Abstract
Sleep–wake disruption is frequently observed and often one of the earliest reported symptoms of many neurodegenerative disorders. This provides insight into the underlying pathophysiology of these disorders, as sleep–wake abnormalities are often accompanied by neurodegenerative or neurotransmitter changes. However, in addition to being a symptom of the underlying neurodegenerative condition, there is also emerging evidence that sleep disturbance itself may contribute to the development and facilitate the progression of several of these disorders. Due to its impact both as an early symptom and as a potential factor contributing to ongoing neurodegeneration, the sleep–wake cycle is an ideal target for further study for potential interventions not only to lessen the burden of these diseases but also to slow their progression. In this review, we will highlight the sleep phenotypes associated with some of the major neurodegenerative disorders, focusing on the circadian disruption associated with Alzheimer’s disease, the rapid eye movement behavior disorder and sleep fragmentation associated with Parkinson’s disease, and the insomnia and circadian dysregulation associated with Huntington’s disease.
Introduction
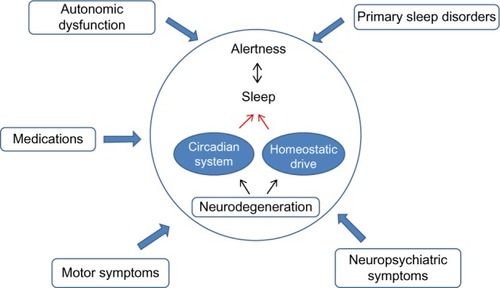
Sleep and neurodegenerative disorders are highly intertwined, and this link is bidirectional. As will be illustrated in this review, sleep disturbances are often early markers of ongoing neurodegeneration. However, it is unclear whether these sleep disturbances may be the cause of, or are simply a symptom of, the underlying disease. In this review, we will present evidence pointing toward both sides of this causal pathway. In addition, each of the major neurodegenerative disorders has its own sleep “phenotype” or characteristic aspects of the sleep–wake cycle that are disrupted in the disorder which we will review ().
Sleep disruption as a symptom of neurodegenerative disease
Sleep and wake are distinct phenomena regulated by specific brain regions and sets of neurotransmitters. Wakefulness is controlled by multiple brain regions, including cholinergic neurons in the basal forebrain and laterodorsal and pedunculopontine tegmental nuclei and norepinephrine-containing neurons in the locus coeruleus. The brain regions are innervated by neurons containing the peptide orexin, which promotes wakefulness. Sleep is initiated by GABA-containing neurons in the ventrolateral preoptic nucleus and can be divided into NREM and REM sleep. NREM sleep is initiated through ventrolateral preoptic nucleus-mediated inhibition of the ascending arousal pathways, while REM is mediated primarily by activation of cholinergic neurons in the laterodorsal and pedunculopontine tegmental nuclei.Citation1
Sleep disturbances are a common early symptom of neurodegenerative disease, ranging from the increased sleep fragmentation and irregular sleep–wake patterns seen in Alzheimer’s disease (AD) to the increased risk for REM sleep behavior disorder (RBD) in the synucleinopathies, including Parkinson’s disease (PD), multiple system atrophy, and Lewy body dementia to the circadian dysregulation and insomnia seen in Huntington’s disease (HD).
Sleep disruption as an early symptom of AD
It has long been recognized that as AD progresses, sleep disturbances increase. AD has been associated with increased fragmentation of the overall sleep–wake pattern, increased sleep during the daytime, increased frequency of nocturnal awakenings, and a decrease in both slow wave and REM sleep.Citation2 Individuals often develop irregular sleep–wake rhythm disorder, in which sleep is fragmented into at least three distinct bouts throughout the 24-hour period, and individuals also frequently develop “sundowning” whereby confusion and agitation worsen later in the day.Citation3 These sleep disturbances do appear to be associated with adverse clinical outcomes, as both greater sleep disturbance and greater time spent in bed have been associated with greater functional impairment in individuals with AD.Citation4
In addition, there does appear to be progressive circadian dysfunction in these individuals, which can in turn result in significant sleep disruption. In the Tg2576 mouse model of AD, which exhibits age-dependent amyloid-β deposition in the brain, animals demonstrate a significantly longer circadian period of wheel running rhythms when compared to control mice, and they also lacked the normally observed increase in delta power following sleep deprivation.Citation5 Based on caregiver reports, individuals with AD have a decrease in the overall amplitude of the rest–activity rhythm that corresponds with abnormal patterns of the core body temperature rhythm.Citation6 Use of objective actigraphy data to measure rest–activity patterns in AD has demonstrated increased fragmentation and decreased amplitude of activity that correlates with the severity of dementiaCitation7 and the degree of impairment in cognitive performance.Citation8
Interestingly, there is increasing evidence that the sleep and circadian disturbances may actually develop prior to the clinical symptoms of AD being evident. In a large actigraphy study of over 1,200 healthy women, a decrease in the amplitude and robustness of the circadian rest–activity rhythm at baseline was associated with an increased risk for developing either mild cognitive impairment or dementia over the next 5 years, suggesting that these sleep disturbances are either an early symptom or contribute to the underlying pathophysiology.Citation9
Furthermore, the use of hypnotics to treat sleep disturbances may further contribute to the development of dementia through unclear mechanisms. There is evidence that in individuals with traumatic brain injury, those with complaints of insomnia who are treated with hypnotics have a higher risk of developing dementia when compared to control individuals, either with or without insomnia.Citation10 Neuroleptic medications are also frequently used to treat sleep disturbances in patients with dementia; however, these have also been associated with an increased risk of morbidity and mortality in these patients.Citation11 This suggests that simply using medication to treat the underlying sleep disturbance will not necessarily improve risk, and may actually do more harm.
Sleep and circadian disruption in movement disorders
Impaired sleep and alertness are frequently encountered in movement disorders such as PD and HD. Sleep disturbances are one of the most common and disabling nonmotor manifestations of PD, affecting as many as 90% of patients.Citation12 The most common sleep disturbances observed include sleep fragmentation, excessive daytime sleepiness, and RBD. Increasing evidence also suggests significant modifications of the circadian system, likely in turn affecting sleep–wake homeostasis in the PD population.Citation13
In most cases, poor sleep is related to the underlying pathology of PD; however in some individuals, the dopamine agonists used to treat the motor symptoms may also contribute to the development of excessive sleepiness. Excessive sleepiness was reported in 13%–36% of PD participants in pivotal clinical trials of levodopa and dopamine agonists.Citation14,Citation15 Levodopa monotherapy confers the lowest risk for sleepiness, while the combination therapy with levodopa and dopamine agonist presents the highest risk.Citation16 In these individuals, the addition of stimulating medications such as armodafinil, buproprion, or one of the more traditional stimulants may be beneficial.Citation17
Poor sleep affects up to 90% of HD patientsCitation18 with symptoms including sleep initiation and maintenance insomnia, reduced sleep efficiency, and reduced total sleep time.Citation19 Circadian rhythms also become progressively more disrupted as the HD-specific neurodegenerative process advances, resulting in further sleep–wake disruption.Citation20 Interventions directed toward sleep consolidation, in this case by using hypnotics to impose a regular sleep–wake schedule do seem to be effective in improving circadian function and cognitive performance in HD.Citation21
RBD is a parasomnia characterized by abnormal motor and vocal behaviors in the context of vivid dreams, associated with loss of muscle atonia during REM sleep. The disorder is strongly associated with synuclein-related disorders and is considered one of its earliest manifestations.Citation22 This is likely reflective of early involvement of neuroanatomic networks responsible for the regulation of REM sleep by synuclein-related neurodegeneration. Idiopathic RBD confers a strong risk for the development of Parkinsonism or cognitive dysfunction, with the risk estimated to be over 50% over a decade of having RBD.Citation23
Neurodegeneration and sleep disturbance
At a structural level, there is growing evidence for an underlying neuroanatomical basis for these observed sleep–wake disruptions. In a community-based study of AD patients, actigraphy was obtained prior to death, and postmortem analysis was performed looking at the number of galanin-positive neurons in the intermediate nucleus, the human equivalent to the ventrolateral preoptic area, the primary wake promoting nucleus in rodents. This investigation demonstrated a correlation between the loss of neurons in the intermediate nucleus and increased sleep fragmentation on actigraphy.Citation24 More recently, it has also been demonstrated that there is a correlation between the circadian amplitude of rest–activity, and the number of vasoactive intestinal peptide expressing neurons in the suprachiasmatic nucleus, the primary circadian pacemaker in mammals.Citation25 In PD, sleep disturbance frequently precede motor symptoms, and this is presumed to reflect the degeneration of areas such as the raphe nucleus and locus coeruleus that constitute preclinical stages 1 and 2 of the pathological staging proposed by Braak et al.Citation26,Citation27 These data suggest that the observed sleep–wake and circadian disturbances in neurodegenerative disease are secondary to pathological degeneration of primary brain regions important for regulating the sleep–wake and circadian cycles.
Melatonin changes with neurodegenerative disease
Melatonin is a hormone released by the pineal gland in a circadian pattern, with levels rising several hours before sleep onset, and peaking in the middle of the night. The pattern and amplitude of melatonin release is a reflection of the overall circadian state of the individual. As the sleep–wake cycle is closely tied to the circadian cycle, circadian disruptions are in turn closely linked to disrupted sleep. Many neurodegenerative disorders have been associated with sleep and circadian disruption as reflected in changes in the pattern of melatonin secretion.
In patients with PD, initial studies comparing untreated patients to treated patients with or without motor complications demonstrated an overall advance in the timing of melatonin secretion in treated patients compared to controls, as well as a decrease in the amplitude of melatonin secretion in treated patients with motor complications.Citation28 Later studies have demonstrated that PD patients have a decrease in the amplitude of melatonin secretion compared to controls,Citation29,Citation30 and this effect is more pronounced in patients with excessive daytime sleepiness.Citation29 In addition, the pattern of expression of clock genes, particularly Bmal1, appears to be altered in these individuals.Citation30 Patients with AD have also exhibited a decrease in the amplitude of the melatonin rhythm.Citation31,Citation32
Interestingly, in addition to reflecting underlying sleep and circadian dysfunction, alterations in melatonin secretion patterns may contribute to the underlying pathophysiology of these disorders. Melatonin, known to have antioxidant properties, has been demonstrated in in vivo models to protect against oxidative stress,Citation33 and can protect against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced neurotoxicity in mouse models of PD.Citation34 In addition, in vitro models have demonstrated that melatonin can inhibit α-synuclein assembly and toxicity, which is believed to be an important step in the pathophysiology of PD.Citation35 This again supports the idea that changes in melatonin release may not only reflect the observed disruption of sleep and circadian rhythms but also may contribute to further progression of the disease.
Orexin levels/neuronal loss in neurodegenerative disease
Orexin (hypocretin) is an important neuropeptide involved in the maintenance of wakefulness and also stabilizes sleep–wake transitions.Citation36 While orexin represents the primary peptide that is deficient in patients with narcolepsy,Citation37 changes in orexin levels may also play a role in the sleep fragmentation and excessive sleepiness frequently observed in neurodegenerative disorders. Orexin-A levels can be measured in the cerebral spinal fluid (CSF) of patients. In addition, at autopsy, the number of orexin-positive neurons can be assessed.
In HD, mouse models have demonstrated a significant decrease in the number of orexin-positive neurons in the hypothalamus.Citation38,Citation39 Similar results have also been found in autopsy studies from humans with HD.Citation40 Interestingly, several studies looking at CSF orexin levels in patients with HD did not find any significant difference when compared with controls,Citation41–Citation44 suggesting that it takes substantial neuronal loss before significant changes are reflected in the CSF profile.
Similarly, several studies in patients with PD have demonstrated normal CSF orexin levels on average,Citation45–Citation47 though some differences have been noted if measured at the level of the ventricle in late-stage PD, with lower levels correlating with increased disease severity.Citation48 Similar to patients with HD, even in PD patients with normal CSF orexin levels, there does appear to be a 25%–50% loss of orexin-containing cells on postmortem autopsy.Citation49 In vitro, there is evidence that orexin can play a neuroprotective role in a cellular model of PD, suggesting that loss of these cells may play a role in the underlying sleep–wake disturbances, but in turn, cell loss may also contribute to the progression of disease.Citation50
The story becomes more complicated in patients with AD, where results have been more variable. In at least one study, CSF levels of orexin were found to be no different than those in controls,Citation51 while another group looking at postmortem brains in patients with advanced AD found both decrease in the number of orexin neurons and a decrease in the CSF orexin levels. Furthermore, lower CSF levels correlated with those who complained of greater daytime sleepiness.Citation52 Of note, another recent study found increased CSF orexin levels in patients with moderate-to-severe AD when compared to controls. This patient population also had a decrease in sleep efficiency, with increased wake after sleep onset on polysomnography (PSG) that correlated with the increased orexin levels.Citation53 In a mouse model of AD, knocking out orexin results in a decrease in amyloid-β deposition. This decrease seems to be related to effects on sleep, rather than direct effects of the orexin peptide, as selective rescue with local injections of orexin does not affect amyloid-β deposition, while sleep deprivation results in increased amyloid-β deposition in orexin knockout mice.Citation54 Overall, this suggests that in neurodegenerative disorders the orexin-containing neurons in the lateral hypothalamus are often affected as part of the underlying neurodegenerative process. In turn, the loss of these neurons can accelerate the underlying pathology, either through loss of direct neuroprotective effects, as in PD, or by further disrupting and fragmenting sleep, resulting in increased amyloid-β deposition, as will be detailed in the following section.
Sleep disruption as a potential cause of neurodegenerative disease
While there is growing evidence that sleep disruption can be an early symptom in the development of neurodegenerative disease (as already discussed), sleep disturbances often predate the onset of cardinal diagnostic clinical symptoms, suggesting that the sleep disruption may actually also play a role in the development and progression of neurodegenerative diseases. This is an exciting concept because it positions the sleep–wake cycle as a novel, readily modifiable factor in its efforts to develop and test neuroprotective/disease-modifying strategies.
The glymphatic system and neurodegenerative disease
One of the primary factors thought to contribute to the development of AD is the accumulation of amyloid-β. This accumulation increases during wakefulness, with further increases observed following sleep deprivation.Citation55 More recently, the mechanism behind these changes in levels has been identified. Throughout the body, systems have been developed to clear the waste products of normal metabolism and function. A process for promoting this clearance from the central nervous system was recently discovered, and named the “glymphatic system”. As implied by the name, this system works similarly to the previously well-described lymphatic system. The CSF circulates and removes by-products from the interstitial fluid, including proteins like amyloid-β that have been linked to neurodegeneration.Citation56 Interestingly, it has recently been demonstrated that this process is up to two times more active during either natural or anesthetic-induced sleep when compared to wakefulness.Citation57 In vivo studies have also demonstrated an increased buildup of insoluble tau protein in response to sleep deprivation.Citation58 As most neurodegenerative disorders are associated with a toxic buildup of waste products in the nervous system, these findings support both the importance of sleep in the prevention of neurodegenerative disorders and a potential mechanism by which sleep disruption could be contributing to the development of these disorders.
Chronic sleep fragmentation, hypoxemia, and the development of neurodegenerative disease
Other factors that may contribute to the development of neurodegenerative disease include the presence of other comorbid sleep disorders. Obstructive sleep apnea (OSA) is a very common disorder affecting up to 19% of men and 15% of women.Citation59 The obstructive events seen in this disorder result in intermittent hypoxemia, as well as sleep fragmentation secondary to the arousals related to respiratory events, both of which may contribute to the development of neurodegenerative disease. Sleep fragmentation has been associated with impaired performance on tasks of vigilance, to a similar degree as is seen with total sleep deprivation.Citation60 In mouse models, chronic mild sleep restriction has been associated with impairments in contextual and cued memory, and an increase in amyloid-β and insoluble tau.Citation61 Neuroimaging studies have demonstrated that the sleep fragmentation associated with OSA has been associated with decreased gray matter volume in the hippocampus, a brain region associated with memory formation.Citation62
In mice, intermittent hypoxia has been associated with increased accumulation of both amyloid-β and insoluble tau protein, two factors that contribute to the development of neurodegenerative disease. These increases corresponded with impaired special learning and memory.Citation63 In humans, in the Apnea Positive Pressure Long-term Efficacy Study (APPLES) it was demonstrated that neurocognitive performance deficits were weakly associated with the apnea–hypopnea index and severity of oxygen desaturation during events.Citation64 Treatment of OSA with continuous positive airway pressure (CPAP) did result in mild improvement in executive and frontal-lobe function for those with severe disease at baseline, suggesting that at least some of the cognitive changes associated with OSA may be reversible.Citation65
Sleep deprivation and motor performance in PD
Sleep deprivation may not be entirely bad when it comes to neurodegenerative disorders. Of interest is the observation from the Nurses’ Health study that individuals who worked night shifts for >15 years had a significantly decreased risk of developing PD. The risk of developing PD was also much lower in individuals sleeping <6 hours/night when compared to those sleeping >9 hours/night.Citation66 Cause and effect are unclear however, as it is not clear at this point whether working night shifts and short sleep duration are protective for developing PD, or if those who are predisposed to develop PD have a lower tolerance for working the night shift. A few small studies have demonstrated that sleep deprivation transiently improves the motor deficits seen in PD, possibly mediated by potentiating dopaminergic activity, though these findings have been somewhat inconsistent, and may reflect different subtypes of the disease.Citation67,Citation68 These observations are certainly in contrast with “sleep benefit” in PD, a clinical phenomenon whereby improved motor function and responsiveness to dopaminergic treatments are noted among PD patients after a restful night of sleep.Citation69 Regardless, these findings do suggest that the link between sleep and neurodegenerative disease is complex and more sleep may not always be better.
Conclusion
Disruptions of sleep–wake cycles are common, yet underdiagnosed and underreported manifestations of neurodegenerative disorders. Further research is needed to clarify whether sleep disruption is primarily a cause of, or a symptom of, the underlying neurodegenerative process. Regardless, it appears that efforts to normalize sleep–wake schedules in patients with neurodegenerative diseases should be beneficial for this population and further may have an impact in slowing the overall disease course.
Disclosure
Dr Videnovic received funding from NIH/NINDS (K23 NS072283). The authors report no other conflicts of interest in this work.
References
- EspanaRAScammellTESleep neurobiology from a clinical perspectiveSleep201134784585821731134
- VitielloMVBorsonSSleep disturbances in patients with Alzheimer’s disease: epidemiology, pathophysiology and treatmentCNS Drugs2001151077779611602004
- AbbottSMZeePCIrregular sleep-wake rhythm disorderSleep Med Clin201510451752226568126
- TractenbergRESingerCMKayeJASymptoms of sleep disturbance in persons with Alzheimer’s disease and normal elderlyJ Sleep Res200514217718515910515
- WisorJPEdgarDMYesavageJSleep and circadian abnormalities in a transgenic mouse model of Alzheimer’s disease: a role for cholinergic transmissionNeuroscience2005131237538515708480
- OkawaMMishimaKHishikawaYHozumiSHoriHTakahashiKCircadian rhythm disorders in sleep-waking and body temperature in elderly patients with dementia and their treatmentSleep19911464784851798879
- WittingWKwaIHEikelenboomPMirmiranMSwaabDFAlterations in the circadian rest-activity rhythm in aging and Alzheimer’s diseaseBiol Psychiatry19902765635722322616
- LimASYuLCostaMDIncreased fragmentation of rest-activity patterns is associated with a characteristic pattern of cognitive impairment in older individualsSleep2012355633B640B22547889
- TranahGJBlackwellTStoneKLCircadian activity rhythms and risk of incident dementia and mild cognitive impairment in older womenAnn Neurol201170572273222162057
- ChiuHYLinEYWeiLHypnotics use but not insomnia increased the risk of dementia in traumatic brain injury patientsEur Neuropsychopharmacol Epub2015108
- BohlkenJSchulzMRappMABatzing-FeigenbaumJPharmacotherapy of dementia in Germany: results from a nationwide claims databaseEur Neuropsychopharmacol Epub2015103
- VidenovicAGolombekDCircadian and sleep disorders in Parkinson’s diseaseExp Neurol2013243455622935723
- VidenovicALazarASBarkerRAOvereemS‘The clocks that time us’–circadian rhythms in neurodegenerative disordersNat Rev Neurol2014101268369325385339
- RascolOBrooksDJKorczynADDe DeynPPClarkeCELangAEA five-year study of the incidence of dyskinesia in patients with early Parkinson’s disease who were treated with ropinirole or levodopa. 056 Study GroupN Engl J Med2000342201484149110816186
- PSGPramipexole vs levodopa as initial treatment for Parkinson disease: a randomized controlled trialJAMA20002841931193811035889
- PausSBrechtHMKosterJSeegerGKlockgetherTWullnerUSleep attacks, daytime sleepiness, and dopamine agonists in Parkinson’s diseaseMov Disord200318665966712784269
- RyeDBSleepiness and unintended sleep in Parkinson’s diseaseCurr Treat Options Neurol20035323123912670412
- GoodmanAOMortonAJBarkerRAIdentifying sleep disturbances in Huntington’s disease using a simple disease-focused questionnairePLoS Curr20102RRN118920972477
- MortonAJCircadian and sleep disorder in Huntington’s diseaseExp Neurol2013243344423099415
- MortonAJWoodNIHastingsMHHurelbrinkCBarkerRAMaywoodESDisintegration of the sleep-wake cycle and circadian timing in Huntington’s diseaseJ Neurosci200525115716315634777
- PallierPNMaywoodESZhengZPharmacological imposition of sleep slows cognitive decline and reverses dysregulation of circadian gene expression in a transgenic mouse model of Huntington’s diseaseJ Neurosci200727297869787817634381
- ZanigniSCalandra-BuonauraGGrimaldiDCortelliPREM behaviour disorder and neurodegenerative diseasesSleep Med201112Suppl 2S54S5822136901
- PostumaRBGagnonJFVendetteMFantiniMLMassicotte-MarquezJMontplaisirJQuantifying the risk of neurodegenerative disease in idiopathic REM sleep behavior disorderNeurology200972151296130019109537
- LimASEllisonBAWangJLSleep is related to neuron numbers in the ventrolateral preoptic/intermediate nucleus in older adults with and without Alzheimer’s diseaseBrain2014137Pt 102847286125142380
- WangJLLimASChiangWYSuprachiasmatic neuron numbers and rest-activity circadian rhythms in older humansAnn Neurol201578231732225921596
- BraakHDel TrediciKInvited article: nervous system pathology in sporadic Parkinson diseaseNeurology200870201916192518474848
- BraakHDel TrediciKRubUde VosRAJansen SteurENBraakEStaging of brain pathology related to sporadic Parkinson’s diseaseNeurobiol Aging200324219721112498954
- BordetRDevosDBriqueSStudy of circadian melatonin secretion pattern at different stages of Parkinson’s diseaseClin Neuropharmacol2003262657212671525
- VidenovicANobleCReidKJCircadian melatonin rhythm and excessive daytime sleepiness in Parkinson diseaseJAMA Neurol201471446346924566763
- BreenDPVuonoRNawarathnaUSleep and circadian rhythm regulation in early Parkinson diseaseJAMA Neurol201471558959524687146
- OhashiYOkamotoNUchidaKIyoMMoriNMoritaYDaily rhythm of serum melatonin levels and effect of light exposure in patients with dementia of the Alzheimer’s typeBiol Psychiatry199945121646165210376127
- SkeneDJSwaabDFMelatonin rhythmicity: effect of age and Alzheimer’s diseaseExp Gerontol2003381–219920612543278
- SaravananKSSindhuKMMohanakumarKPMelatonin protects against rotenone-induced oxidative stress in a hemiparkinsonian rat modelJ Pineal Res200742324725317349022
- Acuna-CastroviejoDCoto-MontesAGaia MontiMOrtizGGReiterRJMelatonin is protective against MPTP-induced striatal and hippocampal lesionsLife Sci1997602PL23PL299000122
- OnoKMochizukiHIkedaTEffect of melatonin on alpha-synuclein self-assembly and cytotoxicityNeurobiol Aging20123392172218522118903
- HaganJJLeslieRAPatelSOrexin A activates locus coeruleus cell firing and increases arousal in the ratProc Natl Acad Sci U S A19999619109111091610485925
- PeyronCFaracoJRogersWA mutation in a case of early onset narcolepsy and a generalized absence of hypocretin peptides in human narcoleptic brainsNat Med20006999199710973318
- WilliamsRHMortonAJBurdakovDParadoxical function of orexin/hypocretin circuits in a mouse model of Huntington’s diseaseNeurobiol Dis201142343844521324360
- PetersenAGilJMaat-SchiemanMLOrexin loss in Huntington’s diseaseHum Mol Genet2005141394715525658
- van WamelenDJAzizNARoosRASwaabDFHypothalamic alterations in Huntington’s disease patients: comparison with genetic rodent modelsJ Neuroendocrinol2014261176177525074766
- BjorkqvistMPetersenANielsenJCerebrospinal fluid levels of orexin-A are not a clinically useful biomarker for Huntington diseaseClin Genet2006701787916813610
- BaumannCRHersbergerMBassettiCLHypocretin-1 (orexin A) levels are normal in Huntington’s diseaseJ Neurol200625391232123316598614
- GausSELinLMignotECSF hypocretin levels are normal in Huntington’s disease patientsSleep200528121607160816408421
- MeierAMollenhauerBCohrsSNormal hypocretin-1 (orexin-A) levels in the cerebrospinal fluid of patients with Huntington’s diseaseBrain Res20051063220120316263095
- OvereemSvan HiltenJJRipleyBMignotENishinoSLammersGJNormal hypocretin-1 levels in Parkinson’s disease patients with excessive daytime sleepinessNeurology200258349849911839864
- BaumannCFerini-StrambiLWaldvogelDWerthEBassettiCLParkinsonism with excessive daytime sleepiness – a narcolepsy-like disorder?J Neurol2005252213914515729517
- YasuiKInoueYKanbayashiTNomuraTKusumiMNakashimaKCSF orexin levels of Parkinson’s disease, dementia with Lewy bodies, progressive supranuclear palsy and corticobasal degenerationJ Neurol Sci20062501–212012317005202
- DrouotXMoutereauSNguyenJPLow levels of ventricular CSF orexin/hypocretin in advanced PDNeurology200361454054312939433
- FronczekROvereemSLeeSYHypocretin (orexin) loss in Parkinson’s diseaseBrain2007130Pt 61577158517470494
- FengYLiuTLiXQNeuroprotection by Orexin-A via HIF-1alpha induction in a cellular model of Parkinson’s diseaseNeurosci Lett2014579354025038418
- RipleyBOvereemSFujikiNCSF hypocretin/orexin levels in narcolepsy and other neurological conditionsNeurology200157122253225811756606
- FronczekRvan GeestSFrolichMHypocretin (orexin) loss in Alzheimer’s diseaseNeurobiol Aging20123381642165021546124
- LiguoriCRomigiANuccetelliMOrexinergic system dysregulation, sleep impairment, and cognitive decline in Alzheimer diseaseJAMA Neurol201471121498150525322206
- RohJHJiangHFinnMBPotential role of orexin and sleep modulation in the pathogenesis of Alzheimer’s diseaseJ Exp Med2014211132487249625422493
- KangJELimMMBatemanRJAmyloid-beta dynamics are regulated by orexin and the sleep-wake cycleScience200932659551005100719779148
- NedergaardMNeuroscience. Garbage truck of the brainScience201334061401529153023812703
- XieLKangHXuQSleep drives metabolite clearance from the adult brainScience2013342615637337724136970
- Di MecoAJoshiYBPraticoDSleep deprivation impairs memory, tau metabolism, and synaptic integrity of a mouse model of Alzheimer’s disease with plaques and tanglesNeurobiol Aging20143581813182024629673
- YoungTPaltaMDempseyJSkatrudJWeberSBadrSThe occurrence of sleep-disordered breathing among middle-aged adultsNew Engl J Med199332817123012358464434
- BonnetMHArandDLClinical effects of sleep fragmentation versus sleep deprivationSleep Med Rev20037429731014505597
- RothmanSMHerdenerNFrankolaKAMughalMRMattsonMPChronic mild sleep restriction accentuates contextual memory impairments, and accumulations of cortical Abeta and pTau in a mouse model of Alzheimer’s diseaseBrain Research2013152920020823856323
- MaceyPMHendersonLAMaceyKEBrain morphology associated with obstructive sleep apneaAm J Respir Crit Care Med2002166101382138712421746
- ZhangXLiLZhangXPrenatal hypoxia may aggravate the cognitive impairment and Alzheimer’s disease neuropathology in APPSwe/PS1A246E transgenic miceNeurobiol Aging201334366367822795785
- QuanSFChanCSDementWCThe association between obstructive sleep apnea and neurocognitive performance – the Apnea Positive Pressure Long-term Efficacy Study (APPLES)Sleep2011343303B314B21358847
- KushidaCANicholsDAHolmesTHEffects of continuous positive airway pressure on neurocognitive function in obstructive sleep apnea patients: the Apnea Positive Pressure Long-term Efficacy Study (APPLES)Sleep201235121593160223204602
- ChenHSchernhammerESchwarzschildMAAscherioAA prospective study of night shift work, sleep duration, and risk of Parkinson’s diseaseAm J Epidemiol2006163872673016495472
- HoglBPeraltaCWetterTCGershanikOTrenkwalderCEffect of sleep deprivation on motor performance in patients with Parkinson’s diseaseMov Disord200116461662111481684
- ReistCSokolskiKNChenCCCoskinasEDemetEMThe effect of sleep deprivation on motor impairment and retinal adaptation in Parkinson’s diseaseProg Neuropsychopharmacol Biol Psychiatry19951934454547624495
- van GilstMMvan MierloPBloemBROvereemSQuantitative motor performance and sleep benefit in Parkinson diseaseSleep201538101567157325902811