Abstract
Aim
The aim of this study is to assess the willingness, motivation, satisfaction levels, and expectations of participants engaged in clinical trials, while also exploring factors that influence compliance from the participants’ standpoint. Furthermore, this research aims to enhance the understanding of all stakeholders involved in clinical trials about the needs of participants and to offer evidence-based and pragmatic recommendations for effectively managing clinical trial participants.
Methods
An electronic questionnaire covers various aspects of Traditional Chinese Medicine (TCM) clinical trials. The distribution of the electronic questionnaire was facilitated through WeChat groups, WeChat Moments, and individual interactions. Count data was expressed in terms of frequency (or rate), and subsequent analysis was conducted utilizing the standard chi-squared test. A p value≤0.05 was considered significant. By drawing a Pareto chart, the priority of factors influencing participants’ willingness to participate in clinical trials was systematically assessed.
Results
88 participants answered the questionnaire. A decline in education level corresponded to a decreasing comprehension of participants’ grasp of “clinical trial” and “clinical trial participant” (p<0.05). The primary reason for participants’ reservations about joining clinical trials stems from their inadequate comprehension of clinical trial details. The key factors contributing to participants’ reluctance to engage in clinical trials encompass aspects of trial design, the operational procedures, insufficient understanding of clinical trial particulars, concerns about potential harm to their bodies and opposition from family members. Importantly, these areas of participants’ hesitation and unwillingness to partake in clinical trials mirror the factors participants’ dissatisfaction contributing to.
Conclusion
We found four factors significantly influencing participants’ willingness to engage and their overall satisfaction: the participant’s profile, trial design, trial institution setup, and the qualifications of the researchers. Derived from these critical factors, this paper presents a series of constructive measures.
Introduction
In the context of clinical trials, compliance pertains to the adherence of all involved parties to pertinent requirements, standards, and applicable laws and regulations.Citation1 As key stakeholders in clinical trials, participants bear the responsibility of maintaining a foundational level of compliance. Furthermore, the participants are partners in new drug innovation, and they need recognition from society and families. The protection of the participants should always be the top priority.
An ethical and scientific clinical trial, without considering the participants’ experience and needs, will greatly reduce its acceptance and compliance, while also having adverse effects on the health of participants. In addition, it will also increase the management costs of clinical research institutions for participants. A meta-analysis of 84 Chinese studies conducted between December 2018 and January 2020, focusing on participant compliance in clinical trials, unveiled a lack of dependable strategies for enhancing compliance.Citation2
Effective management of participants and their adherence to trial protocols serves to mitigate noncompliance and bias, thus underpinning the trial’s success. However, instances of non-compliance are notably common among participants in clinical trials.Citation3 Importantly, existing research on participant compliance in clinical trials inadequately explores the systematic and comprehensive factors that influence participant compliance and satisfaction.
Given the variations in educational backgrounds, participant motivations, and interests, achieving complete alignment among sponsors, researchers, and participants in clinical trials proves challenging. Not only is it unethical to impose mandates on participants without considering their rights and interests, it also tends to yield unsatisfactory outcomes. Consequently, enhancing the quality of clinical trials necessitates an initial grasp of participants’ viewpoints and requirements. In pursuit of this understanding, we conducted a small-scale pilot study aimed at comprehending participant compliance and satisfaction within Traditional Chinese Medicine (TCM) clinical trials at Beijing Hospital of Traditional Chinese Medicine, and provide reference for participant management.
The aim of this study is to assess the willingness, motivation, satisfaction levels, and expectations of participants engaged in clinical trials, while also exploring factors that influence compliance from the participants’ standpoint. Furthermore, this research aims to enhance the understanding of all stakeholders involved in clinical trials about the needs of participants and to offer evidence-based and pragmatic recommendations for effectively managing clinical trial participants.
Research Methods
Questionnaire Design
“Questionnaire Star” (https://www.wjx.cn) is currently the largest professional online platform in China for surveys, online exams, evaluations, and voting, with users covering over 90% of universities and research institutes in China.Citation4,Citation5 An electronic questionnaire was developed using “Questionnaire Star”, comprising a total of 32 questions, including 3 fill-in-the-blank, 27 multiple-choice, and 2 open-ended questions. The questionnaire is structured into three distinct sections: (1) gathering basic demographic information about the research participants (Q1-Q6); (2) assessing participants’ comprehension of and willingness to engage in clinical trials (Q7-Q14), which examines five critical dimensions: understanding of fundamental concepts, intent to participate in clinical trials, perceived benefits of participation, and trust in the efficacy of clinical trials; and (3) evaluating participant satisfaction with their involvement in clinical trials (Q15-Q32). This comprehensive survey covers various aspects of Traditional Chinese Medicine (TCM) clinical trials, encompassing the clinical trial environment, Clinical Trial Staff and associated personnel, trial procedures, medical outcomes, participant requirements during the trial, and suggestions for enhancing satisfaction with the clinical trial process. The completion of the questionnaire is estimated to take between 15 to 30 minutes. Further detailed information is available in Appendix 1. For assessing participant satisfaction with the clinical trial experience (Q15-Q25, Q27, Q28), a 5-point Likert scale was employed, with scores ranging from 1 (Very Dissatisfied) to 5 (Very Satisfied). The distribution of the electronic questionnaire was facilitated through WeChat groups, WeChat Moments, and individual interactions.
Research Participants
This study meticulously adhered to stringent eligibility criteria. Inclusion criteria encompassed individuals aged 18 or above, participants involved in Phase II and Phase III Traditional Chinese Medicine (TCM) clinical trials at the Beijing Hospital of Traditional Chinese Medicine within the past year, completion of at least one clinical trial visit, and volunteering to participate in the survey. Exclusion criteria included clinical trials involving participants with cognitive impairment, individuals lacking proficiency in using mobile phones or computers for questionnaire responses, clinical trials outside the scope of Phase II and Phase III TCM clinical trials (eg, Phase IV TCM clinical trials, trials related to the protection of TCM varieties, trials involving chemical drugs, biological agents, or medical devices), and participants who signed informed consents but did not engage in any trial visits.
The study involving 88 participants from 20 phase II and phase III TCM clinical trials conducted at the Beijing Traditional Chinese Medicine Hospital between November 18, 2021, and March 14, 2022. The trials spanned 10 diverse TCM research domains: Gynecology, Orthopedics, Pulmonology, Rheumatology, Gastroenterology, Otolaryngology, Cardiology, Surgery, Pediatrics, and Dermatology.
Questionnaire Completion
Of the 102 questionnaires distributed, we received a total of 88 completed responses. Notably, all 88 participants provided comprehensive answers to all 32 questions, displaying a lack of logical errors in their responses.
Statistical Analysis
Following the retrieval of data from the “Questionnaire Star” online platform, a meticulous process of data organization, review, and cross-validation was undertaken to ensure the fidelity of the data. Count data was expressed in terms of frequency (or rate), and subsequent analysis was conducted utilizing the standard chi-squared test.
The Pareto chart, recognized for its efficacy in optimization and improvement endeavors, was employed to identify pivotal factors contributing to participants’ reluctance to engage in clinical trials. This methodology serves as a cornerstone in the formulation of effective management strategies. By drawing a Pareto chart, the priority of factors influencing participants’ willingness to participate in clinical trials was systematically assessed. This assessment was carried out through the depiction of both bar and line charts. The bar chart represented the frequency of responses corresponding to factors impacting participation willingness, while the line chart illustrated the cumulative percentages attributed to these influencing factors. By combining the bar and line charts along the horizontal axis, a comprehensive Pareto chart was generated. In congruence with the Pareto principle, the influencing factors were categorized as follows: major factors within Category A (cumulative percentage 0–80%), minor factors in Category B (cumulative percentage 80–90%), and general factors within Category C (cumulative percentage 90–100%).Citation6
Results
Demographic Information of the Participants
The study encompassed a total of 88 participants, each of whom had actively engaged in at least one clinical trial. Predominantly, their participation was concentrated in a single trial. This participant cohort was predominantly situated within the age bracket of 18 to 40 years. Furthermore, a notable proportion of participants held employment status, primarily possessing a technical secondary educational background, or beyond. Remarkably, their annual income distribution was characterized by a balanced dispersion. This demographic exhibited ample cognitive capacity to comprehend the survey questionnaire and consequently provide comprehensive responses. Further details can be found in .
Table 1 Basic Demographic Information of the Participants (n=88)
Awareness and Willingness to Participate in Clinical Trials
Understanding of “Clinical Trial” and “Clinical Trial Participants”
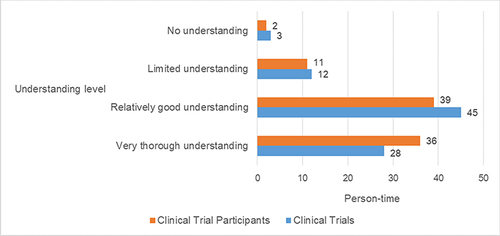
The comprehension levels of the terms “clinical trials” and “clinical trial participants” were assessed among the cohort of 88 respondents. The distribution of understanding among these respondents was as follows for “Clinical Trials”: 28 individuals exhibited a “very thorough understanding”, 45 individuals demonstrated a “relatively good understanding”, 12 individuals had a “limited understanding”, and 3 individuals possessed “no understanding”. For “Clinical Trial Participants”, 36 individuals showcased a “very thorough understanding”, 39 individuals presented a “relatively good understanding”, 11 individuals displayed a “limited understanding”, and 2 individuals had “no understanding”. Further details and graphical representation can be found in .
Figure 1 Understanding of “Clinical Trials” and “Clinical Trial Participants” among 88 Participants.
No significant differences were observed in the comprehension of “clinical trials” and “clinical trial participants” across various participant attributes, including sex, age, occupation, annual income, and number of participated clinical trial projects (p > 0.05).
Statistically significant differences were, however, identified in the understanding level of the term “clinical trial” within distinct educational backgrounds (χ² = 34.68, p = 0.001) (see ). Remarkably, participants with a master’s degree or higher exhibited a “very thorough understanding” at a rate of 51.61%. Conversely, 58.62% of those with a Bachelor’s degree and 87.50% of participants with a high school education demonstrated a “relatively good understanding”. Notably, 100% of participants with a junior high school education or lower indicated a “limited understanding” of the concept.
Table 2 Levels of Understanding of the Concept of “Clinical Trials” and “Clinical Trial Participants” Among 88 Participants with Differing Educational Backgrounds
Likewise, statistically significant difference was evident in the understanding level of the term “clinical trial participants” among individuals with differing educational backgrounds (χ² = 33.75, p = 0.001) (refer to ). Specifically, participants with a master’s degree or above displayed a “very thorough understanding” at a rate of 54.84%, while 51.72% of those with a Bachelor’s degree and 87.50% of individuals with a high school education demonstrated a “relatively good understanding”. Conversely, 100% of participants with a junior high school education or below conveyed a “limited understanding” of the concept.
Willingness to Participate in Clinical Trials and Contributing Factors
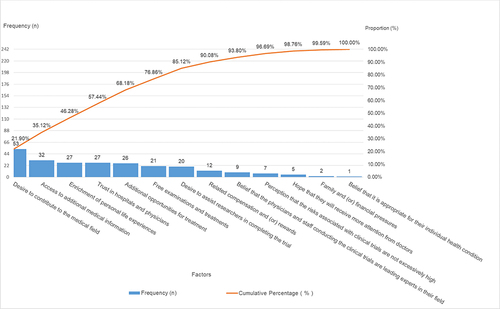
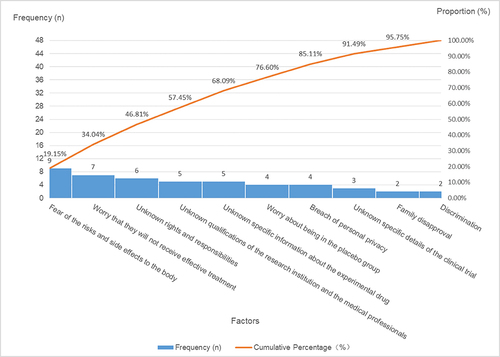
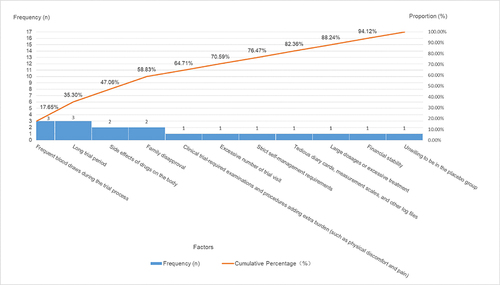
No statistically significant differences were noted in the willingness to engage in clinical trials based on factors such as sex, educational background, age, occupation, annual income, or the number of prior clinical trial involvements (p > 0.05). When queried about their willingness to participate in clinical trials, the 88 participants provided responses as follows: 74 individuals (84.09%) expressed a willingness to participate (1 of them added reasons for the hesitance and unwillingness to partake), 12 individuals (13.64%) exhibited some degree of hesitance (2 of them added reasons for their unwillingness to partake), and 2 individuals (2.27%) conveyed an unwillingness to partake. A comprehensive insight into the factors underpinning participants’ willingness to engage in clinical trials is expounded upon in and further depicted through .
Table 3 Factors Influencing Participants’ Willingness to Partake in Clinical Trials: Category a (Major Factors), Category B (Minor Factors), and Category C (General Factors)
Table 4 Factors Influencing Participants’ Hesitation to Partake in Clinical Trials: Category a (Major Factors), Category B (Minor Factors), and Category C (General Factors)
Table 5 Factors Influencing Participants’ Unwillingness to Partake in Clinical Trials: Category a (Major Factors), Category B (Minor Factors), and Category C (General Factors)
Advantages of Participation in Clinical Trials
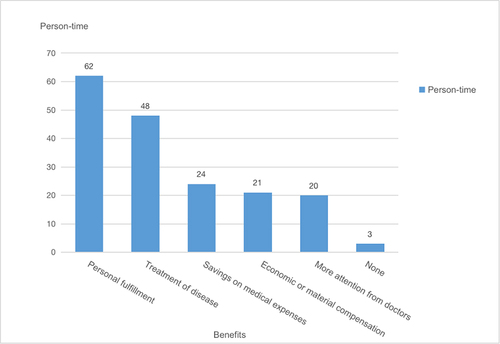
The majority of participants experienced benefits in various aspects, including improvements in mental well-being, treatment effectiveness, and decreased medical expenditures. Among the response choices, “Personal fulfillment” was chosen by 62 individuals, “Treatment of disease” by 48 individuals, “Savings on medical expenses” by 24 individuals, “Economic or material compensation” by 21 individuals, and “More attention from doctors” by 20 individuals. Only a small fraction of respondents, specifically 3 individuals, selected “None” as their perceived benefit. Notably, no statistically significant variations were identified in the perceived advantages of participating in clinical trials across participants with different gender, age, educational background, occupation, annual income levels, or the number of prior clinical trial involvements (p > 0.05), as illustrated in .
Confidence in Clinical Trial Efficacy
No significant differences were detected in the levels of confidence pertaining to the effectiveness of experimental drugs or other trial therapies across participants of diverse sexes, ages, educational levels, annual incomes, and the number of clinical trials in which they participated (p > 0.05).
Statistically significant differences however, emerged in the confidence levels regarding the efficacy of experimental drugs among distinct occupational groups (χ² = 17.90, p = 0.022) (refer to ). The spectrum of higher confidence in clinical trial efficacy was predominantly observed among employed individuals and students. Specifically, 50% of unemployed individuals, 38.81% of employed individuals, 35.71% of students, and 33.33% of freelancers expressed “high confidence. “Additionally, 57.14% of students, 50.75% of employed individuals, and 33.33% of freelancers conveyed “confidence”. Notably, 100% of retirees, 50% of unemployed individuals, 33.33% of freelancers, 10.54% of employed individuals, and 7.14% of students indicated a “neutral” stance. Importantly, no participants selected “unconfident” or “highly unconfident” positions.
Table 6 Confidence Levels Regarding the Efficacy of Experimental Drugs Among Participants with Different Occupations
A statistically significant variation is observed in the willingness to engage in clinical trials across distinct participant groups based on their confidence levels in the effectiveness of such trials (χ2 = 20.92, p=0.000) (). The inclination to participate increases in tandem with the level of trust in the therapeutic efficacy of clinical trials: 33 participants (100%) who exhibit “high confidence”, 35 participants (81.40%) who possess “confidence”, and 6 participants (50%) who maintain a “neutral” degree of trust in clinical trial efficacy express a “willing” disposition toward participation. Among those with “confidence”, 6 participants (13.95%) show “slight hesitancy”, and the same proportion of 6 participants (50%) among those with “neutral” confidence are “slightly hesitant” to participate. Additionally, 2 participants (4.65%) among those who have “confidence” in clinical trial efficacy express an “unwillingness” to participate.
Table 7 Willingness Level to Participate in Clinical Trials Among Different Population Groups Based on Their Level of Confidence in the Effectiveness of Drugs Trials
Satisfaction of Participants in Clinical Trials
To assess the overall satisfaction of participants in clinical trials, a comprehensive analysis was conducted using questions Q15-Q25, Q27, and Q28. No significant disparity was found in the satisfaction levels among the 13 question items (p>0.05) ().
Table 8 Overall Satisfaction Ratings of 88 Participants in Clinical Trials
Satisfaction with Medical Environment During Clinical Trial Participation
Among the cohort of 88 participants, a significant proportion of 45 individuals (51.14%) reported being “very satisfied” with the medical environment encountered during their clinical trial participation. Additionally, 37 participants (42.05%) conveyed a sentiment of being “satisfied”, while a minor segment of 6 participants (6.82%) maintained a “neutral” stance towards this aspect.
Satisfaction with Staff Involved in Clinical Trials
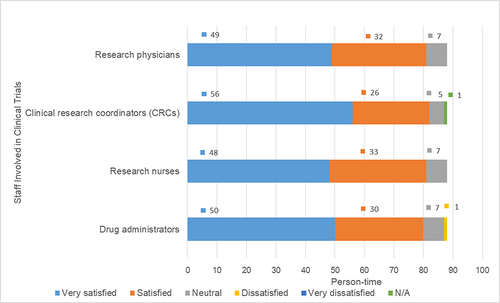
Within the group of 88 participants, an overwhelming majority expressed contentment with the various staff members involved in the clinical trial process. This satisfaction extended to clinical trial research physicians, Clinical Research Coordinators (CRCs), research nurses, and drug administrators. Notably, only a solitary participant (1.14%) communicated a sense of “dissatisfaction” specifically towards the drug administrators. The cited reason for this dissatisfaction pertained to a perceived absence of guidance regarding medication usage. For an in-depth depiction, please consult .
Satisfaction Among Clinical Trial Process
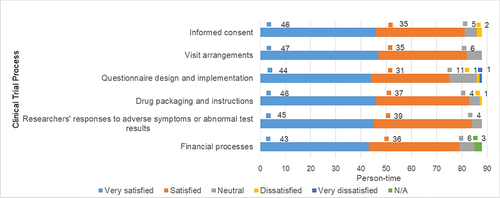
Within the cohort of 88 participants, a prevailing sentiment of contentment emerged that encompassed diverse facets of the clinical trial process. This encompassing satisfaction was observed in crucial aspects such as informed consent, visit arrangements, the adept design and execution of questionnaires (inclusive of diary cards and scales), drug packaging, and associated instructions, researchers’ responsiveness in addressing adverse symptoms or anomalous test outcomes, and the fiscal processes integral to clinical trials (refer to ). However, only a marginal fraction of participants exhibited instances of dissatisfaction: in the case of informed consent, a total of 2 individuals (2.27%) communicated dissatisfaction, attributing it to perceived ineffective communication and inadequate explanations. Likewise, two participants (2.27%) expressed discontent with the questionnaire design and its execution, particularly related to components such as diary cards and scales. The dissatisfaction stemmed from a perceived lack of clarity on how to effectively complete these components. Additionally, one participant (1.14%) cited dissatisfaction with the size of the drug packaging, deeming it excessively large.
Medical Outcomes and Benefits
Out of the total 88 participants, a substantial contingent of 38 individuals (43.18%) conveyed being “very satisfied” with the medical outcomes ensuing from their participation in the clinical trial. Moreover, 36 participants (40.91%) expressed a sentiment of being “satisfied”, while 11 individuals (12.5%) cited “neutral” outcomes. Notably, 3 participants (3.41%) articulated a sense of “dissatisfaction”, attributed to “uncertainty regarding the ultimate effectiveness”.
In consideration of the benefits and potential drawbacks stemming from the clinical trials, a resounding majority of 63 participants (71.59%) attested that the “benefits outweighed the drawbacks”. Contrarily, 14 participants (15.91%) perceived a balance between benefits and drawbacks. Solely 1 participant (1.14%) communicated that the “drawbacks outweighed the benefits”, and 10 participants (11.36%) expressed being “uncertain”.
Satisfaction with Researcher Interactions
Throughout the trial, a comprehensive assessment of participant satisfaction was conducted concerning their interactions with researchers, encompassing the resolution of concerns and the researchers’ responsiveness to those concerns. The findings disclosed that 44 individuals (50%) were “highly satisfied”, and 40 participants (45.45%) expressed a sense of contentment. A marginal portion of 3 participants (3.41%) maintained a “neutral” disposition, while a sole participant (1.14%) reported a state of “dissatisfaction”, which stemmed from occasional challenges in locating a researcher.
Regarding the statement affirming participants’ right to decline participation at any trial stage without impacting researchers’ perceptions, 83 participants (94.32%) affirmed their “understanding” of this principle. Conversely, 5 individuals (5.68%) communicated their ”non-understanding”. In response to inquiries about knowing the designated department to address study-related concerns or demands, 76 participants (86.36%) were aware, whereas 12 participants (13.64%) were unsure.
Additional Unmet Needs
Among the cohort of 88 participants, a substantial proportion of 72 individuals (81.82%) voiced no additional unmet needs. Two participants (2.27%) respectively indicated satisfaction with the service attitude and the comprehensiveness of the questionnaire. In parallel, 14 participants (15.91%) conveyed specific needs. By consolidating similar responses and excluding entries that were unclear, ambiguous, or irrelevant, a concise list of other unmet needs is presented in .
Table 9 Additional Unmet Needs
Recommendations for Enhancing Satisfaction Among Clinical Trial Participants
When queried regarding potential areas for improvement aimed at improving participant satisfaction in clinical trials, a substantial 70 respondents (79.55%) conveyed “no opinion”. Conversely, 18 participants (20.45%) proffered precise recommendations aimed at addressing this aspect (refer to ).
Table 10 Recommendations for Enhancing Satisfaction Among Clinical Trial Participants
Discussion
Analysis of Participants’ Understanding of Clinical Trials
According to the survey outcomes concerning participants’ grasp of “clinical trial” and “clinical trial participant”, roughly 15% of the respondents exhibited unfamiliarity with these terms. The participants’ educational attainment emerged as a significant factor influencing their comprehension, with greater education levels associated with a more profound understanding of these fundamental notions. Conversely, a decline in education level corresponded to a decreasing comprehension of these concepts. Notably, respondents who lacked understanding of these concepts were mainly individuals with a junior high school education or lower.
Analysis of Participants’ Willingness to Engage in Clinical Trials
In the statistical assessment of participants’ willingness to participate in clinical trials, the findings reveal the following trends. First, participants exhibit notable eagerness to partake in clinical trials due to motivations such as contributing to medical advancements, gaining access to additional medical insights, placing trust in medical institutions and professionals, availing additional treatment opportunities, and receiving complimentary medical examinations and therapies. This high enthusiasm for clinical trial involvement is apparent. Second, the primary reason for participants’ reservations about joining clinical trials stems from their inadequate comprehension of clinical trial details. Concerns include apprehension regarding potential bodily risks and side effects, uncertainty about receiving effective treatment versus being assigned to a placebo group, unfamiliarity with rights and responsibilities, lack of specific information about the experimental drug, and uncertainty about the qualifications of the research institution and medical personnel. These apprehensions could be linked to participants’ education levels and the quality of information provided by sponsors and researchers. Finally, the key factors contributing to participants’ reluctance to engage in clinical trials encompass aspects of trial design (such as the nature of laboratory tests, trial duration, and logistical aspects of visits) and the operational procedures. Insufficient understanding of clinical trial particulars (such as unawareness of potential risks and side effects) also plays a role, along with concerns about potential harm to their bodies. Importantly, opposition from family members significantly influences participants’ unwillingness to participate.
Analysis of Participant Satisfaction
The assessment of participant satisfaction revealed that the majority of participants derived benefits from their engagement in clinical trials. They expressed confidence in the therapeutic effects and conveyed contentment with various aspects of the clinical trial process, including the trial environment, personnel, procedural efficiency, medical outcomes, and overall gains. Additionally, participants indicated that their needs throughout the trial were adequately addressed. However, certain areas were identified for potential enhancement. Firstly, while participants did not overtly express dissatisfaction with the medical environment, they did voice concerns regarding aspects of resource allocation procedures. These concerns encompassed changes in research personnel, prolonged wait times for laboratory tests, and delays in receiving test results. Secondly, aspects of trial design were identified as areas of dissatisfaction, including: ① participant dissatisfaction with certain trial design elements, such as the utilization of diary cards or scales; ② participant discontent with drug packaging and administration methods, a desire for simplified administration, and improved efficacy; ③ a lack of transparency in financial processes and transportation allowances, leading participants to seek increased compensation even though they have already received it; ④ apprehensions about frequent blood draws impacting physical well-being; ⑤ extended treatment durations and a preference for shorter visit durations. Thirdly, participants expressed dissatisfaction with research personnel, citing: ① inadequate explanations during the informed consent process; ② difficulties in obtaining medical consultations when experiencing discomfort, receiving abnormal test results, or encountering other issues; ③ insufficient guidance from drug management personnel regarding medication usage; ④ a desire for researchers to be more attentive and patient, with increased responsibilities for research physicians and improved communication with participants, including attention to mental health considerations; ⑤ a wish for clearer communication of trial outcomes. Importantly, these areas of dissatisfaction mirror the factors contributing to participants’ hesitation and unwillingness to partake in clinical trials.
Conclusion
A comprehensive grasp of clinical trials, coupled with a proactive willingness to participate and a heightened level of satisfaction, stands as pivotal driving forces propelling individuals to actively enroll in and successfully complete clinical trials. Simultaneously, stakeholders vested in clinical trials must exercise prudent measures to mitigate the risk factors contributing to participants’ hesitance to engage and their subsequent discontent with the trial process. Through the development of efficacious management strategies, researchers can ensure sustained participant enthusiasm for involvement, thereby substantially elevating the overall quality of clinical trials. Notably, this study has pinpointed four factors significantly influencing participants’ willingness to engage and their overall satisfaction: the participant’s profile, trial design, trial institution setup, and the qualifications of the researchers. Derived from these critical factors, this paper presents a series of constructive measures aimed at enhancing future TCM clinical trials.
Strengthening Training for Participants and Cultivating Qualified Candidates
The survey findings concerning participants’ comprehension and willingness to participate in clinical trials imply that, beyond individual factors such as knowledge accumulation and comprehension abilities, the dissemination of clinical trial information and participant training stands as significant contributing elements.
To bolster participants’ confidence in undertaking clinical trials, targeted information campaigns should be orchestrated for both potential participants and their family members. Training initiatives could encompass the following aspects: (1) Disseminating fundamental knowledge about clinical trials, elucidating the distinctions between clinical trials and other forms of studies, stipulating the objectives of clinical trials, and outlining participants’ rights and responsibilities; (2) Focusing on enhancing comprehension and acceptance of clinical trials, particularly among participants with lower educational backgrounds, through tailored training modules that are lucid, comprehensive, and easily understandable; (3) Introducing essential insights into clinical research projects, including details about regulatory approvals, adherence to regulations, qualifications of researchers and institutions, findings from pharmacokinetic, pharmacological, and toxicological studies, drug dosages, efficacy, safety aspects, and the rationale behind scheduled visits and laboratory tests; (4) Ensuring participants are clearly cognizant of the medical benefits, financial processes and transportation allowances they are entitled to during the trial; (5) Underlining the significance of adhering to medical advice and outlining possible risks along with corresponding solutions for behaviors that deviate from compliance, such as skipping tests, medications, or visits.
To enhance the accessibility of professional knowledge, diverse formats should be employed for clinical trial education. Generally, older participants, individuals with lower educational backgrounds, and those without medical backgrounds exhibit reduced awareness of clinical trials.Citation7 Presently, initiatives to raise clinical trial awareness by hospitals, researchers, non-profit organizations, and pharmaceutical companies encounter constraints such as geographical limitations, intricate content, and reliance on singular dissemination channels.Citation8 Therefore, it is imperative to foster innovation in educational formats by harnessing internet and social media platforms to cultivate a more engaging, adaptable, and accessible comprehension of clinical trials. This can be achieved through mediums like e-handbooks, printed materials, videos, comics, expert lectures, and live broadcasts. Such an approach can enable the public to intuitively and conveniently grasp the concepts of clinical trials.
Optimizing Experimental Design
To conduct clinical trials, a well-crafted clinical trial plan is of paramount importance.Citation9 The design should seamlessly merge scientific rigor with the feasibility of execution.Citation10 The clinical trial design can be refined by addressing factors that might lead to participant dissatisfaction: (1) Formulate trial documents that are lucid, succinct, and easily comprehensible, accounting for participants’ capacity to accept and understand the information; (2) Provide suitable compensation to cover diverse costs borne by participants, including income loss, trial-related time, transportation to and from the research facility, and other associated expenditures. At the same time, inform the participants of the specific content and basis of compensation. This approach caters to varying participant needs during their involvement in clinical trials;Citation11,Citation12 (3) Traditional Chinese Medicine (TCM) trials often involve bulky packaging due to the distinct nature of drug formulations, leading to participant inconvenience. It is imperative to optimize drug formulations and packaging, supply convenient and adequate treatment courses to enhance participants’ satisfaction and adherence; (4) Set rational schedules and intervals for participant visits and blood sample collection; (5) In cases of invasive trial procedures, meticulously assess the associated risks and burdens, and devise strategies to minimize discomfort and pain during such processes. The goal is to mitigate or prevent participant distress, fear, discomfort, and anguish linked to these procedures.Citation13,Citation14
Rational Allocation of Personnel and Resources in Clinical Trial Institutions
Researchers shoulder the responsibility of upholding the reliability of the research team.Citation1,Citation15 Clinical trial institutions should undertake qualification assessments and ongoing training for their investigators and coordinators, while also optimizing personnel deployment. The clinical trial project team should consist of an appropriate number of participants, accompanied by a distinct allocation of duties, ensuring team stability throughout the project’s execution. This not only facilitates participants in promptly reaching out to investigators and study teams but also bolsters their sense of affiliation.
Regarding resource allocation, the following optimizations can be implemented: (1) Integrating the electronic systems of clinical trial institutions to effectively connect medical record order systems with examination and inspection, drug management, and clinical trial management, enabling seamless data transmission and automation. This will result in reduced waiting times for participants, improved result inquiries, and enhanced data traceability for facilities. (2) Implementing centralized management of experimental drugs by dedicated personnel. This entails overseeing the entire process of experimental drug storage, distribution, issuance, collection, storage, and returns, making it convenient for participants to receive and return experimental drugs while also obtaining medication guidance.
Improve Clinical Trial Communication Skills Among Researchers
Improvements can be made in the following aspects: (1) Ensuring comprehensive and informed consent from participants. Researchers should use easily understandable language to thoroughly inform participants about the trial’s content, purpose, rights, responsibilities, potential risks, and benefits. This enables participants to understand the departments, personnel, and medical assistance available to them in case of adverse events or requirements. (2) Enhancing communication between researchers and participants. Researchers should attentively address participants’ concerns, providing detailed and comprehensive guidance on medication, visit schedules, trial results, and health advice. (3) Addressing participants’ psychological needs. Throughout the trial, participants’ mental well-being can be impacted by factors like challenging medical procedures, health conditions, frequent visits, and complex trial processes. Researchers should maintain consistent communication with participants and offer appropriate support, allowing them to recognize the advantages of clinical trial involvement. Increased trust and a heightened sense of safety can positively influence participants’ compliance.
The development of each clinical trial drug relies on the participation and cooperation of participants. Enhancing public awareness and satisfaction necessitates the guidance of policies and regulations, the dissemination of scientific knowledge, well-designed trials, the rational allocation of resources within trial institutions, and improvements in the quality of researchers. It’s important to note that the study sample in this investigation is limited to participants in clinical trial projects at the Beijing Traditional Chinese Medicine Hospital. While both the scope of the research and the number of respondents are restricted, the research findings still carry indicative significance. The objective of this study is to encourage all stakeholders engaged in clinical trials to recognize the valuable contributions of participants and to actively comprehend their needs within their respective roles. Through attentive listening to participants’ perspectives and the provision of practical services, trust can be cultivated and overall satisfaction with clinical trials can be improved.
Strengths and Limitations
This study analyzes the factors that influence participant compliance and satisfaction, and offers recommendations to enhance Traditional Chinese Medicine (TCM) clinical trials. Four key strengths characterize this study. Firstly, it closely aligns with the contemporary strategies of global drug regulatory agencies, adhering to guidelines for drug research and development that prioritize patient-centered approaches, including “patient-centered drug development” (Food and Drug Administration, FDA), “listening to patient opinions, obtaining patient perspectives” (The International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use, ICH), “incorporating patient preferences for benefits and risks into drug decision-making” (European Medicines Agency, EMA), and “patient-centered” drug clinical trial design, implementation, and drug benefit-risk assessment (Center for Drug Evaluation, CDE). This reflects a commitment to patient-centric clinical trial concepts. Secondly, the questions included in the questionnaire itself prioritized the participant’s perspectives and needs by collecting data on the demographics, knowledge levels, and motivations of the participants. The experimental design was also created with the participants’ perspectives in mind and was written to be easily understood. Thirdly, the study systematically examines participant compliance, shedding light on their satisfaction. The factors influencing participant contentment in clinical trials are grouped into four domains: participant profile, trial design, trial institution setup, and qualifications of investigators and research teams. The key elements of clinical trial execution processes are comprehensively analyzed. Lastly, the study presents actionable measures for researchers to consider when designing TCM clinical trials. The enhancement of participant recruitment and training, experimental design, resource allocation, and interpersonal communication are vital steps to streamline TCM clinical trial execution, ensuring both efficiency and effectiveness.
Despite these strengths, there are several weaknesses in the study. First, the limited sample size of 88 participants may restrict the generalizability of the findings. A larger sample size would enhance the broader applicability and significance of the results. Second, this study’s single-hospital setting might not accurately represent practices in other healthcare institutions, potentially limiting the external validity of the findings. Third, the study focus on phase II and phase III TCM clinical trials may not extend to other types of clinical trials, potentially constraining the generalizability of the conclusions. Fourth, the study did not comprehensively assess participants’ comprehension of terms such as “clinical trial” and “clinical trial participants”. Future research could adopt more robust evaluation methods to gauge participants’ understanding of these concepts. Lastly, the reliance on self-reported survey responses introduces the potential for response bias, a concern that could be mitigated in subsequent studies by incorporating objective measures or multiple sources of input.
Ethics Approval
This study was approved by the Institutional Research Review Board of Beijing Traditional Chinese Medicine Hospital, Capital Medical University, and conducted in accordance with the Declaration of Helsinki. The survey participants provided informed consent.
Disclosure
The authors report no conflicts of interest in this work.
Acknowledgments
Acknowledgement to Xiao Michelle Androulakis for formal analysis and reviewing the English language edition.
Data Sharing Statement
The data that support the findings of this study may be provided by the corresponding author upon reasonable request.
Additional information
Funding
References
- National Medical Products Administration, National Health Commission. Regulations for the quality management of drug clinical trials; 2020. Available from: https://www.gov.cn/gongbao/content/2020/content_5525106.htm. Accessed May 29, 2024.
- Yu MK, Xu Y, Hu R, et al. Analysis of characteristics of Chinese research on subject’s compliance methodology in clinical trials. Chin J EBM. 2019;19(06):708–714.
- Zhou W, Lou XH, Wei H, et al. Assessment of factors influencing subject’s compliance and countermeasure study in drug clinical trials. Chin J Pharmacoepidemiol. 2020;29(05):347–351.
- Xin MY, Batool H, Huang SZ. The effect of e-commerce livestreaming services on customer loyalty: a test of the chain mediation model. J Innov Entrep. 2023;12(41):1–21.
- Kan SH, Li JM, Wang ZQ, et al. The application of questionnaire star in teaching human anatomy to international students. Basic Medical Education. 2023;25(8):704–707.
- Xian L, Wang YY, Pang H, et al. Analysis and improvement measures of telephone drug consultation in a specialized hospital of obstetrics and gynaecology in China. J Clin Pharm Ther. 2021;46(1):78–85. doi:10.1111/jcpt.13253
- Zhou WJ, Zeng XH, Zhang XD, et al. A survey of clinical trial awareness and participation among 2029 respondents. Chin J Clin Pharmacol. 2022;38(09):1002–1006.
- 2022 clinical trial participants and family members survey report; 2022. Available from: https://mp.weixin.qq.com/s/Sc-_JuMXPzHbVvaiFEFiaQ. Accessed May 29, 2024.
- Gui YL, Chen Z, Tian GX, et al. Some considerations on clinical trial protocol design. Chin J Cardiovasc EBM. 2017;9(06):641–643.
- Fan DC. Key points of clinical trial protocol design. J Chin Pres Drug. 2009;09:60–61+68.
- Sun TB, Yin M, Zhang X, et al. Protection of the rights and interests of drug clinical trial participants from a legal perspective. Chin Hosp Manag. 2016;36(04):73–74.
- Wu J, Tang HC, Jiang H. Discussion on the problems and improvement measures of clinical trial participant compensation. J Med Philos. 2020;41(22):33–36.
- Cheng JL, Tan G, Zhao XL, et al. Ethical considerations in pain management for clinical trial participants. Drug Eval Res. 2021;44(02):298–304.
- Cheng JL, Tan G, Zhao XL, et al. Ethical considerations in pain management for pediatric population in clinical trials. Drug Eval Res. 2021;44(06):1201–1206.
- ICH E6 (R2): guideline for Good Clinical Practice; 2016. Available from: https://ichgcp.net/6-clinical-trial-protocol-and-protocol-amendments. Accessed May 29, 2024.