Abstract
Purpose
The aim of this study was to investigate the relationship between ARF and coagulopathy in patients with sepsis and to explore the prognostic value of these conditions.
Patients and Methods
The data of 271 patients with sepsis-associated coagulopathy admitted from June 2021 to June 2022 were reviewed. The patients were divided into a survival group and a nonsurviving group according to patient prognosis. Independent sample t tests were utilized to compare laboratory parameters within 24 hours of admission, as well as the APACHE II and SOFA scores, between the two patient groups. According to the sepsis-associated coagulation dysfunction (SAC) grading criteria for grading, Spearman correlation analysis was used to study the relationship between blood creatinine and SAC grading and assignment scores, and receiver operating characteristic (ROC) curves and Cox’s proportional risk regression model were used to explore the factors affecting the prognosis of SAC patients.
Results
Spearman correlation analysis revealed strong associations between serum creatinine (Scr) concentration, SAC classification, and SAC score, with coefficients above 0.7. SAC classification outcomes varied significantly with severity: mild severity had a 77.6% survival rate versus 22.4% mortality; moderate severity had 21.5% survival versus 78.5% mortality; and severe cases had a 0.7% survival rate versus 99.3% mortality (P<0.01 for all). Multivariate analysis revealed significant predictors of outcome, including multiple organ dysfunction syndrome (MODS), with an OR of 2.070 (P=0.019); the SOFA score (OR=1.200, P<0.01); the international normalized ratio (INR) (OR=0.72, P=0.013); and the Scr level (OR=0.995, P<0.01). The areas under the ROC curves for the SOFA score, APACHE II score, and SAC classification were >0.8, all P < 0.05.
Conclusion
In patients with sepsis, SAC grade 3 or a SAC score of 4 or higher is associated with poorer prognosis, and the interaction of acute kidney injury exacerbates the degree of SAC, consequently affecting prognosis.
Plain Language Summary
To investigate the relationship between acute renal dysfunction and coagulation dysfunction in patients with sepsis and to explore the prognostic value of these conditions. We collected information and laboratory indicators from 271 patients, classified these two groups of patients according to the grading criteria for sepsis-associated coagulation dysfunction (SAC), and compared the differences between them. We utilized Spearman correlation analysis to investigate the relationship between blood creatinine and the severity of sepsis-associated coagulation dysfunction (SAC). Additionally, we employed a Cox proportional hazards regression model to study the factors influencing the prognosis of SAC patients. This study revealed a significant positive correlation between blood creatinine levels and SAC grade. Furthermore, the presence of MODS, INR, blood creatinine, and SOFA score can serve as independent predictive factors for mortality. We can infer that there is a significant correlation between coagulation function parameters and blood creatinine levels, which play a crucial role in the diagnosis and prognostic analysis of sepsis. In patients with sepsis, a higher grade of SAC or an SAC score of 4 or higher indicates a poorer prognosis. Additionally, the interaction with acute kidney injury exacerbates the severity of SAC, thereby impacting patient prognosis.
Introduction
Sepsis is a highly prevalent and critical condition that affects millions of individuals worldwide annually. It leads to a significant number of fatalities, comparable in severity to polytrauma, acute myocardial infarction, and acute ischaemic stroke. Therefore, enhancing the capability for early recognition and management of sepsis is paramount.
According to the latest definition of sepsis (Sepsis 3.0), sepsis refers to life-threatening organ dysfunction caused by a dysregulated host response to infection.Citation1 Common complications of sepsis include acute hepatic and renal failure, acute respiratory failure, cerebral oedema, and coagulopathy, often accompanied by shock, and in severe cases, multiple organ dysfunction syndrome may ensue. Common complications of sepsis include acute hepatic and renal failure, acute respiratory failure, cerebral oedema, and coagulation dysfunction. These conditions can be accompanied by shock and may progress to severe multiple-organ dysfunction, with the kidney being one of the earliest and most vulnerable organs to be affected. Indeed, more than 40% of sepsis patients experience acute kidney injury (AKI), and the mortality rate of sepsis patients with concurrent acute kidney injury is significantly greater than that of patients without acute kidney injury.Citation2,Citation3 The pathogenesis of sepsis-induced acute kidney injury is complex and is considered associated with various factors, including haemodynamic changes, fluid retention, coagulation dysfunction, and inflammatory cascade reactions.Citation4–6
In patients with sepsis, the presence of two or more concurrent complications is not uncommon, and these complications occur frequently. Some scholars speculate that coagulation dysfunction in sepsis may be associated with acute kidney injury. During sepsis, the initiation of coagulation and the production of thrombin are triggered by the expression of tissue factors on activated monocytes and endothelial cells.Citation7,Citation8 Activation of tissue factor triggers activation of the extrinsic coagulation pathway, followed by activation of the intrinsic coagulation pathway, leading to widespread disseminated intravascular coagulation (DIC) in the body.Citation9 Furthermore, pathogens induce the production and release of inflammatory mediators, known as pathogen-associated molecular patterns (PAMPs). Proinflammatory substances are also activated or released from damaged host cells, leading to the production of damage-associated molecular patterns (DAMPs).Citation10 These two factors exacerbate the inflammatory response and activate neutrophil extracellular traps (NETs).Citation11–13 NETs not only facilitate the formation of platelet thrombi but also interact with extracellular vesicles and extracellular vesicles carrying NETs, thereby promoting thrombus formation.Citation12,Citation14 However, research regarding coagulation grading and mortality rates in patients with sepsis-related coagulopathy and subsequent acute kidney failure is still lacking.
Therefore, the objective of this study was to investigate the relationship between acute kidney failure (AKF) and coagulation dysfunction in patients with sepsis and to explore its ability to predict patient prognosis.
Materials and Methods
Study Design and Population Selection
This study selected septic patients admitted to the Emergency Intensive Care Unit (EICU) between June 2021 and June 2022 as the research subjects. The patients were categorized into groups of those with acute kidney injury and those without acute injury. The patient coagulation parameters included prothrombin time (PT), international normalized ratio (INR), fibrinogen degradation product concentration (FIB), activated partial thromboplastin time (APTT), plasma D-dimer, platelet count (Plt), serum creatinine (Scr), sequential organ failure assessment (SOFA) score, and acute physiology and chronic health evaluation II (APACHE II) score. The two groups of subjects were matched based on the aforementioned factors. shows the patient selection process and an illustration of the process.
Diagnostic Criteria
Diagnostic criteria for sepsis and septic shock.
Sepsis:Citation1 In patients with infection or suspected infection, a diagnosis of sepsis can be made when the SOFA score is ≥2 points greater than the baseline score.
Diagnostic Criteria for Acute Kidney Injury
Acute kidney injuryCitation3,Citation5,Citation15 (AKI) is characterized by a sudden decline in renal function occurring within 7 days or less. An increase in Scr of ≥26.5 μmol/L within 48 hours or a ≥50% increase in Scr within 7 days compared to baseline levels is considered to indicate an increase in serum creatinine (Scr) within 48 hours.
Diagnostic Criteria for Sepsis-Induced Coagulopathy
Sepsis-induced coagulopathyCitation8,Citation16: A sepsis-induced coagulopathy (SIC) score ≥4 points can indicate sepsis-induced coagulopathy. The SIC is calculated based on the PLT, prothrombin time ratio, and Sequential Organ Failure Assessment (SOFA) score.
Diagnostic Criteria for Sepsis-Associated Acute Kidney Injury (AKI)
Sepsis-associated AKI: Diagnostic criteria for both sepsis and AKI were necessary for simultaneous diagnosis, while other noninfectious factors contributing to AKI were excluded.
Diagnostic Criteria for Multiple Organ Dysfunction Syndrome (MODS)
MODS:Citation17,Citation18 Occurrence of potentially life-threatening physiological injuries that may lead to reversible physiological imbalances involving two or more organ systems unrelated to the imbalance, resulting in admission to the intensive care unit.
Inclusion Criteria
Diagnostic criteria for sepsis 3.0, specifically sepsis and septic shock, as outlined in 2016, were met.Citation1 For patients with infection or suspected infection, sepsis can be diagnosed when there is an increase of 2 or more points in the SOFA score from baseline, indicating infection-related organ dysfunction.
Diagnostic criteria for AKI aligned with kidney disease: KDIGO guidelines are met:Citation3,Citation5,Citation15 AKI is characterized by a sudden decline in renal function occurring within 7 days or less. The diagnostic criteria for AKI include an increase in Scr of ≥26.5 μmol/L within 48 hours or an increase in Scr of ≥50% from baseline within 7 days.
The diagnostic criteria for sepsis-associated coagulopathy (SAC) were met.Citation8,Citation16 SIC was diagnosed when the SOFA score was equal to or greater than 4.
Exclusion Criteria
Patients younger than 18 years old or older than 95 years old;
Patients who were diagnosed with chronic renal failure or who received long-term renal replacement therapy;
Patients with coagulation disorders caused by a medical history of haematological disorders, malignant tumours, or severe hepatic insufficiency;
Patients who received blood products such as platelets, plasma, and fibrinogen prior to admission;
Lactating or pregnant;
Individuals who are poisoned, including those affected by drug or food poisoning;
Use of antiplatelet or anticoagulant medications within the six months prior to admission;
Patients who were automatically discharged or lost to follow-up before completing a hospitalization period of 24 hours.
Methods and Groups
The present study employed a retrospective case‒control design to gather relevant data on coagulopathy in septic patients. The data collection period spans one year. The patients were stratified based on renal function into groups with acute kidney injury (AKI) and without AKI and further subdivided into survival and mortality groups based on prognostic outcomes. The primary focal points of the study included the overall mortality rate and the change in severity of SIC, which is associated with impaired coagulation function.
Classification and Scoring of SIC
This study employed the SAC classification criteria proposed by Iba et alCitation8 to establish different levels of scoring criteria. The specifics are as follows. 1. When the PLT is greater than 150×10^9/L and the international normalized ratio (INR) is less than 1.2, the score is zero. A score of 1 is assigned when the PLT is between 100×10^9/L and 150×10^9/L or when the INR is between 1.2 and 1.4. A score of 2 was allocated when the PLT fell within the range of 80×10^9/L to 100×10^9/L or when the INR was in the range of 1.4 to 1.6. A score of 3 was assigned when the PLT was less than 80×10^9/L or when the INR was equal to or greater than 1.6. The SAC grade was assigned as follows: 1–3 points were classified as mild (Grade 1), 4–5 points as moderate (Grade 2), and ≥6 points as severe (Grade 3).
Observation Indicators
This study collected data on patient sex, age, coagulation and complete blood analysis parameters within 24 hours after admission. These parameters included PT, INR, APTT, FIB, D-dimer, and PLT. In addition, relevant indicators, such as the Scr level, SOFA score, APACHE II score, SAC score, and SAC classification, were recorded.
Statistical Analysis
Data analysis for this study was conducted using SPSS 25.0 statistical software. Comparisons between two groups were performed using an independent sample t-test. To investigate the relationship between the blood creatinine concentration and SAC grade, this study employed Spearman correlation analysis. Furthermore, to explore factors that influence the prognosis of SAC patients, this study utilized the Cox proportional hazards regression model. Receiver operating characteristic (ROC) curves were plotted to evaluate the ability of indicators such as the SOFA score, APACHE II score, and SAC grade to predict the prognosis of sepsis patients with coagulation dysfunction. A P value <0.05 indicated that the difference was statistically significant.
Results
General Information
This study included a total of 352 patients with sepsis-associated coagulopathy. Among them, 21 patients were excluded due to age differences, 10 patients were excluded due to preexisting conditions such as malignancy or haematological disorders, 30 patients were excluded due to intoxication, and 20 patients were excluded due to a hospitalization duration of less than 24 hours. In the end, 271 patients who met the inclusion criteria were included.
Clinical data were compared between the survival group and the deceased group. Multiple factors, including the presence of MODS, acute kidney failure, PLT, PT, INR, fibrinogen degradation product levels, APTT, D-dimer levels, Scr levels, SOFA score, SAC classification, APACHE II score, and others, were compared between the two groups. Differences in various aspects were statistically significant (P<0.05). However, when comparing the two groups of patients in terms of sex and age, the differences were not significant (P>0.05). For specific data, please refer to .
Table 1 Comparison of Clinical Data Between the Survival Group and the Deceased Group
Correlations Between Serum Creatinine Levels and Sepsis-Induced Coagulopathy Grade and Score
The Scr concentration has an impact on the grading and scoring of sepsis-associated coagulation dysfunction in patients. In this study, Spearman correlation analysis revealed a significant correlation (P < 0.05) between the Scr level and the grading and scoring of SAC in the study subjects. Based on the calculation of the Spearman correlation coefficient, there was a positive correlation between Scr and SAC grade. For detailed data, please refer to .
Table 2 Correlation Analysis of Serum Creatinine Levels with the Severity and Severity of Sepsis-Associated Coagulation Dysfunction
Effects of the Sequential Organ Failure Assessment Score, Acute Physiology and Chronic Health Evaluation II Score, and Severity of Sepsis-Associated Coagulopathy on the Prognosis of Patients with SAC
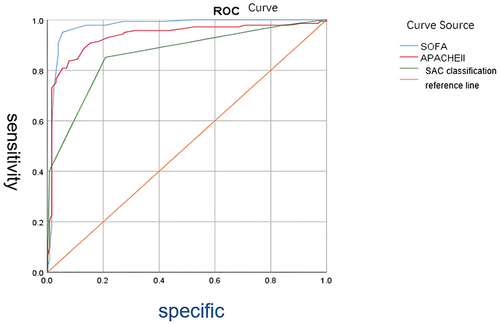
The findings of this study demonstrate the diagnostic accuracy in descending order as follows. The Results of this study indicate diagnostic accuracy, ranked from highest to lowest, as follows: SOFA score (AUC=0.972, 95% CI: 0.950–0.994, P<0.05), APACHE II score (AUC=0.934, 95% CI: 0.901–0.967, P<0.05), and SAC classification (AUC=0.863, 95% CI: 0.819–0.907, P<0.05). For specific data, please refer to .
Analysis of Factors Influencing the Prognosis of SAC Patients
The study findings indicate that certain factors possess independent predictive value for adverse prognosis in sepsis-complicated coagulopathy (MODS) patients. These factors included the presence of MODS (hazard ratio [HR] = 2.070), INR (HR = 0.727), Scr (HR = 0.995), and SOFA score (HR = 1.200). For detailed information, please refer to .
Table 3 Analysis of Factors Influencing the Prognosis of Patients with SAC
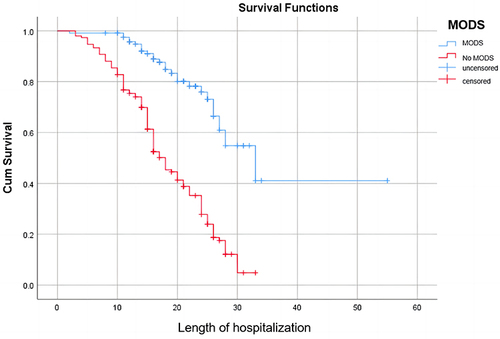
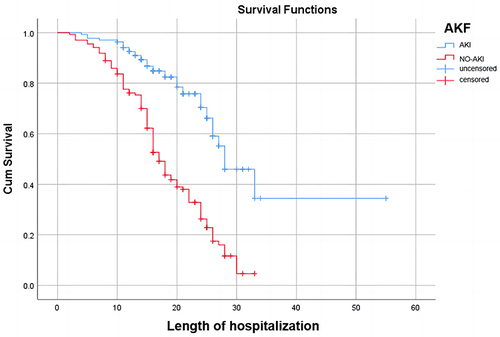
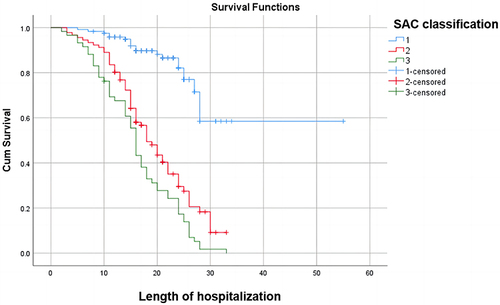
Cox curve analysis further demonstrated a rapid decrease in survival rates among patients with MODS within 30 days, decreasing from 100% to nearly 0%. By the 30th day, the survival rate remained within the range of 0% to 20%, with the ultimate survival time not exceeding 40 days. In contrast to the other group, the decline was more substantial, with shorter survival times observed. It is evident that in the group without MODS, the ultimate survival time ranged from 50 to 60 days, with the survival rate stabilizing near 40%. In the acute kidney injury cohort, patients exhibited final survival times ranging between 30 and 40 days, with a survival rate approaching zero. Within 30 days, their survival rate declines from 100% to near zero. Concurrently, patients without acute kidney injury demonstrate longer final survival times, ranging between 50 and 60 days, with a final survival rate stabilizing between 20% and 40%. Their decline within 30 days is comparatively less pronounced. In this study, Cox proportional hazards analysis revealed the survival rates of patients stratified by SAC grade. Patients with mild disease had a median survival time of 50–60 days, whereas those with moderate-to-severe disease had a median survival time of less than 40 days. The survival rate within 30 days decreased from 100% to approximately 60% for mild cases, while the decline was more significant for moderate to severe cases, all dropping from 100% to below 20%. Additional information can be found in .
Discussion
Sepsis is a syndrome of inflammatory response caused by infection and is commonly observed in critically ill patients. This disease progresses rapidly and is often accompanied by primary infection. A lack of timely and effective control measures can lead to septic shock and even further development of MODS, posing a significant threat to patient health.Citation1,Citation11 In this paper, we verified that MODS was an independent risk factor affecting the prognosis of patients with septic coagulation dysfunction (SAC) by multifactorial regression analysis (HR = 2.07, P < 0.05), which was also reflected in the Cox curves and that patients with MODS had a lower survival rate within 30 days than those without MODS. Organ dysfunction frequently manifests in severe septic shock, and its pathophysiological mechanisms are intricate and intertwined with haemodynamics, apoptosis, and PAMPs associated with the infectious agent. When the body experiences an infection-induced decrease in blood volume, endothelial cell apoptosis increases, and permeability subsequently changes. This leads to the translocation of bacterial flora and the release of toxins, resulting in the generation of PAMPs and the production of inflammatory mediators and cytokines.Citation6,Citation19,Citation20 Under the influence of multiple factors, the hepatic, renal, respiratory, and nervous systems of patients can be severely damaged.Citation21 For more details, please refer to .
Figure 6 Illustrates the mechanism of multiorgan dysfunction in septic shock.
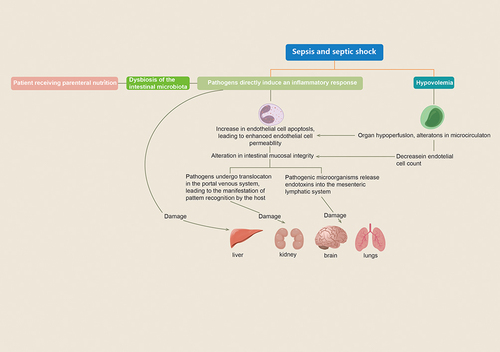
A review of sepsis-associated acute kidney injury (S-AKI) revealed that renal failure occurs as a result of multiple combined mechanisms. The pathophysiological basis underlying the development of S-AKI can be categorized as follows. The pathophysiological basis underlying the development of S-AKI includes a cascade of inflammatory responses, impairment of both large and small blood vessels, and aberrant cellular reactions.Citation22,Citation23 In this study, Cox curve analysis revealed that patients with S-AKI had a significantly lower survival rate at 30 days than those without AKI and verified that the blood creatinine (Scr) was an independent risk factor affecting the prognosis of SAC patients. Sepsis triggers a systemic inflammatory response, leading to the release of many inflammatory mediators, such as cytokines and chemokines, which directly damage renal tubules and glomeruli.Citation2 Dilation of renal blood vessels and intrarenal shunting are classified as macrovascular disturbances that can result in a decrease in renal perfusion and subsequently impact renal function.Citation4,Citation24 Additionally, animal models have shown that with increased production of proinflammatory factors and activation of leukocytes during sepsis, renal capillary thrombosis increases. The formation of microthrombi further leads to inadequate renal perfusion, as well as the diffusion of inflammatory factors and tissue oedema.Citation25 These tissues generate reactive oxygen species, which disrupt the epithelial cell barrier and exacerbate epithelial cell leakage,Citation26,Citation27 possibly resulting in endothelial cell dysfunction and exacerbating thrombosis.Citation27–29
Endothelial cell injury is highly prevalent in sepsis-associated organ dysfunction and plays a crucial role in this process. Disruption of normal cell-to-cell connections increases the permeability of the endothelial barrier.Citation30–32 A consequent increase in permeability will inevitably lead to a series of complications, including decreased microvascular perfusion and thrombus formation. The coagulation system contributes to the disruption of endothelial function through the activation of protease-activated receptor 1 (PAR1).Citation33–35 Hence, endothelial dysfunction serves as a pivotal link between S-AKI and SAC.
Sepsis-induced acute renal failure can affect the progression of coagulation dysfunction. The blood creatinine value (Scr) was correlated with the SAC classification and SAC assignment, which were positively correlated according to the Spearman correlation coefficient. Andres-Hernando et alCitation36 found in a murine animal model that cytokines and their receptors, such as IL-2, IL-6, IL-8, IL-1 beta, IL-12, and TNF-α, exhibit an early surge following AKI, leading to proinflammatory responses, neutrophil activation, endothelial cell apoptosis, and endothelial dysfunction. Moreover, sepsis often presents with coagulation dysfunction characterized by widespread microvascular thrombosis and the consumption of platelets and clotting proteins, ultimately leading to bleeding or disseminated intravascular coagulation (DIC). The coagulation dysfunction that accompanies sepsis is mainly due to the excessive inflammatory response of the organism to pathogens, leading to the overexpression of inflammatory mediators. Nicola Semeraro discussed three pathophysiological mechanisms in which inflammatory mediators, along with pathogenic microorganisms and their products, lead to the formation of a significant amount of thrombin and the deposition of fibrin:Citation37 (1) abnormal expression of TF is primarily attributed to monocyte–macrophage dysfunction; (2) dysfunction of endothelial cells (ECs) orchestrates the onset of anticoagulation pathway dysfunction; and (3) excessive production of plasminogen activator inhibitor-1 (PAI-1) by ECs and thrombin-mediated activation of clotting factor-induced fibrinolysis inhibitors result in fibrinolysis inhibition.Citation38 In conclusion, in sepsis, inflammatory mediators and cytokines can be regarded as the bridge between acute renal failure and coagulation dysfunction, as both are interconnected due to endothelial dysfunction.
The internationalized normative ratio (INR) plays an important role in determining coagulation indicators and is closely related to exogenous coagulation. Exogenous coagulation releases many coagulation factors, leading to hyperfibrinolysis and reduced coagulation factor synthesis, which is mainly reflected in elevated PT, decreased FIB and elevated INR.Citation39 In this study, the INR was found to be an independent risk factor for predicting the prognosis of patients with sepsis and coagulation disorders (OR=0.727, P<0.05). In a retrospective study analysis, the authors demonstrated through Kaplan‒Meier survival curves that an elevated INR was associated with a cumulative survival rate of 21.6%, which was associated with a significant decrease in survival, and that the superiority ratio (OR) of the INR for predicting the risk of in-hospital death in their all-cause mortality independent independent risk factor logistic analysis was 10.691.Citation40
Multiple scores are widely used in the diagnosis and management of sepsis and are essential for the assessment of this disease. These include the Acute Physiology and Chronic Health Score (APACHE II), Sequential Organ Failure Score (SOFA), and Sepsis-Associated Coagulation Dysfunction Score (SAC). The APACHE II scoring system was introduced by Knaus et al in 1985 and features comprehensive clinical parameters.Citation41 It is commonly utilized in intensive care units to assess the severity of patients’ conditions and predict their prognosis. Previous literature indicates that when assessing patients’ conditions and prognosis using the APACHE II score, a mean score of 20 ± 7.3 points was associated with an observed mortality rate of 58.2%.Citation42 In this study, ROC curve analysis of the APACHE II score yielded an AUC of 0.934, with a significance level of p < 0.05. In contrast to the SOFA score, there was no significant difference in the APACHE II score, a widely used clinical tool for predicting the severity of critical illness, between patients with a more severe form of sepsis and those who develop MODS within the first 48 hours. This might be attributed to the fact that the SOFA score is primarily designed to predict organ failure and that the APACHE II score is more suitable for predicting the length of hospital stay and severity of the disease.Citation43 The SOFA score, a diagnostic indicator of sepsis, has been widely used in clinical practice and has been increasingly well studied. In a retrospective study, Emanuel Eguia et al found a correlation between the SOFA score at admission and the incidence of sepsis.Citation44 In a randomized controlled trial based on 869 patients, Insiyah Campwala et al reported multiple organ failure to be a serious complication in patients with sepsis, the occurrence of which is usually associated with higher SOFA scores, higher shock indices, and lower GCS scores.Citation45 In this paper, it was demonstrated that the SOFA score was an independent risk factor for predicting death in patients with sepsis (HR = 1.2, p < 0.05), and the value of the SOFA score in predicting the prognosis of patients was also demonstrated by the ROC curve (AUC = 0.972, p < 0.05), which is in line with previous experimental studies as described in the argumentation. Compared to the first two, the SAC score has been used less often, but research on this score is gradually catching fire. In a retrospective study evaluating the value of SAC in predicting the risk of in-hospital death, SAC scores incorporating both the PLT and INR were used to categorize patients with sepsis into four classes based on the INR and PLT: mild, moderate, severe SAC and no SAC. Their results showed that SAC scores were strongly associated with patient mortality at admission, with the odds ratio (OR) for predicting the risk of in-hospital mortality ranging from 1.33 to 2.14.Citation46 Zhipeng Xu discussed the relationship between coagulation markers and highlighted the positive correlation between APTT and PT, D-dimer, platelet count, and SOFA score. Thus, the SAC classification should be included as part of the organ dysfunction scoring system.Citation47
Conclusion
In summary, patients with sepsis are prone to acute kidney injury and coagulation dysfunction, which interact with each other, and elevated blood creatinine is positively correlated with the SAC classification and assignment score. Patients with sepsis who are classified as having SAC grade 3 or have an SAC score of 4 or above tend to have a poor clinical prognosis.
Abbreviations
EICU, Emergency Intensive Care Unit; SAC, Sepsis-Associated Coagulopathy; APTT, Activated Partial Thromboplastin Time; PLT, Platelet Count; PT, Prothrombin Time; FIB, Fibrinogen Degradation Product; INR, International Normalized Ratio; Scr, Serum Creatinine; SOFA, Sequential Organ Failure Assessment; AKI, Acute Kidney Injury; DIC, Disseminated Intravascular Coagulation; PAMPs, Pathogen-associated Molecular Patterns; DAMPs, Damage-associated Molecular Patterns; NETs, Neutrophil Extracellular Traps; AKF, Acute Kidney Failure; APACHE II, Acute Physiology And Chronic Health Evaluation II; SIC, Sepsis-induced Coagulopathy; MODS, Multiple Organ Dysfunction Syndrome; ROC, Receiver Operating Characteristic; LPS, Lipopolysaccharide; TLR4, Toll-like Receptor 4; LBP, Lipopolysaccharide-binding Protein; HMGB1, High Mobility Group Box 1; S-AKI, Sepsis-associated Acute Kidney Injury; PAR1, Protease-activated Receptor 1; TF, Tissue Factor; t-PA, Tissue Plasminogen Activator; PAI-1, Plasminogen Activator Inhibitor-1; SAGES, the Society of American Gastrointestinal and Endoscopic Surgeons; ACS, Acute Care Surgery; Ecs, Endothelial Cells; SAPS, The Simplified Acute Physiology Score; RCT, Randomized Clinical Trial.
Ethics Approval and Informed Consent
This study was approved by the Ethics Committee of the Third Hospital of Hebei Medical University (Shijiazhuang, Hebei, China). All patients provided informed consent. The research was conducted in accordance with the ethical standards of the 1964 Declaration of Helsinki. Ethics approval number W2022-044-1.
Consent for Publication
I understand and agree that copyright will transfer to Open Access Emergency Medicine upon acceptance of my thesis. I confirm that this thesis is original and has not been published elsewhere. This thesis does not violate the intellectual property or other rights of others. I agree to abide by Open Access Emergency Medicine’s publication policy and related regulations, including but not limited to, revisions, edits, and formatting adjustments. If the paper involves collaboration with others, I have obtained the consent of all collaborators and will clearly state the contributions of each collaborator to the paper. I agree to follow the open access emergency medicine review process and to provide any additional information or documentation to support the publication of my paper. I hereby acknowledge and agree with the above.
Disclosure
The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Data Sharing Statement
The datasets used and/or analysed during the current study are available from the corresponding author upon reasonable request.
Additional information
Funding
References
- Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA. 2016;315(8):801–810. doi:10.1001/jama.2016.0287
- Shum HP, Kong HHY, Chan KC, Yan WW, Chan TM. Septic acute kidney injury in critically ill patients – a single-centre study on its incidence, clinical characteristics, and outcome predictors. Renal Failure. 2016;38(5):706–716. doi:10.3109/0886022X.2016.1157749
- Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120(4):C179–84. doi:10.1159/000339789
- Prowle JR, Bellomo R. Sepsis-associated acute kidney injury: macrohemodynamic and microhemodynamic alterations in the renal circulation. Semin Nephrol. 2015;35(1):64–74. doi:10.1016/j.semnephrol.2015.01.007
- Zarbock A, Nadim MK, Pickkers P, et al. Sepsis-associated acute kidney injury: consensus report of the 28th acute disease quality initiative workgroup. Nat Rev Nephrol. 2023;19(6):401–417. doi:10.1038/s41581-023-00683-3
- Fourrier F, Chopin C, Goudemand J, et al. Septic shock, multiple organ failure, and disseminated intravascular coagulation. Compared patterns of antithrombin III, protein C, and protein S deficiencies. Chest. 1992;101(3):816–823. doi:10.1378/chest.101.3.816
- Gando S, Saitoh D, Ishikura H, et al. A randomized, controlled, multicentre trial of the effects of antithrombin on disseminated intravascular coagulation in patients with sepsis. Crit Care. 2013;17:R297. doi:10.1186/cc13163
- Iba T, Nisio MD, Levy JH, Kitamura N, Thachil J. New criteria for sepsis-induced coagulopathy (SIC) following the revised sepsis definition: a retrospective analysis of a nationwide survey. BMJ Open. 2017;7(9):e017046. doi:10.1136/bmjopen-2017-017046
- Scarlatescu E, Tomescu D, Arama SS. Sepsis-associated coagulopathy. J Crit Care Med. 2016;2(4):156–163. doi:10.1515/jccm-2016-0024
- Annane D, Bellissant E, Cavaillon JM. Septic shock. Lancet. 2005;365(9453):63–78. doi:10.1016/S0140-6736(04)17667-8
- Angus DC, Van Der Poll T. Severe sepsis and septic shock. N Engl J Med. 2013;369(9):840–851. doi:10.1056/NEJMra1208623
- Allison SJ. Sepsis: NET-induced coagulation induces organ damage in sepsis. Nat Rev Nephrol. 2017;13(3):133. doi:10.1038/nrneph.2017.7
- Semeraro N, Ammollo CT, Semeraro F, Colucci M. Coagulopathy of acute sepsis. Semin Thromb Haemost. 2015;41(6):650–658. doi:10.1055/s-0035-1556730
- Camicia G, Pozner R, De Larranaga G. Neutrophil extracellular traps in sepsis. Shock. 2014;42(4):286–294. doi:10.1097/SHK.0000000000000221
- Chawla LS, Bellomo R, Bihorac A, et al. Acute disease quality initiative workgroup, acute kidney disease and renal recovery: consensus report of the acute disease quality initiative (ADQI) 16 workgroup. Nat Rev Nephrol. 2017;13(4):241–257. doi:10.1038/nrneph.2017.2
- Iba T, Connors JM, Nagaoka I, Levy JH. Recent advances in the research and management of sepsis-associated DIC. Int J Haematol. 2021;113(1):24–33. doi:10.1007/s12185-020-03053-y
- Ramirez M. Multiple organ dysfunction syndrome. Curr Probl Pediatr Adolesc Health Care. 2013;43(10):273–277. doi:10.1016/j.cppeds.2013.10.003
- Mizock BA. The multiple organ dysfunction syndrome. Dis Mon. 2009;55(8):476–526. doi:10.1016/j.disamonth.2009.04.002
- Cavaillon JM, Adire C. Sepsis and Non-Infectious Systemic Inflammation: From Biology to Critical Care. Weinheim: Wiley; 2007.
- Muth H, Maus U, Wygrecka M, et al. Pro- and antifibrinolytic properties of human pulmonary microvascular versus artery endothelial cells: impact of endotoxin and tumour necrosis factor-alpha. Crit Care Med. 2004;32(1):217–226. doi:10.1097/01.CCM.0000104941.89570.5F
- Greco E, Lupia E, Bosco O, Vizio B, Montrucchio G. Platelets and multiorgan failure in sepsis. Int J Mol Sci. 2017;18(10):2200. doi:10.3390/ijms18102200
- Fani F, Regolisti G, Delsante M, et al. Recent advances in the pathogenetic mechanisms of sepsis-associated acute kidney injury. J Nephrol. 2018;31(3):351–359. doi:10.1007/s40620-017-0452-4
- Chang YM, Chou YT, Kan WC, Shiao CC. Sepsis and acute kidney injury: a review focusing on the bidirectional interplay. Int J Mol Sci. 2022;23(16):9159. doi:10.3390/ijms23169159
- Yang L. Acute kidney injury in Asia. Kidney Dis. 2016;2(3):95–102. doi:10.1159/000441887
- Yue S, Li S, Huang X, et al. Construction and validation of a risk prediction model for acute kidney injury in patients suffering from septic shock. Dis Markers. 2022;2022:9367873. doi:10.1155/2022/9367873
- Maneta E, Aivalioti E, Tual-Chalot S, et al. Endothelial dysfunction and immunothrombosis in sepsis. Front Immunol. 2023;14:1144229. doi:10.3389/fimmu.2023.1144229
- Walborn A, Rondina M, Mosier M, Fareed J, Hoppensteadt D. Endothelial dysfunction is associated with mortality and severity of coagulopathy in patients with sepsis and disseminated intravascular coagulation. Clin Appl Thromb Haemost. 2019;25:1076029619852163. doi:10.1177/1076029619852163
- Paulus P, Jennewein C, Zacharowski K. Biomarkers of endothelial dysfunction: can they help us deciphering systemic inflammation and sepsis? Biomarkers. 2011;16 Suppl 1:S11–S21. doi:10.3109/1354750X.2011.587893
- López-Aguirre Y, Páramo JA. Endothelial cell and haemostatic activation in relation to cytokines in patients with sepsis. Thromb Res. 1998;94(2):95–101. doi:10.1016/S0049-3848(98)00200-X
- Joffre J, Hellman J, Ince C, Ait-Oufella H. Endothelial responses in sepsis. Am J Respir Crit Care Med. 2020;202(3):361–370. doi:10.1164/rccm.201910-1911TR
- Ait-Oufella H, Maury E, Lehoux S, Guidet B, Offenstadt G. The endothelium: physiological functions and role in microcirculatory failure during severe sepsis. Intensive Care Med. 2010;36(8):1286–1298. doi:10.1007/s00134-010-1893-6
- Chelazzi C, Villa G, Mancinelli P, De Gaudio AR, Adembri C. Glycocalyx and sepsis-induced alterations in vascular permeability. Crit Care. 2015;19(1):26. doi:10.1186/s13054-015-0741-z
- Zeerleder S, Schroeder V, Hack CE, Kohler HP, Wuillemin WA. TAFI and PAI-1 levels in human sepsis. Thromb Res. 2006;118(2):205–212. doi:10.1016/j.thromres.2005.06.007
- Iba T, Levy JH. Derangement of the endothelial glycocalyx in sepsis. J Thromb Haemost. 2019;17(2):283–294. doi:10.1111/jth.14371
- Tressel SL, Kaneider NC, Kasuda S, et al. A matrix metalloprotease-PAR1 system regulates vascular integrity, systemic inflammation and death in sepsis. EMBO Mol Med. 2011;3(7):370–384. doi:10.1002/emmm.201100145
- Andres-Hernando A, Dursun B, Altmann C, et al. Cytokine production increases and cytokine clearance decreases in mice with bilateral nephrectomy. Nephrol Dial Transplant. 2012;27(12):4339–4347. doi:10.1093/ndt/gfs256
- Semeraro N, Ammollo CT, Semeraro F, Colucci M. Sepsis, thrombosis and organ dysfunction. Thromb Res. 2012;129(3):290–295. doi:10.1016/j.thromres.2011.10.013
- Iba T, Umemura Y, Wada H, Levy JH. Roles of coagulation abnormalities and microthrombosis in sepsis: pathophysiology, diagnosis, and treatment. Arch Med Res. 2021;52(8):788–797. doi:10.1016/j.arcmed.2021.07.003
- Levi M, Poll T. Coagulation in patients with severe sepsis. Semin Thromb Haemost. 2015;41(1):9–15. doi:10.1055/s-0034-1398376
- Pan L, Mo M, Huang A, et al. Coagulation parameters may predict clinical outcomes in patients with septic acute kidney injury. Clin Nephrol. 2021;96(5):253–262. doi:10.5414/CN110459
- Knaus WA, E. D, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818–829. doi:10.1097/00003246-198510000-00009
- Freitas F. Profile and severity of the patients of intensive care units: prospective application of the APACHE II index. Rev Lat Am Enfermagem. 2010;2010:1.
- Giannoni C, Chelazzi C, Villa G, De Gaudio AR. Organ dysfunction scores in ICU. Trends Anaesth Crit Care. 2013;3(3):89–96.
- Eguia E, Cobb AN, Baker MS, et al. Risk factors for infection and evaluation of Sepsis-3 in patients with trauma. Am J Surg. 2019;218(5):851–857. doi:10.1016/j.amjsurg.2019.03.005
- Campwala I, Guyette FX, Brown JB, et al. Evaluation of critical care burden following traumatic injury from two randomized controlled trials. Sci Rep. 2023;13:1. doi:10.1038/s41598-023-28422-5
- Lyons PG, Micek ST, Hampton N, Kollef MH. Sepsis-associated coagulopathy severity predicts hospital mortality. Crit Care Med. 2018;46(5):736–742. doi:10.1097/CCM.0000000000002997
- Xu Z, Cheng B, Fu S, et al. Coagulative biomarkers on admission to the ICU predict acute kidney injury and mortality in patients with septic shock caused by intra-abdominal infection. Infect Drug Resist. 2019;12:2755–2764. doi:10.2147/IDR.S218592