Abstract:
A 51-year-old woman, who intentionally ingested a massive dose of ~60 g of valproic acid which she was using as a mood stabilizer for bipolar affective disorder, presented within 30 minutes of ingestion to the emergency department. The patient was asymptomatic and was immediately started on decontamination therapy with activated charcoal (AC). Drug serum levels, liver functions, and ammonia levels were tested and followed up during treatment. Due to the massive ingestion and continuous rise in serum drug levels, the patient received regular multiple doses of AC, as well as l-carnitine for liver protection. The patient was started on extracorporeal therapy in the form of renal replacement therapy in the intensive care unit (ICU), followed by intermittent hemodialysis. Drug serum levels dropped significantly. Ammonia levels showed improvement with treatment. The patient was discharged from the ICU after 14 days of treatment. She was stable and in good condition with no residual hepatic or central nervous system (CNS) manifestations.
Introduction
Valproic acid (VPA) has been primarily used as an anticonvulsant since 1967, and later, Lambert et al reported an additional antimanic property. Further studies in Germany and the United States demonstrated its efficacy, and by 1995, the US Food and Drug Administration approved the use of VPA for such indications.Citation1 VPA works by increasing the availability of gamma-aminobutyric acid (GABA) in the brain. It is metabolized primarily in the liver and has an average half-life of 9ߝ19 hours in a healthy adult. Time to peak concentration differs according to the product (regular, delayed, and extended-released) from 2 to 17 hours and it is mainly excreted in urine. Trough levels are used to assess the therapeutic effect of the medication. Therapeutic dosages between 50ߝ100 µg/mL and 50ߝ125 µg/mL are adequate for epilepsy and mania, respectively. Levels >450 mg/L have been found to cause severe life-threatening clinical manifestations such as severe central nervous system (CNS) depression,Citation2 hepatotoxicity, hyperammonemia, and cerebral edema which can be clinically apparent as early as 12 hours following ingestion.Citation3ߝCitation5
Case presentation
A 51-year-old woman known to have bipolar affective disorder presented to the emergency department (ED) having overdosed on 120 tablets of VPA (DepakeneÒ) 30 minutes prior to her arrival. Each tablet was of 500 mg strength; a total of 60 g was ingested. She had a history of attempting suicide and had been treated with VPA as a mood stabilizer. The patient had no specific complaints. Initial patient vital signs were as follows: temperature, 37°C; blood pressure, 147/75 mmHg; heart rate, 102 bpm; oxygen saturation, 99% on room air; and respiratory rate, 20 breaths per minute. On physical examination, the patient was conscious, alert, and oriented to time, place, and person. The rest of the physical examination was unremarkable. An electrocardiogram was done and showed sinus tachycardia. Blood was drawn and sent for testing for VPA levels at 0 and 4-hour intervals. Complete blood count, renal and liver functions, ammonia levels, and venous blood gas were also done, and the results are shown in .
Table 1 Laboratory tests day by day during the hospital admission
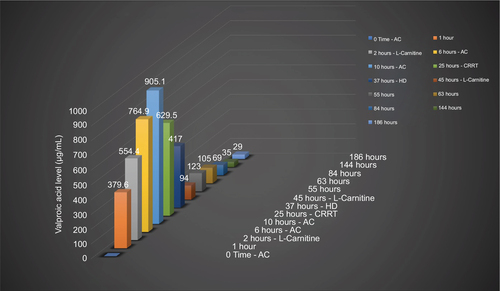
The patient was managed initially in the ED by 50 g of activated charcoal (AC). The lab reported that her VPA level was 379.6 µg/mL, and additional multiple doses of AC were started at a dose of 0.5 g/kg every 4 hours. Also, a loading dose of l-carnitine was administered (100 mg/kg intravenously over 3 minutes). This was followed by maintenance doses of l-carnitine at 15 mg/kg every 4 hours over 10ߝ30 minutes to be continued during admission. The patient was referred to the intensive care unit (ICU) team for further management and admission. VPA levels were repeatedly tested every 4 hours. At 4 hours following presentation, VPA levels were measured to be 764 µg/mL and were on the rise.
The patient developed seizures and a depressed level of consciousness 8 hours following presentation and was then intubated. Twelve hours after presentation VPA level reported from the laboratory was 905 µg/mL. At the time, continuous renal replacement therapy (CRRT) was planned, which was started almost 24 hours after presentation. Eight hours following CRRT, VPA levels were tested and showed a significant drop to 417 µg/mL. At this point, hemodialysis (HD) was initiated for 8 hours, and the level dropped further to 94 µg/mL. A computed tomography (CT) scan of the brain was done, and the results were unremarkable. Ammonia levels were initially elevated, reaching up to 393 mmol/L by the second day, but improved with management. With further VPA level drops and clinical improvement, sedation was stopped, and the patient was successfully extubated on the 12th day of admission. By day 14, she was transferred to the psychiatry ward in good and stable condition for further management of her primary illness. shows the serial level for valproic acid and shows laboratory test results over days of treatment.
Figure 1 Valproic acid level in association with intervention.
The patientߣs stay in the psychiatry unit was uneventful; during the stay she was started on olanzapine, lorazepam, and escitalopram. The patient was discharged after 2 weeks and a follow-up appointment in psychiatry outpatient clinic was scheduled.
Ethical statement
The patient provided written informed consent for the case details to be published.
Discussion
VPA is metabolized in the liver. In the case of overdose, it binds to carnitine within the hepatocytes to form the VPAߝcarnitine complex leading to carnitine deficiency. This impairs fatty acid oxidation causing steatosis and liver failure. Furthermore, VPAߝcarnitine oxidation in the mitochondria produces 2-propyl-4-pentenoic acid (4-EN-VPA), which interferes with the urea cycle and causes hyperammonemia.Citation6,Citation7
We report a case of a middle-aged woman who intentionally ingested a massive dose of ~60 g of VPA, which she had been using as a mood stabilizer for the preceding year to treat her bipolar affective disorder. Her course of treatment required ICU admission and intubation to protect her airway due to CNS depression which is a known complication of high doses of such medication. Her care was managed using the best available information on drug toxicity, multi doses of AC, L-carnitene, HD, intensive ventilation and other supportive and ICU bundle measures. She improved after 12 days and was extubated. Two days later she was discharged from the ICU.
Management of VPA toxicity varies due to the lack of well-designed controlled studies supporting any of the interventions used currently such as AC, l-carnitine, and HD. The only available guidelines for VPA poisoning management were published in 2008 and were primarily based on a review of the American Association of Poison Control Centerߣs data of over 9,000 ingestions. All C and D recommendations were the highest grade of evidence of these guidelines, which means recommendations are extracted from case series, single case reports, or poor-quality cohort and caseߝcontrol studies for the C recommendations, and expert opinion without explicit critical appraisal or based on physiology or bench research for the D recommendations.Citation7 shows some of these reported cases and studies in the literature with different management approaches and outcomes.
Table 2 Review of published case reports about valproic acid toxicity
In our case, we used the three main interventions (multidose AC, l-carnitine, and HD) that have been reported in the literature. In regard to AC, one prospective observational study investigated its efficacy on VPA absorption in volunteers. The results showed a significant decrease in the peak serum level by 65%; P<0.01 when 50 g of AC was used within 5 minutes of ingestion. Compared to our case, AC was started within the first 30 minutes then multidoses of AC were continued; however, the levels continued to get higher, but we were unsure whether an increased level would have been reported higher if intervention was not started.Citation8
The hypothesis behind the use of l-carnitine, our second intervention, is that VPA toxicity causes an inhibiting effect of carnitine synthesis during VPA toxicity by decreasing the concentration of α-ketoglutarate. Because of this, adding l-carnitine may increase the β-oxidation of VPA, thereby decreasing the production of toxic metabolites that may cause liver toxicity. In our scenario, treatment with l-carnitine started within the first 6 hours of toxicity, and we are of the opinion that despite a very high level of VPA and an increased level of ammonia, the liver function was treading a benign course, possibly due to the early use of l-carnitene.
The last option for therapy used in managing our patient was HD. The role of HD in some overdoses is clearly studied, with proven efficacy. For VPA toxicity, the role of HD is not well defined. In our case, HD was started after almost 24 hours due to the continuous rise in the drug serum levels and worsening of our patientߣs CNS function. Eight hours after starting HD in the form of CRRT, VPA levels dropped significantly to 417 µg/mL, and then treatment switched to intermittent HD and the levels dropped further to 94 µg/mL. We strongly believe that HD was the most effective intervention in the management of this patient and that it led to the rapid drop in her drug serum level and improvement of her clinical condition. For a drug to be dialyzable, it should be less protein bound. VPA at therapeutic levels is found to be ~90% plasma protein binding, but in the case of overdose or higher serum levels, the protein-binding property decreases and more of the drug is left free in the serum. In levels of >500 mg/L, possibly <10% is protein bound.Citation9
Intermittent HD is the preferred modality of renal replacement therapy in VPA poisoning. If HD is not available, then intermittent hemoperfusion or CRRT is an acceptable alternative. The end point of HD includes clinical stabilization and VPA levels < 100 mg/L.Citation9 As in many other cases of intoxication, it is important to monitor VPA levels after the cessation of HD, since redistribution of the medication can cause reemergence of toxicity.
Conclusion
We report a fortunate outcome for a case of a life-threatening intentional overdose of VPA. The patient showed progressive deterioration of her central neurological functions and was managed by a combination of supportive measures of airway protection, mechanical ventilation plus early decontamination with AC, l-carnitine therapy for her liver toxicity protection, and finally, extracorporeal therapy in the form of CRRT and intermittent HD. We believe that the early aggressive combination of these interventions had a great impact and led to a benign course for this patient during her ICU and hospital stay with complete recovery with no adverse sequelae.
Disclosure
The authors report no conflicts of interest in this work.
References
- Lempérière T. [Brief history of the development of valproate in bipolar disorders]. Encephale. 2001;27(4):365–372. French.
- Spiller HA, Krenzelok EP, Klein-Schwartz W, et al. Multicenter case series of valproic acid ingestion: serum concentrations and toxicity. J Toxicol Clin Toxicol. 2000;38:755–760.
- Björnsson E. Hepatotoxicity associated with antiepileptic drugs. Acta Neurol Scand. 2008;118:281–290.
- McCall M, Bourgeois M. Valproic acid-induced hyperammonemia. J Clin Psychopharmacol. 2004;24:521–526.
- Zafrani ES, Berthelot P. Sodium valproate in the induction of unusual hepatotoxicity. Hepatology. 1982;2:648–649.
- Hoffman RS, Howland MA, Lewin NA, Nelson LS, Goldfrank LR. Goldfrank’s Toxicologic Emergencies. 10th ed. New York: McGraw Hill Education; 2015.
- Lexicomp Inc. [webpage on the Internet], Valproate: Drug Information. UpToDate. Available from: https://www.uptodate.com/contents/valproate-drug-information?source=preview&search=valproic%20acidȶanchor=F232992#F232992. Accessed October 21, 2017.
- Manoguerra AS, Erdman AR, Woolf AD, et al. American Association of Poison Control Centers. Valproic acid poisoning: an evidence-based consensus guideline for out-of-hospital management. Clin Toxicol (Phila). 2008;46(7):661–676.
- Neuvonen PJ, Kannisto H, Hirvisalo EL. Effect of activated charcoal on absorption of tolbutamide and valproate in man. Eur J Clin Pharmacol. 1983;24:243–246.
- Licari E, Calzavacca P, Warrillow SJ, Bellomo R. Life-threatening sodium valproate overdose: a comparison of two approaches to treatment. Crit Care Med. 2009;37(12):3161–3164.
- Ghannoum M, Laliberté M, Nolin TD, et al. Extracorporeal treatment for valproic acid poisoning: systematic review and recommendations from the EXTRIP workgroup. Clin Toxicol (Phila). 2015;53(5):454–465.
- Ingels M, Beauchamp J, Clark RF, Williams SR. Delayed valproic acid toxicity: a retrospective case series. Ann Emerg Med. 2002;39(6):616–621.
- Mestrović J, Filipović T, Polić B et al. Life-threatening valproate overdose successfully treated with haemodialysis. Arch Indus Hyg Toxicol. 2008;59(4):295–298.
- Nasa P, Sehrawat D, Kansal S, Chawla R. Effectiveness of HD in a case of severe valproate overdose. Indian J Crit Care Med. 2011;15(2):120–122.
