepsy and other neurological conditionsNeurology200157122253225811756606
- DauvilliersYBaumannCRCarlanderBCSF hypocretin-1 levels in narcolepsy, kleine-levin syndrome, and other hypersomnias and neurological conditionsJ Neurol Neurosurg Psychiatry200374121667167314638887
- DeuschleMSchillingCLewekeFMHypocretin in cerebrospinal fluid is positively correlated with tau and pTauNeurosci Lett2014561414524373987
- SchmidtFMKratzschJGertzHJCerebrospinal fluid melanin-concentrating hormone (MCH) and hypocretin-1 (HCRT-1, orexin-A) in Alzheimer’s diseasePLoS One201385e6313623667582
- WennstromMLondosEMinthonLNielsenHMAltered CSF orexin and alpha-synuclein levels in dementia patientsJ Alzheimers Dis201229112513222207004
- SlatsDClaassenJALammersGJMelisRJVerbeekMMOvereemSAssociation between hypocretin-1 and amyloid-beta42 cerebrospinal fluid levels in Alzheimer’s disease and healthy controlsCurr Alzheimer Res20129101119112522742854
- DauvilliersYALehmannSJaussentIGabelleAHypocretin and brain beta-amyloid peptide interactions in cognitive disorders and narcolepsyFront Aging Neurosci2014611924966833
- ClaustratBLestonJMelatonin: physiological effects in humansNeurochirurgie2015612–3778425908646
- MishimaKTozawaTSatohKMatsumotoYHishikawaYOkawaMMelatonin secretion rhythm disorders in patients with senile dementia of Alzheimer’s type with disturbed sleep-wakingBiol Psychiatry199945441742110071710
- WuYHSwaabDFThe human pineal gland and melatonin in aging and Alzheimer’s diseaseJ Pineal Res200538314515215725334
- ZhouJNLiuRYKamphorstWHofmanMASwaabDFEarly neuropathological Alzheimer’s changes in aged individuals are accompanied by decreased cerebrospinal fluid melatonin levelsJ Pineal Res200335212513012887656
- WuYHFischerDFKalsbeekAPineal clock gene oscillation is disturbed in Alzheimer’s disease, due to functional disconnection from the “master clock.”FASEB J200620111874187616818472
- LiuRYZhouJNvan HeerikhuizeJHofmanMASwaabDFDecreased melatonin levels in postmortem cerebrospinal fluid in relation to aging, Alzheimer’s disease, and apolipoprotein E-epsilon4/4 genotypeJ Clin Endocrinol Metab19998413233279920102
- Hita-YanezEAtienzaMCanteroJLPolysomnographic and subjective sleep markers of mild cognitive impairmentSleep20133691327133423997365
- GottliebDJDeStefanoALFoleyDJAPOE epsilon4 is associated with obstructive sleep apnea/hypopnea: the sleep heart health studyNeurology200463466466815326239
- LinLHuangQXYangSSChuJWangJZTianQMelatonin in Alzheimer’s diseaseInt J Mol Sci2013147145751459323857055
- RoseKMBeckCTsaiPFSleep disturbances and nocturnal agitation behaviors in older adults with dementiaSleep201134677978621629366
- AllenRPKushidaCAAtkinsonMJRLS QoL ConsortiumFactor analysis of the international restless legs syndrome study group’s scale for restless legs severitySleep Med20034213313514592343
- GuarnieriBAdorniFMusiccoMPrevalence of sleep disturbances in mild cognitive impairment and dementing disorders: a multicenter Italian clinical cross-sectional study on 431 patientsDement Geriatr Cogn Disord2012331505822415141
- TalaricoGCanevelliMTostoGRestless legs syndrome in a group of patients with Alzheimer’s diseaseAm J Alzheimers Dis Other Demen201328216517023264651
- RedekerNSSteinSCharacteristics of sleep in patients with stable heart failure versus a comparison groupHeart Lung200635425226116863897
- DignaniLToccaceliALucertiniCPetrucciCLanciaLSleep and quality of life in people with COPD: a descriptive-correlational studyClin Nurs Res Epub201564
- Geiger-BrownJLindbergSKrachmanSSelf-reported sleep quality and acute exacerbations of chronic obstructive pulmonary diseaseInt J Chron Obstruct Pulmon Dis20151038939725759571
- KatzPMargarettenMTrupinLSchmajukGYazdanyJYelinESleep disturbance, depression, obesity, and physical inactivity explain a significant portion of fatigue in rheumatoid arthritisArthritis Care Res (Hoboken) Epub2015316
- FujiwaraYArakawaTFassRGastroesophageal reflux disease and sleepGastroenterol Clin North Am2013421577023452631
- BliwiseDLRosenRCBaumNImpact of nocturia on sleep and quality of life: a brief, selected review for the international consultation on incontinence research society (ICI-RS) nocturia think tankNeurourol Urodyn201433Suppl 1S15S1824729148
- MigliorelliRTesonASabeLPetracchiMLeiguardaRStarksteinSEPrevalence and correlates of dysthymia and major depression among patients with Alzheimer’s diseaseAm J Psychiatry1995152137447802118
- ForalPKnezevichJDewanNMaleskerMMedication-induced sleep disturbancesConsult Pharm201126641442521628140
- MostEIAboudanSScheltensPVan SomerenEJDiscrepancy between subjective and objective sleep disturbances in early- and moderate-stage Alzheimer diseaseAm J Geriatr Psychiatry201220646046722531105
- TractenbergRESingerCMCummingsJLThalLJThe sleep disorders inventory: an instrument for studies of sleep disturbance in persons with Alzheimer’s diseaseJ Sleep Res200312433133714633245
- YesavageJAFriedmanLAncoli-IsraelSDevelopment of diagnostic criteria for defining sleep disturbance in Alzheimer’s diseaseJ Geriatr Psychiatry Neurol200316313113912967054
- Van de WaterATHolmesAHurleyDAObjective measurements of sleep for non-laboratory settings as alternatives to polysomnography – a systematic reviewJ Sleep Res2011201 Pt 218320020374444
- Ancoli-IsraelSCloptonPKlauberMRFellRMasonWUse of wrist activity for monitoring sleep/wake in demented nursing-home patientsSleep199720124279130330
- BrownCABerryRTanMCKhoshiaATurlapatiLSwedloveFA critique of the evidence base for non-pharmacological sleep interventions for persons with dementiaDementia (London)201312221023724336770
- AlessiCAYoonEJSchnelleJFAl-SamarraiNRCruisePAA randomized trial of a combined physical activity and environmental intervention in nursing home residents: do sleep and agitation improve?J Am Geriatr Soc199947778479110404920
- GitlinLNKalesHCLyketsosCGNonpharmacologic management of behavioral symptoms in dementiaJAMA2012308192020202923168825
- McCurrySMPikeKCVitielloMVLogsdonRGLarsonEBTeriLIncreasing walking and bright light exposure to improve sleep in community-dwelling persons with Alzheimer’s disease: results of a randomized, controlled trialJ Am Geriatr Soc20115981393140221797835
- DeschenesCLMcCurrySMCurrent treatments for sleep disturbances in individuals with dementiaCurr Psychiatry Rep2009111202619187704
- ShubDDarvishiRKunikMENon-pharmacologic treatment of insomnia in persons with dementiaGeriatrics2009642222619256583
- MishimaKOkawaMHishikawaYHozumiSHoriHTakahashiKMorning bright light therapy for sleep and behavior disorders in elderly patients with dementiaActa Psychiatr Scand1994891178140901
- Van SomerenEJKesslerAMirmiranMSwaabDFIndirect bright light improves circadian rest-activity rhythm disturbances in demented patientsBiol Psychiatry19974199559639110101
- ItoTYamaderaHItoREndoSEffects of bright light on cognitive disturbances in Alzheimer-type dementiaNihon Ika Daigaku Zasshi199966422923810466338
- YamaderaHItoTSuzukiHAsayamaKItoREndoSEffects of bright light on cognitive and sleep-wake (circadian) rhythm disturbances in Alzheimer-type dementiaPsychiatry Clin Neurosci200054335235311186110
- GrafAWallnerCSchubertVThe effects of light therapy on mini-mental state examination scores in demented patientsBiol Psychiatry200150972572711704081
- Riemersma-van der LekRFSwaabDFTwiskJHolEMHoogendijkWJVan SomerenEJEffect of bright light and melatonin on cognitive and noncognitive function in elderly residents of group care facilities: a randomized controlled trialJAMA2008299222642265518544724
- Ancoli-IsraelSGehrmanPMartinJLIncreased light exposure consolidates sleep and strengthens circadian rhythms in severe Alzheimer’s disease patientsBehav Sleep Med200311223615600135
- DowlingGAHubbardEMMastickJLuxenbergJSBurrRLVan SomerenEJEffect of morning bright light treatment for rest-activity disruption in institutionalized patients with severe Alzheimer’s diseaseInt Psychogeriatr200517222123616050432
- DowlingGABurrRLVan SomerenEJMelatonin and bright-light treatment for rest-activity disruption in institutionalized patients with Alzheimer’s diseaseJ Am Geriatr Soc200856223924618070004
- BurnsAAllenHTomensonBDuignanDByrneJBright light therapy for agitation in dementia: a randomized controlled trialInt Psychogeriatr200921471172119323872
- FigueiroMGPlitnickBALokATailored lighting intervention improves measures of sleep, depression, and agitation in persons with Alzheimer’s disease and related dementia living in long-term care facilitiesClin Interv Aging201491527153725246779
- HanfordNFigueiroMLight therapy and Alzheimer’s disease and related dementia: Past, present, and futureJ Alzheimers Dis201333491392223099814
- ForbesDBlakeCMThiessenEJPeacockSHawranikPLight therapy for improving cognition, activities of daily living, sleep, challenging behaviour, and psychiatric disturbances in dementiaCochrane Database Syst Rev20142CD00394624574061
- Fontana GasioPKrauchiKCajochenCDawn-dusk simulation light therapy of disturbed circadian rest-activity cycles in demented elderlyExp Gerontol2003381–220721612543279
- Ancoli-IsraelSMartinJLGehrmanPEffect of light on agitation in institutionalized patients with severe Alzheimer diseaseAm J Geriatr Psychiatry200311219420312611749
- DowlingGAGrafCLHubbardEMLuxenbergJSLight treatment for neuropsychiatric behaviors in Alzheimer’s diseaseWest J Nurs Res200729896197517596638
- NowakLThe effect of timed blue-green light on sleep-wake patterns in women with Alzheimer’ diseaseDissertation Abstracts Int Sect B Theses Sci Eng2008696-B
- McCleeryJCohenDASharpleyALPharmacotherapies for sleep disturbances in Alzheimer’s diseaseCochrane Database Syst Rev20143CD00917824659320
- NeubauerDNChronic insomniaContinuum (Minneap Minn)2013191 Sleep Disorders506623385694
- CardinaliDPFurioAMReyesMPClinical perspectives for the use of melatonin as a chronobiotic and cytoprotective agentAnn N Y Acad Sci2005105732733616399904
- PolimeniGEspositoEBevelacquaVGuarneriCCuzzocreaSRole of melatonin supplementation in neurodegenerative disordersFront Biosci (Landmark Ed)20141942944624389194
- Di DomenicoFBaroneEPerluigiMButterfieldDAStrategy to reduce free radical species in Alzheimer’s disease: an update of selected antioxidantsExpert Rev Neurother2015151194025243342
- AsayamaKYamaderaHItoTSuzukiHKudoYEndoSDouble blind study of melatonin effects on the sleep-wake rhythm, cognitive and non-cognitive functions in Alzheimer type dementiaJ Nippon Med Sch200370433434112928714
- GehrmanPRConnorDJMartinJLShochatTCorey-BloomJAncoli-IsraelSMelatonin fails to improve sleep or agitation in double-blind randomized placebo-controlled trial of institutionalized patients with Alzheimer diseaseAm J Geriatr Psychiatry200917216616919155748
- SingerCTractenbergREKayeJA multicenter, placebo-controlled trial of melatonin for sleep disturbance in Alzheimer’s diseaseSleep200326789390114655926
- SerfatyMKennell-WebbSWarnerJBlizardRRavenPDouble blind randomised placebo controlled trial of low dose melatonin for sleep disorders in dementiaInt J Geriatr Psychiatry200217121120112712461760
- MahlbergRWaltherSActigraphy in agitated patients with dementia. monitoring treatment outcomesZ Gerontol Geriatr200740317818417565435
- WadeAGFarmerMHarariGAdd-on prolonged-release melatonin for cognitive function and sleep in mild to moderate Alzheimer’s disease: a 6-month, randomized, placebo-controlled, multicenter trialClin Interv Aging2014994796124971004
- DuboisBAlbertMLAmnestic MCI or prodromal Alzheimer’s disease?Lancet Neurol20043424624815039037
- FurioAMBruscoLICardinaliDPPossible therapeutic value of melatonin in mild cognitive impairment: a retrospective studyJ Pineal Res200743440440917910609
- CardinaliDPVigoDEOlivarNVidalMFFurioAMBruscoLITherapeutic application of melatonin in mild cognitive impairmentAm J Neurodegener Dis20121328029123383398
- VermeerenACoenenAMEffects of the use of hypnotics on cognitionProg Brain Res20111908910321531246
- Billioti de GageSMorideYDucruetTBenzodiazepine use and risk of Alzheimer’s disease: case-control studyBMJ2014349g520525208536
- DefrancescoMMarksteinerJFleischhackerWWBlaskoIUse of benzodiazepines in Alzheimer’s disease: a systematic review of literatureInt J Neuropsychopharmacol20151810pyv05525991652
- RosenbergPBBenzodiazepine exposure increases risk of Alzheimer’s diseaseEvid Based Med2015202110117
- WuCSWangSCChangISLinKMThe association between dementia and long-term use of benzodiazepine in the elderly: nested case-control study using claims dataAm J Geriatr Psychiatry200917761462019546656
- ZhangYZhouXHMeranusDHWangLKukullWABenzodiazepine use and cognitive decline in elderly with normal cognitionAlzheimer Dis Assoc Disord Epub201565
- OtmaniSDemazieresAStanerCEffects of prolonged-release melatonin, zolpidem, and their combination on psychomotor functions, memory recall, and driving skills in healthy middle aged and elderly volunteersHum Psychopharmacol200823869370518763235
- BeckerPMSomiahMNon-benzodiazepine receptor agonists for insomniaSleep Med Clin2015101577626055674
- ShihHILinCCTuYFAn increased risk of reversible dementia may occur after zolpidem derivative use in the elderly population: a population-based case-control studyMedicine (Baltimore)20159417e80925929937
- RajiMABradySRMirtazapine for treatment of depression and comorbidities in Alzheimer diseaseAnn Pharmacother20013591024102711573849
- BanerjeeSHellierJDeweyMSertraline or mirtazapine for depression in dementia (HTA-SADD): a randomised, multicentre, double-blind, placebo-controlled trialLancet2011378978940341121764118
- MendelsonWBA review of the evidence for the efficacy and safety of trazodone in insomniaJ Clin Psychiatry200566446947615816789
- CamargosEFLouzadaLLQuintasJLNavesJOLouzadaFMNobregaOTTrazodone improves sleep parameters in Alzheimer disease patients: a randomized, double-blind, and placebo-controlled studyAm J Geriatr Psychiatry201422121565157424495406
- MaustDTKimHMSeyfriedLSAntipsychotics, other psychotropics, and the risk of death in patients with dementia: number needed to harmJAMA Psychiatry201572543844525786075
- TanLTanLWangHFEfficacy and safety of atypical antipsychotic drug treatment for dementia: a systematic review and meta-analysisAlzheimers Res Ther20157120 eCollection 201525897331
- Wirz-JusticeAWerthESavaskanEKnoblauchVGasioPFMuller-SpahnFHaloperidol disrupts, clozapine reinstates the circadian rest-activity cycle in a patient with early-onset Alzheimer diseaseAlzheimer Dis Assoc Disord200014421221511186599
- LippmannSMazourIShahabHInsomnia: therapeutic approachSouth Med J200194986687311592743
- CookeJRLoredoJSLiuLAcetylcholinesterase inhibitors and sleep architecture in patients with Alzheimer’s diseaseDrugs Aging200623650351116872233
- Moraes WdosSPoyaresDRGuilleminaultCRamosLRBertolucciPHTufikSThe effect of donepezil on sleep and REM sleep EEG in patients with Alzheimer disease: a double-blind placebo-controlled studySleep200629219920516494088
- SchredlMHornungORegenFAlbrechtNDanker-HopfeHHeuserIThe effect of donepezil on sleep in elderly, healthy persons: a double-blind placebo-controlled studyPharmacopsychiatry200639620520817124641
- SongHRWooYSWangHRJunTYBahkWMEffect of the timing of acetylcholinesterase inhibitor ingestion on sleepInt Clin Psychopharmacol201328634634823948729
- KitabayashiYUedaHTsuchidaHYamashitaTNarumotoJFukuiKDonepezil-induced nightmares in mild cognitive impairmentPsychiatry Clin Neurosci200660112312416472374
- NaharciMIOzturkAYasarHGalantamine improves sleep quality in patients with dementiaActa Neurol Belg2015115456356825777522
- Ancoli-IsraelSAmatniekJAscherSSadikKRamaswamyKEffects of galantamine versus donepezil on sleep in patients with mild to moderate Alzheimer disease and their caregivers: a double-blind, head-to-head, randomized pilot studyAlzheimer Dis Assoc Disord200519424024516327351
- KauferDIBorsonSKershawPSadikKReduction of caregiver burden in Alzheimer’s disease by treatment with galantamineCNS Spectr200510648148815908902
- YehSBYehPYSchenckCHRivastigmine-induced REM sleep behavior disorder (RBD) in a 88-year-old man with Alzheimer’s diseaseJ Clin Sleep Med20106219219520411699
- RingmanJMSimmonsJHTreatment of REM sleep behavior disorder with donepezil: a report of three casesNeurology200055687087110994012
- BrunettiVLosurdoATestaniERivastigmine for refractory REM behavior disorder in mild cognitive impairmentCurr Alzheimer Res201411326727324597506
- VermaARadtkeRAVanLandinghamKEKingJHHusainAMSlow wave sleep rebound and REM rebound following the first night of treatment with CPAP for sleep apnea: correlation with subjective improvement in sleep qualitySleep Med20012321522311311684
- ChongMSAyalonLMarlerMContinuous positive airway pressure reduces subjective daytime sleepiness in patients with mild to moderate Alzheimer’s disease with sleep disordered breathingJ Am Geriatr Soc200654577778116696743
- Ancoli-IsraelSPalmerBWCookeJRCognitive effects of treating obstructive sleep apnea in Alzheimer’s disease: a randomized controlled studyJ Am Geriatr Soc200856112076208118795985
- MoraesWPoyaresDSukys-ClaudinoLGuilleminaultCTufikSDonepezil improves obstructive sleep apnea in Alzheimer disease: a double-blind, placebo-controlled studyChest2008133367768318198262
- Sukys-ClaudinoLMoraesWGuilleminaultCTufikSPoyaresDBeneficial effect of donepezil on obstructive sleep apnea: a double-blind, placebo-controlled clinical trialSleep Med201213329029622281004
- PearsonVEAllenRPDeanTGamaldoCELesageSREarleyCJCognitive deficits associated with restless legs syndrome (RLS)Sleep Med200671253016198145
- GamaldoCEBenbrookARAllenRPOguntimeinOEarleyCJA further evaluation of the cognitive deficits associated with restless legs syndrome (RLS)Sleep Med20089550050517869573
- SrinivasanVKaurCPandi-PerumalSBrownGMCardinaliDPMelatonin and its agonist ramelteon in Alzheimer’s disease: Possible therapeutic valueInt J Alzheimers Dis2010201174197421197086
- Pandi-PerumalSRSpenceDWVersterJCPharmacotherapy of insomnia with ramelteon: Safety, efficacy and clinical applicationsJ Cent Nerv Syst Dis20113516523861638
- RothTSeidenDSainatiSWang-WeigandSZhangJZeePEffects of ramelteon on patient-reported sleep latency in older adults with chronic insomniaSleep Med20067431231816709464
- Guardiola-LemaitreBDe BodinatCDelagrangePMillanMJMunozCMocaerEAgomelatine: mechanism of action and pharmacological profile in relation to antidepressant propertiesBr J Pharmacol2014171153604361924724693
- TaylorDSparshattAVarmaSOlofinjanaOAntidepressant efficacy of agomelatine: meta-analysis of published and unpublished studiesBMJ2014348g188824647162
- DubovskySLWarrenCAgomelatine, a melatonin agonist with antidepressant propertiesExpert Opin Investig Drugs2009181015331540
- SansoneRASansoneLAAgomelatine: a novel antidepressantInnov Clin Neurosci20118111014
- KasperSHajakGThe efficacy of agomelatine in previously-treated depressed patientsEur Neuropsychopharmacol201323881482123820051
- MacIsaacSECarvalhoAFChaDSMansurRBMcIntyreRSThe mechanism, efficacy, and tolerability profile of agomelatineExpert Opin Pharmacother201415225927424328686
- GahrMAgomelatine in the treatment of major depressive disorder: an assessment of benefits and risksCurr Neuropharmacol201412528739825426008
- NeubauerDNTasimelteon for the treatment of non-24-hour sleep-wake disorderDrugs Today (Barc)2015511293525685859
- EquihuaACDe La Herran-AritaAKDrucker-ColinROrexin receptor antagonists as therapeutic agents for insomniaFront Pharmacol2013416324416019
- BossCOrexin receptor antagonists – a patent review (2010 to august 2014)Expert Opin Ther Pat201424121367138125407283
- ChristopherJAOrexin receptor antagonistsPharm Pat Anal20121332934624236845
- AndrewsSPAvesSJChristopherJANonooROrexin receptor antagonists: Historical perspectives and future opportunitiesCurr Top Med Chem Epub2015929
- RoeckerAJCoxCDColemanPJOrexin receptor antagonists: new therapeutic agents for the treatment of insomniaJ Med Chem Epub2015915
- MiedaMSakuraiTOrexin (hypocretin) receptor agonists and antagonists for treatment of sleep disorders. Rationale for development and current statusCNS Drugs2013272839023359095
- WinrowCJRengerJJDiscovery and development of orexin receptor antagonists as therapeutics for insomniaBr J Pharmacol2014171228329323731216
- MichelsonDSnyderEParadisESafety and efficacy of suvorexant during 1-year treatment of insomnia with subsequent abrupt treatment discontinuation: a phase 3 randomised, double-blind, placebo-controlled trialLancet Neurol201413546147124680372
- JacobsonLHCallanderGEHoyerDSuvorexant for the treatment of insomniaExpert Rev Clin Pharmacol20147671173025318834
- SunHPalczaJCardDEffects of suvorexant, an orexin receptor antagonist, on respiration during sleep in patients with obstructive sleep apneaJ Clin Sleep Med Epub2015615
- HirotaTLeeJWLewisWGHigh-throughput chemical screen identifies a novel potent modulator of cellular circadian rhythms and reveals CKIalpha as a clock regulatory kinasePLoS Biol2010812e100055921179498
- ChenZYooSHParkYSIdentification of diverse modulators of central and peripheral circadian clocks by high-throughput chemical screeningProc Natl Acad Sci U S A2012109110110622184224
- Gerhart-HinesZLazarMARev-erbalpha and the circadian transcriptional regulation of metabolismDiabetes Obes Metab201517Suppl 1121626332963
- MehtaNChengAHChiangCKGRK2 fine-tunes circadian clock speed and entrainment via transcriptional and post-translational control of PERIOD proteinsCell Rep20151281272128826279567
- PritchettDJagannathABrownLADeletion of metabotropic glutamate receptors 2 and 3 (mGlu2 and mGlu3) in mice disrupts sleep and wheel-running activity, and increases the sensitivity of the circadian system to lightPLoS One2015105e012552325950516
- SerchovTJilgAWolfCTRadtkeIStehleJHHeumannRRas activity oscillates in the mouse suprachiasmatic nucleus and modulates circadian clock dynamicsMol Neurobiol Epub2015312
- SoltLAWangYBanerjeeSRegulation of circadian behaviour and metabolism by synthetic REV-ERB agonistsNature20124857396626822460951
