Abstract
Premenstrual Dysphoric Disorder (PMDD) is a severe form of premenstrual syndrome (PMS) affecting up to 7% of reproductive age women. Women with PMDD are of reproductive age; therefore, contraception and treatment of PMDD are important considerations. The disorder as described in the DSM-V is characterized by moderate to severe psychological, behavioral and physical symptoms beginning up to two weeks prior to menses, resolving soon after the onset of menstruation and significantly interfering with daily functioning. PMDD develops in predisposed individuals after they are exposed to progesterone at the time of ovulation. It has been hypothesized that PMDD is in part attributable to luteal phase abnormalities in serotonergic activity and to altered configuration of ℽ-aminobutyric acid subunit A (GABAA) receptors in the brain triggered by the exposure to the neuroactive steroid progesterone metabolite, allopregnanolone (Allo). A large body of evidence suggests that selective serotonin reuptake inhibitors (SSRIs) can be effective in the treatment of PMDD. Combined hormonal contraceptive (CHC) pills, specifically the 20 mcg ethinyl estradiol/3mg drospirenone in a 24/4 extended cycle regimen has been shown to significantly improve the emotional and physical symptoms of PMDD. Other combined monophasic, extended cycle hormonal contraceptive pills with less androgenic progestins may also be helpful, although not well studied. Copper intrauterine devices (IUDs) are recommended for those not seeking hormonal contraceptives. Progestin-only methods including the progestin-only pill (POP), levonorgestrel (LNG) IUD, etonorgestrel implant or depot medroxyprogesterone acetate (DMPA) have the potential to negatively affect mood symptoms for women with or without baseline mood disorders, including PMDD. Careful counseling and close follow-up is recommended for patients with PMDD seeking these contraceptive methods.
Introduction
Premenstrual Dysphoric Disorder (PMDD) is characterized by the cyclical occurrence of psychological, behavioral and physical premenstrual symptoms that resolve within the first week of menstruation.Citation1–Citation5 Symptoms can start up to 14 days prior to menses, at the time of ovulation or any time during the luteal phase. The cardinal mood symptom of PMDD is irritability but anxiety, depression and mood swings are typical. Physical and behavioral symptoms, especially fatigue and difficulty concentrating, also contribute to disruption of daily activities and relationships ().Citation5,Citation6 An individual may desire or be counseled to utilize effective contraception to avoid unplanned pregnancy or to reduce pregnancy-related maternal risks, for example, if they have an underlying medical or psychiatric disorder. Women also utilize hormonal contraceptives for non-contraceptive benefits such as improving acne, dysmenorrhea, endometriosis or abnormal menstrual bleeding. The most effective contraceptives however, with the exception of the copper intrauterine device (IUD) (Paragard®), contain progestins which can potentially exacerbate PMDD symptoms.Citation7
Table 1 DSM-V premenstrual dysphoric disorder symptomsCitation6
The etiology of PMDD is multifactorial. Symptoms are triggered by the rise and fall of ovarian sex steroids at the time of ovulation. The most widely accepted hypothesis suggests that there is a relationship between progesterone, the neuroactive steroid metabolites of progesterone such as allopregnanolone (Allo), the neurotransmitter serotonin and the occurrence of PMDD symptoms.Citation7–Citation11 A limited number of combined contraceptive formulations, in particular one preparation containing 20 mcg of ethinyl estradiol (EE) and 3 mg drospirenone (DSRP) in a 24/4 regimen,Citation12,Citation13 and another containing 20 mcg EE and 90 mg levonorgestrel (LNG) daily extended regimen,Citation14 have been studied for the treatment of severe PMS and PMDD. There is a dearth of literature on tolerability and side effects of other combined hormonal contraceptive (CHC) pills, rings or patches, progestin-only pills (POPs), LNG IUDs, injections or implants in women with PMDD, either for symptom management or contraception.Citation12,Citation13,Citation15–Citation17
This review presents the clinical problem of PMDD and more broadly of severe premenstrual disorders and summarizes the literature pertaining to coitus independent, effective contraceptive methods studied in this population. Finally, recommendations based on the available literature and on expert opinion for screening, contraceptive prescribing and counseling are provided.
Diagnostic criteria for Premenstrual Dysphoric Disorder
The Diagnostic and Statistical Manual of Mental Disorders (5th edition) defines PMDD as a collection of symptoms () that must be present in the final week before the onset of menses, improve in the week after the onset of menses and become minimal or absent in the postmenstrual week.Citation6 The patient must experience at least 5 symptoms, with at least 1 symptom being a mood symptom, and these symptoms must cause clinically significant distress and interference with school, work, relationships or social activities.Citation12
The above symptoms must be present for the majority of menstrual cycles for the past year and should not be the result of medications, illicit substances, another medical condition or other mental disorder, although a co-existent mental disorder does not rule out a possible secondary PMDD diagnosis. In order to confirm the diagnosis, a daily prospective rating of symptoms must be documented over at least two menstrual cycles to confirm the relationship between the timing of symptom onset and the luteal phase of the menstrual cycle. Ratings should also reflect resolution of symptoms by the end of menses and a symptom-free interval during the postmenstrual follicular phase.Citation6 Retrospective recall can be biased by “menstrual awareness” or the tendency to link adverse symptoms to the occurrence of menses. No objective measure or laboratory test can confirm the diagnosis of PMDD. Another relevant set of diagnostic criteria for severe premenstrual disorders was developed for the International Society for Premenstrual Disorders (ISPMD) because many women experience distress and impairment that are below the threshold for diagnosis for PMDD but are more severe than a PMS diagnosis.Citation18–Citation21 The criteria guidelines are useful in guiding treatment decisions.Citation22
The most commonly used diagnostic tool, the Daily Record of Severity of Problems (DRSP), first published by J. Endocott, is available for free to download on the internet.Citation18 Another questionnaire, the Premenstrual Symptoms Screening Tool (PSST), is designed to be used retrospectivelyCitation23 and has an adolescent version.Citation24 The PSST is not a prospective tool and therefore may not accurately reflect the temporal changes in symptoms across the menstrual cycle.Citation23 The PSST is best used to screen patients for PMDD to be followed by further evaluation using a daily prospective questionnaire such as the DRSP for confirmation.Citation25
Epidemiology of PMDD
Most menstruating women (80–95%) experience physiological changes in the premenstrual period, but the number of women that meet criteria for PMDD is much smaller.Citation6 Estimates range from 1.2% to 6.4% according to one source and 3–7% according to another.Citation5,Citation26 A third study followed a total of 1246 rural and urban women over 2 cycles, but only 11 (1.3%) met criteria for PMDD, though if only retrospective subject reports of symptoms were used, far more would have had the diagnosis.Citation27 As the diagnosis can only be made by prospective recording of symptoms over multiple cycles, and must not be an exacerbation of an underlying psychiatric disorder, it is difficult to determine the true prevalence. However, prevalence estimates from multiple studies in communities across the globe generally fall between 1.2% and 7% depending on the study.Citation5 Symptoms of PMDD can be as debilitating as major depressive disorder (MDD).Citation1,Citation28 Up to 20% of women will experience severe sub-syndromal premenstrual mood and physical symptoms.Citation29 If an individual has a current or past depression or anxiety diagnosis, it may be difficult to rule out premenstrual exacerbation of the underlying psychiatric disorder versus a coexistent diagnosis of PMDD. In this setting, consultation with a mental health provider is important.Citation26 There is an increased risk of developing MDD or postpartum depression in women with PMDD.Citation30,Citation31 A history of MDD has been reported in 30–70% of women with PMDD.Citation32,Citation33 Women with a past medical history of MDD and postpartum depression are also at risk for developing PMDD.Citation34
Pathophysiology
Fluctuation in gonadal steroids, in particular exposure to progesterone, is necessary for triggering PMDD.Citation35 Before menarche, during pregnancy and after menopause (without hormone replacement), PMDD does not occur. Symptoms are also unusual during naturally anovulatory cycles and after ovarian suppression with gonadotropin-releasing hormone (GnRH) agonists.Citation35 While serum estradiol and progesterone levels do not differ between women with and without PMDD, the central and peripheral responses to the rise and fall of sex steroids are characteristic. The most accepted hypothesis suggests a relationship between exposure to progesterone, Allo, serotonin and occurrence of PMDD symptoms. These relationships also are the basis for some of the PMDD treatments.
Physiology of the menstrual cycle
The menstrual cycle functions as a product of a complex set of interactions between the hypothalamus, anterior pituitary, ovary and endometrium.Citation36 The hypothalamus secretes a neurohormone called gonadotropin-releasing hormone (GnRH), which is then transported to the anterior pituitary. GnRH is secreted in a pulsatile fashion and stimulates cells in the anterior pituitary to release the gonadotropins follicle-stimulating hormone (FSH) and luteinizing hormone (LH). FSH stimulates the production of estradiol within the inner granulosa cells of the follicle. Rising estradiol levels in the late follicular phase provide positive feedback to enable the mid-cycle surge of LH, which in turn stimulates ovulation. The luteal phase follows ovulation, and during this interval, the cells of the ovulated follicle become the corpus luteum which secretes progesterone in preparation for implantation. The development of the corpus luteum shifts the cycle from an estradiol-dominant to a progesterone-dominant process. The progesterone suppresses follicular growth and initiates secretory changes in the endometrium. Peak progesterone production occurs 7–8 days after the LH surge. In the absence of pregnancy, the corpus luteum rapidly declines which results in a decline in the progesterone and estradiol levels, which then results in menstruation.Citation36 The menstrual cycle is composed of four phases: the follicular phase, the ovulation phase, the luteal phase and menstruation. Ovulation thus bridges the follicular and luteal phases and menstruation is the beginning of the follicular phase. The follicular phase is variable in length beginning with day 1 of menses until ovulation. The luteal phase is restricted to 12–14 days prior to menses. During CHC use, ovulation and the luteal phase are suppressed but the active hormone phase of all CHC regimens is dominated by progestin (see below).
Progesterone and allopregnanolone (Allo)
Allo is a 3 alpha, 5 alpha reduced metabolite of progesterone produced by the ovaries and the brain during the luteal phase. It appears that Allo and possibly other reduced progesterone metabolites are the significant drivers of the psychological and behavioral symptoms of PMDD.Citation10 Allo is a positive allosteric ℽ-aminobutyric acid subunit A (GABAA) receptor modulator with potent sedative and anxiolytic properties. It does not appear that an excess or deficiency of Allo acting on GABAA receptors causes PMDD symptoms but rather, an abnormal paradoxical response to fluctuation in Allo, possibly due to alterations of the subunit composition (and therefore reduced sensitivity) of the GABAA receptor after exposure to Allo.Citation10 The duration of the GABA subunit changes is unknown, but based on clinical observations, begin to resolve as the levels of Allo decline in the late luteal phase. In pivotal studies, Backstrom and colleagues showed evidence for GABAergic deficiency in women with PMDD using the model of the saccadic eye velocity (SEV).Citation37 SEV is a measure of GABAA receptor sensitivity. In the luteal phase, after administration of a GABAergic agent such as progesterone metabolite pregnanolone, alcohol or a benzodiazepine, women with PMDD demonstrate altered sensitivity compared with healthy controls.Citation38 This finding suggests a cyclical tolerance for GABAA receptor agonists with diminution of GABA inhibitory effects in women with PMDD. Administration of the SSRI citalopram to women with PMDD women restored the GABAergic sensitivity and increased the pregnanolone sensitivity during this experimental treatment.Citation39 Thus, dose-dependent exposure to Allo causes paradoxical anxiety, irritability, depression and aggression in susceptible women and these effects seem to be ameliorated by increasing synaptic serotonin.Citation38
There is also substantial evidence for GABAergic dysfunction in MDD and postpartum depression.Citation40,Citation41 MDD sufferers have reduced central nervous system GABA levels and altered GABAA subunit expression. GABA also has an important role in controlling stress, a vulnerability factor in depression.Citation40
Blocking the binding of Allo on the GABA receptor has potential to decrease PMDD symptoms; however, available options are limited.Citation10 Hormonal contraceptives that prevent ovulation obliterate the cyclic production of progesterone and ovarian derived Allo. It is not known why the hormonal contraceptives that suppress ovulation are not always effective for PMDD, but the progestin in the hormonal contraceptive and possibly the duration of the pill-free interval are thought to play a role.Citation42 Many women with PMDD continue to have cyclical or daily PMDD-like symptoms while taking cyclical or continuous active oral contraceptive pill formulations.Citation14 One explanation for the persistence of PMDD-like symptoms during ovulation suppression with a contraceptive containing a progestin is that synthetic progestational compounds (progestins) can also be metabolized to Allo or similar neuroactive compounds that bind the GABAA receptor and alter the subunit composition, with resultant symptoms in susceptible individuals.Citation43
Ovarian hormone fluctuations across the menstrual cycle, in particular progesterone, alter binding of the serotonin 5-HT2A receptor and serotonin transporter.Citation44 SSRIs can also alter Allo levels, which may contribute to their effectiveness for the treatment of PMDD and MDD.Citation55,Citation45,Citation46
Serotonin
Abnormalities in serotonergicsted contribute to PMDD as well as to mood disorders like depression. Estrogen and progesterone and sex steroid receptors are found in many areas of the brain, particularly the amygdala, and can modulate serotonin transmission. Multiple studies have shown lowered serotonergic transmission in women with severe premenstrual symptoms and such symptoms can be brought on by dietary depletion of the serotonin pre-cursor tryptophan.Citation11,Citation47,Citation48 These findings are the basis for PMDD treatment using medications that increase synaptic serotonin such as SSRIs and serotonin-norepinephrine reuptake inhibitors (SNRIs) and may account for the treatment failure with non-serotonergic antidepressants.Citation5,Citation49
Knowledge of the etiology of complex mood symptoms is evolving. The monoamine hypothesis for MDD arose with the observation that medications that lowered serotonin levels caused depressive symptoms and was then supported by positive treatment response to serotonergic drugs. A direct role for serotonin in MDD has been challenged recently on the basis of lack of universal efficacy of SSRIs and SNRIs as well as the 2–4-week delayed onset of action of treatment (characteristic of MDD, but not PMDD). A more nuanced understanding of MDD is informed by neuroimaging studies and recent progress in basic molecular neuroscience. Further investigations into the role of neuroplasticity and stress in MDD may be relevant for the understanding and treatment of PMDD and MDD.Citation50,Citation51
Treatment of PMDD
As PMDD is a hormonally modulated and serotonin sensitive disorder, most evidence-based treatments are aimed at obliterating ovarian cyclicity or augmentation of serotonin. GABA receptor modulation is more complex and clinically available treatments are still lacking.Citation10 SSRIs, SNRIs and cognitive behavioral therapy (CBT) are the main psychiatric-focused treatments.Citation26 Hormonal regulation, primarily with certain hormonal contraceptives or high-dose transdermal estrogen with added progestin for endometrial protection, and in extreme cases and for limited duration, GnRH agonists, address the effects of ovulation on symptoms.Citation26 Other lifestyle modifications, such as diet, exercise, vitamin, mineral and herbal supplements and stress reduction fall into a third category.Citation26
Selective serotonin reuptake inhibitors (SSRIs)
SSRIs and SNRIs augment serotonergic transmission and have shown efficacy for the treatment of PMDD.Citation52,Citation53 Psychotropics that increase norepinephrine and dopamine do not significantly improve PMDD symptoms.Citation49 A 2013 Cochrane Review examined 31 randomized placebo-controlled trials for SSRI use in women with severe Premenstrual Syndrome (PMS), including PMDD and found both daily SSRI use or SSRI administered only during the luteal phase (approximately 2 weeks before the onset of menses) were similarly effective for reducing psychological and physical symptoms.Citation54 SSRIs improved psychological, physical and functional symptoms, in particular irritability.Citation55 However, there were not enough high-quality studies to determine whether daily or luteal-phase-only dosing was more effective. Clearly, luteal phase dosing can decrease cost, side effects and stigma of taking a psychiatric medication. Subjects taking SSRIs compared with placebo were more likely to experience nausea and decreased energy, in some cases resulting in discontinuation of the medication.Citation54 Other side effects of SSRIs can include weight gain, lowered libido, headache and gastrointestinal upset.Citation55
Different dosing regimens were examined in the Cochrane Review; however, there were insufficient data to definitively recommend one regimen of medication or dosing regimen over another. Medications studied included sertraline 50–150 mg, fluoxetine 10–20 mg, paroxetine 5–25 mg, escitalopram 10–20 mg and citalopram 10–30 mg.Citation54 Overall, moderate doses of these SSRIs, given either daily or during the luteal phase only, reduced PMS/PMDD symptoms and had low rates of side effects causing discontinuation.Citation54 A few small studies found efficacy for symptom onset dosing.Citation56 Failure to respond to one SSRI should prompt trial of another SSRI before abandoning the use of these agents. However, further studies are needed to determine the optimal medication for a given symptom profile, dose and schedule or use with hormonal contraceptives.Citation54
Cognitive behavioral therapy (CBT)
CBT has been used for other conditions in particular mood, anxiety and pain disorders. The goals of CBT are to train patients to re-frame negative emotions, behaviors and thoughts in order to regulate emotions and help cope with stressors.Citation57 A review of CBT for PMDD revealed seven trials of CBT.Citation58 However, the methodologies for these trials varied tremendously and none was placebo-controlled, though some compared CBT to relaxation techniques or assertiveness training, and some included use of SSRI or hormonal therapy. The results also suggest that the symptom reduction in PMDD patients who participated in CBT may have longer-lasting improvements than medications alone. Conversely, medications may improve symptoms more quickly than with CBT. However, further randomized control trials (RCTs) are needed to determine the most effective duration and method of CBT compared to, or in conjunction with, other treatments.Citation58
Hormonal contraceptives
Some combined oral contraceptive pill formulations have been subjected to RCTs for the treatment of PMDD.Citation15 One caveat is that there are few RCTs of various hormonal contraceptive regimens provided to women specifically diagnosed with PMDD.Citation5 The oral contraceptive with the most evidence for efficacy in treating PMDD is the 24-day active pill and 4-day inert pill formulation of 20 mcg EE with 3 mg of drospirenone. It is also the only hormonal contraceptive that is FDA-approved based on pivotal RCTs specifically for the treatment of PMDD for women who also desire contraception.Citation13,Citation59 This pill formulation is proposed to be more efficacious for women with PMDD for three reasons: lower dose of EE, shorter hormone-free interval and the spironolactone-like activity of drospirenone, with anti-mineralocorticoid and anti-androgenic properties. The 20 mcg of EE as opposed to the 30 or 35 mcg EE is proposed to be less stimulating for the renin-angiotensin system. The four rather than seven-day pill-free interval allows for better suppression of follicle development and more stable hormone levels, as well as decreasing the duration of the hormone-free interval when women have reported more mood symptoms.Citation13,Citation17,Citation59 Drospirenone is a unique progestin that is not a 19-nortestosterone derivative typically found in most other CHCs.
A double-blind, randomized, placebo-controlled, crossover design study was performed by Pearlstein et al to evaluate the effect of the 20 mcg EE/3 mg drospirenone 24/4 formulation specifically in women with a diagnosis of PMDD. The participants who completed the study showed significant improvement in productivity, social activities and social relationships during the premenstrual period (as measured by the Daily Record of Severity of Problems) while taking the medication, compared to baseline. Additionally, 61.7% of subjects reported a positive score on the Clinical Global Impressions-Improvement scale (indicating symptoms were much or very much improved) while taking the drug, compared to just 31.8% of subjects while taking the placebo.Citation13 Another multicenter, double-blind, randomized clinical trial by Yonkers et al prospectively followed 449 women with PMDD, randomized to either active treatment with of 20 mcg EE/3 mg drospirenone 24/4 (231 women) or placebo (218 women), with daily symptom ratings over 3 cycles. Those taking active treatment showed statistically significant improvements compared to the placebo group in Daily Record of Severity of Problems scores, productivity, enhanced social activities, better relationships and self-rated Premenstrual Tension Scales scores. Mood, physical and behavioral scores were all significantly improved. Overall, a 50% decrease in symptom scores was seen in 48% of the active-treatment group and only 36% of the placebo group (relative risk 1.7, 95% CI: 1.1–2.6; P=0.015).Citation59 This trial provides strong support for the use of 20 mcg EE/3 mg drospirenone 24/4 specifically for the treatment of PMDD.
A study of 30 mcg EE/3 mg drospirenone 21/7 for PMS (not PMDD) was found to improve only a few selected PMS symptoms studied as the primary endpoints (food cravings and acne), but subjects given this pill did have greater improvement from baseline compared to placebo on secondary endpoints assessed with the Beck Depression Inventory and the Profile of Mood States questionnaires.Citation12 Another small trial of 30 mcg EE/3 mg drospirenone 21/7 was not effective for the entire constellation of PMS symptoms either, though sexual quality of life and other quality of life indices (including mental health) were improved.Citation28 As reviewed in a 2012 Cochrane Review of oral contraceptives containing drospirenone for PMSs, overall significant improvement in premenstrual symptoms and lower rates of impairment in productivity, social activities and relationships were found for patients taking drospirenone-containing CHCs.Citation60 However, higher rates of nausea, irregular bleeding and breast pain were noted. Overall, the best evidence for using an oral contraceptive to treat PMDD comes from studies of 20 mcg EE/3 mg drospirenone 24/4, but it is still unclear if those positive effects persist after the first 3 cycles, as long-term usage has not been sufficiently studied.Citation13,Citation59
Other studies investigating 20 mcg EE and LNG 90 mcg daily had mixed results and did not meet primary endpoints for efficacy but showed some positive responses. In one study, 52% of subjects taking 20 mcg EE/90 mcg LNG daily for 112 days reported significant improvement from baseline in Daily Record of Severity of Problems during the estimated late luteal phase of the last treatment cycle compared to 40% of subjects taking placebo. Those symptoms included depressive, anger/irritability and physical symptoms.Citation14 Another study compared 30 mcgEE/150 mcg desogestrel with either 30 mcg EE/150 mcg LNG or (triphasic) 30/40/30 mcg EE/50/75/125 mcg LNG in a randomized cross-over design (without placebo) in women with mood changes during their menstrual cycles. They found that negative mood symptoms of tension and irritability were more improved with desogestrel, but that breast tension was more improved when taking the LNG. Overall, however, mood symptom scores were improved from baseline for all three CHCs.Citation61
There are some limited studies of other hormonal methods for PMDD treatment. Small studies of 100 mcg of transdermal 17 beta estradiol to block ovulation combined with LNG-IUD to protect the endometrium and insure contraception appears to be helpful, and might suggest an approach to management of PMDD for women using a LNG-IUD for contraception.Citation34,Citation62 Progesterone therapy alone to block ovulation for the treatment of PMDD such as with depot medroxyprogesterone acetate (DMPA) has not been adequately studied. The few existing studies are small and are heterogeneous in dose, duration, study population and instruments for measuring symptoms.Citation17 GnRH agonists are effective for PMDD and can potentially be successfully combined with hormone addback for menopausal symptoms; however, the significant negative side effects such as vasomotor symptoms and risks of a prolonged hypoestrogenic state make it a much less desirable first-line treatment.Citation5
Hormonal contraceptive mode of action
CHCs suppress ovulation by manipulating the pituitary ovarian axis to prevent pregnancy. The progestin and estrogen components of CHCs suppress the mid-cycle surge of LH and FSH and thus prevent ovulation.Citation63 CHCs can be monophasic (providing the same dose of estrogen and progestin daily) or multiphasic (providing varying doses of hormones throughout a 21- or 28-day cycle). POPs work by suppression ovulation in about half of cycles, suppressing midcycle peaks of LH and FSH, but their primary contraceptive effect is by increasing the cervical mucus, thus resulting in poor sperm penetration.Citation64 The etonorgestrel implant, Nexplanon® inhibits gonadotropin secretion and is quite effective in suppressing ovulation.Citation36 The LNG-IUD has less direct effect on the Hypothalamic-Pituitary-Ovarian axis with ovulation occurring in most women after the first 6–12 months. The contraceptive effect is via thickening of cervical mucous and thinning of the endometrium.Citation65 The long-acting reversible contraceptive (LARC) methods such as the IUD or the Nexplanon® are the most effective contraception methods. 0.2% of women experience an unintended pregnancy in the first year of use for the Mirena® LNG-IUD and 0.05% per year with the Nexplanon®.Citation66 The Copper IUD is also over 99% effective, with a 0.8% unintended pregnancy rate. These methods are most effective as they do not rely on patient compliance, such as with pill-taking or seeking 3-month injection.Citation66
Combined hormonal contraceptives effect on mood
The effects of hormonal contraception on mood, even for women without PMDD, are highly variable. Duke et al did not find an association between CHC use and development or exacerbation of mood disorders,Citation67 and several studies suggested that hormonal contraception was associated with improvement in mood.Citation68–Citation70 Schaffir et al published a systematic review of CHCs and mood. Despite a lack of prospective data and inconsistent methods, they could conclude most women do not have adverse mood symptoms with CHC use, but that the type of progestin, dosing method and predisposition to a mood disorder likely influence adverse mood symptoms in women using CHCs.Citation71 A review of hormonal contraceptives and mood in healthy women that included women with dysmenorrhea and PMDD utilized a prospective recording of mood symptoms and concluded that only 4–10% of the CHC users experienced deterioration of mood or emotional well-being.Citation72 In these prospective trials, mood symptoms were generally improved with CHCs that contained anti-androgenic progestins, such as drospirenone and desogestrel and negative mood symptoms were most prominent during the pill-free interval of the cycle. It can be inferred that elimination or shortening of the pill-free interval is recommended for women with menstrual-related mood symptoms, including PMDD. These findings suggest that although some women experience negative mood symptoms while taking hormonal contraception, more frequently, women experience an overall improvement in their mood and physical symptoms, especially during the premenstrual phase. Some caution is warranted for those with a prior diagnosis of a mood, anxiety or eating disorder as this population had a greater risk of worsening of mood and anxiety symptoms in the intermenstrual phase (between end of menses and up to 7 days prior to menses) in one randomized controlled trial of a novel CHC containing 1.5 mg estradiol and 2.5 mg nomegestrol acetate (a progestin not derived from 19-nortestosterone) in a 24/4 regimen.Citation73
A 2016 Danish prospective cohort study followed over 1 million women from 1995 through 2013.Citation74 The authors examined the relationship of contraceptive use and first use of antidepressants. Overall, compared with nonusers of hormonal contraception, combined oral contraceptive users had an incidence rate ratio of 1.23 (95% CI: 1.22–1.25) for first use of an antidepressant. Users of the CHC patch or vaginal ring, the LNG-IUD and POP users had RR of 2.0, 1.6, 1.4 and 1.34, respectively. The elevated risk ratios for a formal diagnosis of depression from a psychiatric hospital for users of hormonal contraceptives compared to non-users were slightly lower than the risk ratios for antidepressant use, but still statistically significant. This effect was more pronounced for adolescents.Citation74 There are a number of possible confounding factors influencing the results, such as reasons for antidepressant treatment, complexity of the initiation of sexual activity in the teen years and other life circumstances. As no prospective data on mood symptoms or changes in mood symptoms were collected, it is difficult to apply this information specifically to women with PMDD.
Interactions between combined hormonal contraceptives and psychotropic drugs
Concurrent medications can interfere with the metabolism of hormonal contraception. A recent systematic review concluded that although there is scant clinical or pharmacokinetic data, common psychotropic drugs (excluding St. John’s wort) that alter serotonin, dopamine or norepinephrine used to treat anxiety and depressive disorders are unlikely to interact with hormonal contraceptive methods, and hormonal methods do not alter efficacy of antidepressants.Citation75
Progestin-only methods effect on mood
LNG-IUD
LARC methods including the LNG-IUD are currently among the most widely used forms of birth control in North America and Europe.Citation76 As the LNG-IUD works by releasing LNG at a local level, it was assumed that there is a negligible risk of adverse effects on mood. A small study measured mean serum concentrations of LNG over the 5-year course of the LNG-IUD 52 mg (Mirena®), as seen in . These numbers do not correlate with contraceptive efficacy as most of the contraceptive effect is due to the local action of LNG.Citation77 However, clearly, the LNG released by the LNG-IUD enters the bloodstream and crosses the blood-brain barrier, with the potential to cause adverse effects related to mood and depressive symptoms, particularly in the first 6 months of use.Citation78,Citation79 The side effects listed in the package insert of the LNG-IUDs include nausea, breast tenderness, headache, skin problems and mood changes and nervousness.Citation80 The LNG-IUD package insert contains a warning stating that 5% or more of clinical trial subjects reported a depressed mood as an adverse event.
Table 2 Quantitative LNG plasma level measurements in patients with regular and prolonged use of the 52 mg (20 mg/day) LNG-releasing intrauterine systemCitation77
Since most women continue to ovulate (less than 15% of women are anovulatory by the end of their 1st year of use)Citation81 while using the LNG IUDs, the device would not be expected to provide treatment for PMDD; however, the possible effects on mood in women in general and those with depression are relevant to this review. There are no published studies assessing women with prospectively diagnosed PMDD, untreated or treated (with SSRIs for example) and given a LNG IUD for contraception.
Numerous studies evaluated the association between the use of LNG-IUDs and mood disturbances in general.Citation76,Citation78,Citation82–Citation84 There is conflicting evidence regarding LNG-IUDs and their impact on depression. The two randomized studies that evaluated mood or depression in LNG-IUD users reported very low depression rates, between 0.2% and 0.5%.Citation83,Citation84 The most current review comes from the European Journal of Contraception, which published an expert statement on the effects on mood of progestin-only contraceptives.Citation78 In their analysis, they concluded that very few women complain of severe mood alterations as a consequence of using an LND-IUD, either the 20 mg/d or 12 mg/d dose.Citation78 A prospective cohort study found that compared with nonusers, users of LNG-IUD had a relative risk of first use of an antidepressant of 1.4.Citation39 In many studies, changes in mood were not distinguished from underlying or de novo MDD; thus, future studies are indicated to evaluate the nature and severity of the mood changes. A systematic review of the limited evidence from 6 studies demonstrated that among women with a prior diagnosis of depressive or bipolar disorder, the LNG-IUD use was not associated with a worse clinical course of the disease.Citation79 It is important to point out that this study did not include women with PMDD, so at this time, data on the mood effects of progestin-containing IUDs in women with PMDD is lacking. However, since there is a relationship between PMDD and MDD, LNG-IUDs may be an appropriate option for women with PMDD, albeit after careful counseling and with close follow-up. Of note, women with bipolar disorder have a high rate of contraceptive non-compliance, making a LARC a desirable option.Citation85 Additionally, mood stabilizers (anticonvulsants) can lower the efficacy of CHCs and possibly even progestin implants. It is reassuring that in one study, the rates of complications and psychiatric hospitalizations were not different among women using a LND-IUD compared with a copper IUD.Citation85 Lastly, this same study found that women with bipolar disorder were more likely to continue IUD use over a year compared to DMPA injections.
Progestin-only pills
POPs suppress ovulation in about 50% of women and are less effective than CHCs, with approximately 9 out of 100 women becoming pregnant within the first year of use, compared with 3 out of 100 with CHCs.Citation64 A retrospective analysis demonstrated an increased RR of first use of antidepressants with both norethindrone and desogestrel, compared to the nonuse of hormonal contraception.Citation86 However, further studies are needed to evaluate the impact of the POP on mood.
Injectable and subdermal progestins
DMPA intramuscular injection (administered every 3 months) and the etonorgestrel progestin subdermal implant (Nexplanon®) are two forms of contraception that are highly effective. There are no studies of these methods in women with PMDD. A large population-based study evaluated symptoms of depression in DMPA users over 3 years and found increased likelihood of depressive symptoms with resolution after DMPA was discontinued.Citation87 DMPA package insert states that depression is a side effect of the medication and patients who have a history of depression should be carefully observed and that the drug not be re-administered if the depression persists. The Nexplanon® package insert also contains a warning stating that women with a history of depressed mood should be carefully observed. In the implant clinical trials, 5.5% of patients reported depression as an adverse side effect, but only 1% experienced depression of sufficient severity to request implant removal. The most recent systematic review found no correlation between the progestin subdermal implant and depression.Citation87 It is clear that there are no firm conclusions about the relationship between progestin-only hormonal contraception and PMDD or depression.Citation87 It is therefore critical to properly counsel patients on the risks of possible mood symptoms should they choose a progestin-only method.
Adolescents
There are data to suggest that there is an increased risk for younger women.Citation88 Adolescents with elevated levels of depressive symptoms are more likely to select an IUD as compared to those with minimal symptoms.Citation89 By screening adolescent females for depressive symptoms and PMDD, providers can strategize their approach to effective contraception counseling. Adolescents who screen positive should be reevaluated within the 1–2 months of initiation of a hormonal contraceptive and when feasible be managed in concert with a mental health professional.
Postpartum
Postpartum women who are breastfeeding are usually counseled to use a progestin-only method or a LARC.Citation66 The American College of Obstetrics and Gynecology (ACOG) recommends screening of all women in the 2–6 weeks postpartum for postpartum depression using a validated instrument such as the Edinburgh Postnatal Depression Scale (EPDS), the Patient Health Questionnaire 9 or the Postpartum Depression Scale.Citation90 A confirmatory diagnosis should initiate the “collaborative care” model with a mental health practitioner but does not preclude a progestin-only contraceptive method for lactating mothers or a CHC (after 6 weeks) for others.
Contraceptive counseling for women with PMDD
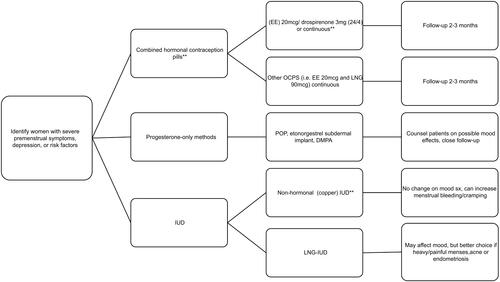
It is important to identify women with history of PMDD or other mood disorders when providing contraceptive counseling (). Clinicians should take a thorough medical history to identify women who have severe premenstrual symptoms, PMDD, current or past history of a mood or anxiety disorder, postpartum depression, previous sensitivity to progestins or prior use of anti-depressants. Those with risk factors should undergo a more thorough current and past psychiatric history. These patients should be counseled about the importance of reporting new or worsening adverse mood symptoms to their health care provider. Current or past history of severe symptoms should initiate a “collaborative care” model that includes a mental health practitioner. Copper IUDs are probably the least controversial highly effective LARC for women with mood diagnoses and symptoms. However, many women, particularly those with dysmenorrhea, heavy menstrual bleeding, irregular cycles, iron deficiency anemia and endometriosis, benefit from the non-contraceptive advantages of a CHC, LNG-IUD or subdermal implant. If a CHC or progestin-only method is initiated in a predisposed woman, the potential effects on mood should be addressed during counseling, and short-term follow-up (1–3 months) is required. Because of the risk of symptom deterioration in women who have already experienced major depression, the LNG-IUD and other progestin-only contraceptive methods may not be the first choice. There are no studies regarding the use of progestin-only contraceptive methods in women with PMDD and as noted, CHCs can have positive effects on affective symptoms in women with PMDD. If a patient has persistent bothersome PMDD symptoms and are otherwise happy with their hormonal method, it is also an option to add a serotonergic antidepressant. In light of the fact that studies report higher rates of contraceptive nonuse, misuse and discontinuation among women with symptoms of some mental health disorders (ie, depression and anxiety; PMDD was not addressed specifically), a LARC may be advisable if unintended pregnancy is the most important issue.Citation70 Women with depression and stress symptoms (again, PMDD was not queried) are at increased risk for user-related contraceptive failures, especially for the most commonly used methods that require greater user effort and diligence.Citation68 Finally, the United States Medical Eligibility Criteria for Contraceptive Use 2016 rates all hormonal contraceptives as level 1, no restriction for women with depressive disorders.Citation91 For those with a higher risk of mood symptoms, it suggests discussion of the potential risks, the expected timing of the onset and duration of mood changes (eg, highest levels of systemic progestin after LNG-IUD placement are within the first three months) and reversibility with removal of the contraceptive agent.
Conclusion
Women with PMDD or who are at risk for mood disorders present a special challenge when seeking hormonal contraceptive care. Contraceptive efficacy, user preferences and non-contraceptive benefits must be weighed against potential mood effects. If PMDD or another mood disorder is suspected when a patient presents for contraceptive counseling or initiation, prospective daily recording of symptoms, using a tool such as the DRSP should be completed for 2 cycles before and after initiating a contraceptive method to evaluate for PMDD or underlying depression and subsequent symptoms. Initial short-term 1–3-month follow-up is recommended to assess the impact of the medication. It has been proposed that a short question such as “Have you noticed any mood changes in the last 1–3 months” can be the first step for highlighting adverse mood symptoms. A discussion of initiation of SSRI or SNRI for those diagnosed with PMDD is also warranted. The assistance of a mental health professional may be indicated. Women with PMDD who are seeking both treatment of PMDD and contraception can be recommended a choice of 20 mcg CHCs preferably containing drospirenone and a shortened pill-free interval (24/4 or continuous active pills). There is also some evidence for continuous low dose CHCs such as EE 20 mcg/LNG 90 mg. Other monophasic CHCs (especially a continuous regimen or with a shorter hormone-free interval) and less androgenic progestin such as desogestrel can also be considered. For women who cannot use estrogen-containing methods or prefer a longer-acting method that does not require daily dosing, the copper IUD is preferred but if the non-contraceptive benefits of less pain and bleeding with menses are desired, a LNG-IUD is a reasonable choice as long as potential mood changes from the progestin are addressed. As with CHC methods, closer follow-up is recommended. Symptoms may be worse in the first 1–3 months and patients should have a plan for follow-up with the provider or mental health consultation in the event of significant mood symptoms. The copper IUD is an excellent choice for women seeking effective contraception who do not have heavy or painful menses and wish to avoid potential adverse effects from an exogenous progestin. Other methods including POPs, DMPA and the etonorgestrel contraceptive implant are also reasonable options for certain women, but again will require counseling about risk of mood deterioration and careful management. Barrier methods and natural family planning methods may be acceptable options for those who accept less effective methods. Balancing family planning goals, mood symptom management and medical considerations are essential to care for women with PMDD seeking contraception.
Disclosure
Dr Rapkin received payment for serving on an independent Data and Safety Monitoring Board for the Berlin Center for Epidemiology and Health funded by Bayer Pharmaceuticals. He is a member of the Speakers Bureau for Abbvie Pharmaceuticals and for legal reviews. Dr Rapkin also reports personal fees from Bayer Pharmaceuticals, during the conduct of the study. The authors report no other conflicts of interest in this work.
References
- Halbreich U, Borenstein J, Pearlstein T, Kahn LS. The prevalence, impairment, impact, and burden of premenstrual dysphoric disorder (PMS/PMDD). Psychoneuroendocrinology. 2003;28(Suppl 3):1–23.
- Hussein Shehadeh J, Hamdan-Mansour AM. Prevalence and association of premenstrual syndrome and premenstrual dysphoric disorder with academic performance among female university students. Perspect Psychiatr Care. 2018;54:176–184. doi:10.1111/ppc.12219
- Sternfeld B, Swindle R, Chawla A, Long S, Kennedy S. Severity of premenstrual symptoms in a health maintenance organization population. Obstet Gynecol. 2002;99:1014–1024. doi:10.1016/s0029-7844(02)01958-0
- Cohen LS, Soares CN, Otto MW, Sweeney BH, Liberman RF, Harlow BL. Prevalence and predictors of premenstrual dysphoric disorder (PMDD) in older premenopausal women. The Harvard Study of Moods and Cycles. J Affect Disord. 2002;70:125–132.
- Yonkers KA, Simoni MK. Premenstrual disorders. Am J Obstet Gynecol. 2018;218:68–74. doi:10.1016/j.ajog.2017.05.045
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington (DC): American Psychiatric Association; 2013.
- Backstrom T, Andreen L, Birzniece V, et al. The role of hormones and hormonal treatments in premenstrual syndrome. CNS Drugs. 2003;17:325–342. doi:10.2165/00023210-200317050-00003
- Halbreich U. The etiology, biology, and evolving pathology of premenstrual syndromes. Psychoneuroendocrinology. 2003;28(Suppl 3):55–99. doi:10.1016/S0306-4530(03)00097-0
- Martinez PE, Rubinow DR, Nieman LK, et al. 5α-reductase inhibition prevents the luteal phase increase in plasma allopregnanolone levels and mitigates symptoms in women with premenstrual dysphoric disorder. Neuropsychopharmacology. 2016;41:1093–1102. doi:10.1038/npp.2015.246
- Bixo M, Ekberg K, Poromaa IS, et al. Treatment of premenstrual dysphoric disorder with the GABAA receptor modulating steroid antagonist Sepranolone (UC1010) – a randomized controlled trial. Psychoneuroendocrinology. 2017;80:46–55. doi:10.1016/j.psyneuen.2017.02.031
- Gracia CR, Freeman EW, Sammel MD, Lin H, Sheng L, Frye C. Allopregnanolone levels before and after selective serotonin reuptake inhibitor treatment of premenstrual symptoms. J Clin Psychopharmacol. 2009;29:403–405. doi:10.1097/JCP.0b013e3181ad8825
- Freeman EW, Kroll R, Rapkin A, et al. Evaluation of a unique oral contraceptive in the treatment of premenstrual dysphoric disorder. J Womens Health Gend Based Med. 2001;10:561–569. doi:10.1089/15246090152543148
- Pearlstein TB, Bachmann GA, Zacur HA, Yonkers KA. Treatment of premenstrual dysphoric disorder with a new drospirenone-containing oral contraceptive formulation. Contraception. 2005;72:414–421. doi:10.1016/j.contraception.2005.08.021
- Halbreich U, Freeman EW, Rapkin AJ, et al. Continuous oral levonorgestrel/ethinyl estradiol for treating premenstrual dysphoric disorder. Contraception. 2012;85:19–27. doi:10.1016/j.contraception.2011.05.008
- Freeman EW, Halbreich U, Grubb GS, et al. An overview of four studies of a continuous oral contraceptive (levonorgestrel 90 mcg/ethinyl estradiol 20 mcg) on premenstrual dysphoric disorder and premenstrual syndrome. Contraception. 2012;85:437–445. doi:10.1016/j.contraception.2011.09.010
- Naheed B, Kuiper JH, Uthman OA, O’Mahony F, O’Brien PM. Non-contraceptive oestrogen-containing preparations for controlling symptoms of premenstrual syndrome. Cochrane Database Syst Rev. 2017;3:Cd010503.
- Ford O, Lethaby A, Roberts H, Mol BW. Progesterone for premenstrual syndrome. Cochrane Database Syst Rev. 2012;Cd003415.
- O’Brien PM, Backstrom T, Brown C, et al. Towards a consensus on diagnostic criteria, measurement and trial design of the premenstrual disorders: the ISPMD Montreal consensus. Arch Womens Ment Health. 2011;14:13–21. doi:10.1007/s00737-010-0201-3
- Nevatte T, O’Brien PM, Backstrom T, et al. ISPMD consensus on the management of premenstrual disorders. Arch Womens Ment Health. 2013;16:279–291. doi:10.1007/s00737-013-0346-y
- Ismaili E, Walsh S, O’Brien PMS, et al. Fourth consensus of the International Society for Premenstrual Disorders (ISPMD): auditable standards for diagnosis and management of premenstrual disorder. Arch Womens Ment Health. 2016;19:953–958. doi:10.1007/s00737-016-0631-7
- Kadian S, O’Brien S. Classification of premenstrual disorders as proposed by the International Society for Premenstrual Disorders. Menopause Int. 2012;18:43–47. doi:10.1258/mi.2012.012017
- Green L, O’Brien PMS, Panay N, Craig M. Royal College of Obstetrics and Gynecologists. Management of premenstrual syndrome. BJOG. 2017;124:e73–e105.
- Steiner M, Macdougall M, Brown E. The premenstrual symptoms screening tool (PSST) for clinicians. Arch Womens Ment Health. 2003;6:203–209. doi:10.1007/s00737-003-0018-4
- Steiner M, Peer M, Palova E, Freeman EW, Macdougall M, Soares CN. The Premenstrual Symptoms Screening Tool revised for adolescents (PSST-A): prevalence of severe PMS and premenstrual dysphoric disorder in adolescents. Arch Womens Ment Health. 2011;14:77–81. doi:10.1007/s00737-010-0202-2
- Henz A, Ferreira CF, Oderich CL, et al. Premenstrual syndrome diagnosis: a comparative study between the Daily Record of Severity of Problems (DRSP) and the Premenstrual Symptoms Screening Tool (PSST). Rev Bras Ginecol Obstet. 2018;40:20–25. doi:10.1055/s-0037-1608672
- Pearlstein T, Steiner M. Premenstrual dysphoric disorder: burden of illness and treatment update. J Psychiatry Neurosci. 2008;33:291–301.
- Gehlert S, Song IH, Chang C-H, Hartlage SA. The prevalence of premenstrual dysphoric disorder in a randomly selected group of urban and rural women. Psychol Med. 2009;39:129–136. doi:10.1017/S003329170800322X
- Halbreich U, Backstrom T, Eriksson E, et al. Clinical diagnostic criteria for premenstrual syndrome and guidelines for their quantification for research studies. Gynecol Endocrinol. 2007;23:123–130. doi:10.1080/09513590601167969
- Yonkers KA, Pearlstein T, Rosenheck RA. Premenstrual disorders: bridging research and clinical reality. Arch Womens Ment Health. 2003;6:287–292. doi:10.1007/s00737-003-0026-4
- Endicott J. Premenstrual Dysphorias: Myths and Realities. Washington (DC): American Psychiatric Press; 1994.
- Critchlow DG, Bond AJ, Wingrove J. Mood disorder history and personality assessment in premenstrual dysphoric disorder. J Clin Psychiatry. 2001;62:688–693.
- Pearlstein TB, Frank E, Rivera-Tovar A, Thoft JS, Jacobs E, Mieczkowski TA. Prevalence of axis I and axis II disorders in women with late luteal phase dysphoric disorder. J Affect Disord. 1990;20:129–134.
- Yonkers KA. The association between premenstrual dysphoric disorder and other mood disorders. J Clin Psychiatry. 1997;58(Suppl 15):19–25.
- Watson NR, Studd JW, Savvas M, Garnett T, Baber RJ. Treatment of severe premenstrual syndrome with oestradiol patches and cyclical oral norethisterone. Lancet. 1989;2:730–732. doi:10.1016/s0140-6736(89)90784-8
- Schmidt PJ, Martinez PE, Nieman LK, et al. Premenstrual dysphoric disorder symptoms following ovarian suppression: triggered by change in ovarian steroid levels but not continuous stable levels. Am J Psychiatry. 2017;174:980–989. doi:10.1176/appi.ajp.2017.16101113
- Hatcher R. Contraceptive Technology. 21st ed. Bridging the Gap Communications; 2018.
- Bixo M, Johansson M, Timby E, Michalski L, Backstrom T. Effects of GABA active steroids in the female brain with a focus on the premenstrual dysphoric disorder. J Neuroendocrinol. 2018;30:e12553. doi:10.1111/jne.12553
- Backstrom T, Bixo M, Johansson M, et al. Allopregnanolone and mood disorders. Prog Neurobiol. 2014;113:88–94. doi:10.1016/j.pneurobio.2013.07.005
- Sundstro II, Backstrom T. Citalopram increases pregnanolone sensitivity in patients with premenstrual dysphoric disorder. Acta Physiol Scand. 1999;167:A6–A7. doi:10.1046/j.1365-201x.1999.0600f.x
- Luscher B, Shen Q, Sahir N. The GABAergic deficit hypothesis of major depressive disorder. Mol Psychiatry. 2011;16:383–406. doi:10.1038/mp.2010.120
- Maguire J. Neuroactive steroids and GABAergic involvement in the neuroendocrine dysfunction associated with major depressive disorder and postpartum depression. Front Cell Neurosci. 2019;13:83. doi:10.3389/fncel.2019.00083
- Lundin C, Danielsson KG, Bixo M, et al. Combined oral contraceptive use is associated with both improvement and worsening of mood in the different phases of the treatment cycle – a double-blind, placebo-controlled randomized trial. Psychoneuroendocrinology. 2017;76:135–143. doi:10.1016/j.psyneuen.2016.11.033
- Giatti S, Melcangi RC, Pesaresi M. The other side of progestins: effects in the brain. J Mol Endocrinol. 2016;57:R109–R126. doi:10.1530/JME-16-0061
- Bixo M, Allard P, Backstrom T, et al. Binding of [3H]paroxetine to serotonin uptake sites and of [3H]lysergic acid diethylamide to 5-HT2A receptors in platelets from women with premenstrual dysphoric disorder during gonadotropin releasing hormone treatment. Psychoneuroendocrinology. 2001;26:551–564.
- Uzunova V, Sheline Y, Davis JM, et al. Increase in the cerebrospinal fluid content of neurosteroids in patients with unipolar major depression who are receiving fluoxetine or fluvoxamine. Proc Natl Acad Sci USA. 1998;95:3239–3244. doi:10.1073/pnas.95.6.3239
- Uzunova V, Sampson L, Uzunov DP. Relevance of endogenous 3alpha-reduced neurosteroids to depression and antidepressant action. Psychopharmacology (Berl). 2006;186:351–361. doi:10.1007/s00213-005-0201-6
- Eriksson E. SSRIs probably counteract premenstrual syndrome by inhibiting the serotonin transporter. J Psychopharmacol. 2014;28:173–174. doi:10.1177/0269881113512910
- Menkes DB, Coates DC, Fawcett JP. Acute tryptophan depletion aggravates premenstrual syndrome. J Affect Disord. 1994;32:37–44.
- Freeman EW, Rickels K, Sondheimer SJ, Polansky M. Differential response to antidepressants in women with premenstrual syndrome/premenstrual dysphoric disorder: a randomized controlled trial. Arch Gen Psychiatry. 1999;56:932–939. doi:10.1001/archpsyc.56.10.932
- Liu B, Liu J, Wang M, Zhang Y, Li L. From serotonin to neuroplasticity: evolvement of theories for major depressive disorder. Front Cell Neurosci. 2017;11:305. doi:10.3389/fncel.2017.00305
- Pittenger C, Duman RS. Stress, depression, and neuroplasticity: a convergence of mechanisms. Neuropsychopharmacology. 2008;33:88–109. doi:10.1038/sj.npp.1301574
- Dimmock PW, Wyatt KM, Jones PW, O’Brien PM. Efficacy of selective serotonin-reuptake inhibitors in premenstrual syndrome: a systematic review. Lancet. 2000;356:1131–1136. doi:10.1016/s0140-6736(00)02754-9
- Wyatt KM, Dimmock PW, O’Brien PM. Selective serotonin reuptake inhibitors for premenstrual syndrome. Cochrane Database Syst Rev. 2002;Cd001396.
- Marjoribanks J, Brown J, O’Brien PM, Wyatt K. Selective serotonin reuptake inhibitors for premenstrual syndrome. Cochrane Database Syst Rev. 2013;Cd001396.>.
- Steiner M, Pearlstein T, Cohen LS, et al. Expert guidelines for the treatment of severe PMS, PMDD, and comorbidities: the role of SSRIs. J Womens Health (Larchmt). 2006;15:57–69. doi:10.1089/jwh.2006.15.57
- Yonkers KA, Holthausen GA, Poschman K, Howell HB. Symptom-onset treatment for women with premenstrual dysphoric disorder. J Clin Psychopharmacol. 2006;26:198–202. doi:10.1097/01.jcp.0000203197.03829.ae
- Busse JW, Montori VM, Krasnik C, Patelis-Siotis I, Guyatt GH. Psychological intervention for premenstrual syndrome: a meta-analysis of randomized controlled trials. Psychother Psychosom. 2009;78:6–15. doi:10.1159/000162296
- Lustyk MK, Gerrish WG, Shaver S, Keys SL. Cognitive-behavioral therapy for premenstrual syndrome and premenstrual dysphoric disorder: a systematic review. Arch Womens Ment Health. 2009;12:85–96. doi:10.1007/s00737-009-0052-y
- Yonkers KA, Brown C, Pearlstein TB, Foegh M, Sampson-Landers C, Rapkin A. Efficacy of a new low-dose oral contraceptive with drospirenone in premenstrual dysphoric disorder. Obstet Gynecol. 2005;106:492–501. doi:10.1097/01.AOG.0000175834.77215.2e
- Lopez LM, Kaptein A, Helmerhorst FM. Oral contraceptives containing drospirenone for premenstrual syndrome. Cochrane Database Syst Rev. 2008;Cd006586.
- Backstrom T, Hansson-Malmstrom Y, Lindhe BA, Cavalli-Bjorkman B, Nordenstrom S. Oral contraceptives in premenstrual syndrome: a randomized comparison of triphasic and monophasic preparations. Contraception. 1992;46:253–268. doi:10.1016/0010-7824(92)90006-f
- Smith RN, Studd JW, Zamblera D, Holland EF. A randomised comparison over 8 months of 100 micrograms and 200 micrograms twice weekly doses of transdermal oestradiol in the treatment of severe premenstrual syndrome. Br J Obstet Gynaecol. 1995;102:475–484. doi:10.1111/j.1471-0528.1995.tb11321.x
- Sondheimer SJ. Oral contraceptives: mechanism of action, dosing, safety, and efficacy. Cutis. 2008;81:19–22.
- McCann MF, Potter LS. Progestin-only oral contraception: a comprehensive review. Contraception. 1994;50:S1–S195.
- Attia AM, Ibrahim MM, Abou-Setta AM. Role of the levonorgestrel intrauterine system in effective contraception. Patient Prefer Adherence. 2013;7:777–785. doi:10.2147/PPA.S36948
- ACOG Committee Opinion No. 735. Adolescents and long-acting reversible contraception: implants and intrauterine devices. Obstet Gynecol. 2018;131:e130–e139. doi:10.1097/AOG.0000000000002632
- Duke JM, Sibbritt DW, Young AF. Is there an association between the use of oral contraception and depressive symptoms in young Australian women? Contraception. 2007;75:27–31. doi:10.1016/j.contraception.2006.08.002
- Toffol E, Heikinheimo O, Koponen P, Luoto R, Partonen T. Hormonal contraception and mental health: results of a population-based study. Hum Reprod. 2011;26:3085–3093. doi:10.1093/humrep/der269
- Keyes KM, Cheslack-Postava K, Westhoff C, et al. Association of hormonal contraceptive use with reduced levels of depressive symptoms: a national study of sexually active women in the United States. Am J Epidemiol. 2013;178:1378–1388. doi:10.1093/aje/kwt188
- Toffol E, Heikinheimo O, Koponen P, Luoto R, Partonen T. Further evidence for lack of negative associations between hormonal contraception and mental health. Contraception. 2012;86:470–480. doi:10.1016/j.contraception.2012.02.014
- Schaffir J, Worly BL, Gur TL. Combined hormonal contraception and its effects on mood: a critical review. Eur J Contracept Reprod Health Care. 2016;21:347–355. doi:10.1080/13625187.2016.1217327
- Poromaa IS, Segebladh B. Adverse mood symptoms with oral contraceptives. Acta Obstet Gynecol Scand. 2012;91:420–427. doi:10.1111/j.1600-0412.2011.01333.x
- Bengtsdotter H, Lundin C, Gemzell Danielsson K, et al. Ongoing or previous mental disorders predispose to adverse mood reporting during combined oral contraceptive use. Eur J Contracept Reprod Health Care. 2018;23:45–51. doi:10.1080/13625187.2017.1422239
- Skovlund CW, Morch LS, Kessing LV, Lidegaard O. Association of hormonal contraception with depression. JAMA Psychiatry. 2016;73:1154–1162. doi:10.1001/jamapsychiatry.2016.2387
- Berry-Bibee EN, Kim M-J, Simmons KB, et al. Drug interactions between hormonal contraceptives and psychotropic drugs: a systematic review. Contraception. 2016;94:650–667. doi:10.1016/j.contraception.2016.07.011
- Aleknaviciute J, Tulen JHM, De Rijke YB, et al. The levonorgestrel-releasing intrauterine device potentiates stress reactivity. Psychoneuroendocrinology. 2017;80:39–45. doi:10.1016/j.psyneuen.2017.02.025
- Seeber B, Ziehr SC, Gschlieβer A, et al. Quantitative levonorgestrel plasma level measurements in patients with regular and prolonged use of the levonorgestrel-releasing intrauterine system. Contraception. 2012;86:345–349. doi:10.1016/j.contraception.2012.01.015
- Merki-Feld GS, Apter D, Bartfai G, et al. ESC expert statement on the effects on mood of the natural cycle and progestin-only contraceptives. Eur J Contracept Reprod Health Care. 2017;22:247–249. doi:10.1080/13625187.2017.1353075
- Pagano HP, Zapata LB, Berry-Bibee EN, Nanda K, Curtis KM. Safety of hormonal contraception and intrauterine devices among women with depressive and bipolar disorders: a systematic review. Contraception. 2016;94:641–649. doi:10.1016/j.contraception.2016.06.012
- Nilsson CG, Lahteenmaki PL, Luukkainen T. Ovarian function in amenorrheic and menstruating users of a levonorgestrel-releasing intrauterine device. Fertil Steril. 1984;41:52–55.
- Kailasam C, Cahill D. Review of the safety, efficacy and patient acceptability of the levonorgestrel-releasing intrauterine system. Patient Prefer Adherence. 2008;2:293–302.
- Tewari R, Kay VJ. Compliance and user satisfaction with the intra-uterine contraceptive device in Family Planning Service: the results of a survey in Fife, Scotland, August 2004. Eur J Contracept Reprod Health Care. 2006;11:28–37. doi:10.1080/13625180500431422
- Andersson K, Odlind V, Rybo G. Levonorgestrel-releasing and copper-releasing (Nova T) IUDs during five years of use: a randomized comparative trial. Contraception. 1994;49:56–72. doi:10.1016/0010-7824(94)90109-0
- Sivin I, el Mahgoub S, McCarthy T, et al. Long-term contraception with the levonorgestrel 20 mcg/day (LNg 20) and the copper T 380Ag intrauterine devices: a five-year randomized study. Contraception. 1990;42:361–378. doi:10.1016/0010-7824(90)90046-x
- Berenson AB, Asem H, Tan A, Wilkinson GS. Continuation rates and complications of intrauterine contraception in women diagnosed with bipolar disorder. Obstet Gynecol. 2011;118:1331–1336. doi:10.1097/AOG.0b013e318233beae
- Smith K, Nayyar S, Rana T, Archibong AE, Looney KR, Nayyar T. Do progestin-only contraceptives contribute to the risk of developing depression as implied by beta-arrestin 1 levels in leukocytes? A pilot study. Int J Environ Res Public Health. 2018;15. doi:10.3390/ijerph15061188
- Worly BL, Gur TL, Schaffir J. The relationship between progestin hormonal contraception and depression: a systematic review. Contraception. 2018;97:478–489. doi:10.1016/j.contraception.2018.01.010
- Gregory ST, Hall K, Quast T, et al. Hormonal contraception, depression, and academic performance among females attending college in the United States. Psychiatry Res. 2018;270:111–116. doi:10.1016/j.psychres.2018.09.029
- Francis J, Presser L, Malbon K, Braun-Courville D, Linares LO. An exploratory analysis of contraceptive method choice and symptoms of depression in adolescent females initiating prescription contraception. Contraception. 2015;91:336–343. doi:10.1016/j.contraception.2014.12.010
- ACOG Committee Opinion No. 757. Summary: screening for perinatal depression. Obstet Gynecol. 2018;132:1314–1316. doi:10.1097/AOG.0000000000002928
- Curtis KM, Tepper NK, Jatlaoui TC, et al. U.S. medical eligibility criteria for contraceptive use, 2016. MMWR Recomm Rep. 2016;65:1–103.