Abstract
Nearly 3 years after the emergence of COVID-19, it remains one of the world’s problems. COVID-19 vaccination is a priority programme for reducing death and severe symptoms. The primary recombinant novel coronavirus ZF2001 vaccine has gone through Phase III clinical trials and demonstrates efficacy against the highly critical COVID-19 globally and in Indonesia (87.6% and 76.0%, respectively). This study aimed to assess immune persistence after three doses of ZF2001. The study monitored and followed up 400 participants 14 days and 6 months after the third dose and investigated immune persistence 6 months after the phase III vaccination (ZF2001). This study was conducted at recruitment locations in Bandung. Participants were divided into the vaccine and placebo groups and entered into the immunogenicity group. The immune persistence of the vaccine was assessed by measuring geometric mean titres (GMT), neutralising antibodies and seropositivity of IgG anti-S-receptor-binding domain (RBD) after 14 days and 6 months. The seropositive antibody rates were nearly the same between 14 days and 6 months. After 6 months, the GMT, seropositivity of IgG anti-S-RBD and neutralising antibodies in the vaccine group decreased significantly, from 6447.63 to 1514.61 with a P-value of 0.001. A booster was considered important after 6 months.
Introduction
The coronavirus disease 2019 (COVID-19) pandemic has occurred for over 3 years. In December 2019, the World Health Organization was informed of pneumonia cases in Wuhan City, Hubei Province of China. The infection quickly spread worldwide. Vaccination remains one of the keys to fighting severe acute respiratory syndrome coronavirus 2 (SARS-COV-2).Citation1–3 According to the latest data, 2.9 million new cases of COVID-19 were reported globally between December 2022 and January 2023. From January 2 to 8, 2023, the highest number of new COVID-19 cases was 3365, which occurred during the Indonesia Epidemiology week.Citation2,Citation3
Therefore, the prevalence of SARS-CoV-2 variants and protection offered by vaccines against serious/life-threatening illnesses brought on by these developing variants are relevant.Citation2,Citation3 In December 2020, the population received the first dose of the Pfizer/BioNTech vaccine.Citation3,Citation4 The first batch of COVID-19 vaccines from the COVAX facility that arrived in Indonesia was AstraZeneca.Citation5,Citation6 From 2022 to January 30, 2023, 34.23% of the general public received their third dose. The highest coverage was in Jakarta, with 57.39% of the population receiving up to the third dose or the first booster dose.Citation5,Citation6
As of November 20, 2022, 13 vaccines were approved for use in Indonesia. ZF2001 has been approved or granted emergency use authorisation in four countries.Citation5,Citation7 The safety and efficacy of ZF2001 have been confirmed in Phase 3 randomised, placebo-controlled trials in China, Ecuador, Indonesia, Pakistan and Uzbekistan. The efficacy rates of ZF2001 against the highly critical COVID-19 in the world and Indonesia were 87.6% and 76.0%, respectively. ZF2001 caused pain at the injection site in 17.3% of the vaccinated individuals, and no severe adverse events were reported, so the vaccine can be said to be safe.Citation7,Citation8 The average levels of antibodies against the receptor-binding domain (RBD) after the COVID-19 vaccine administration were demonstrated by geometric mean titres (GMTs). Fourteen days after ZF2001 administration, the GMT were higher after three doses than after two.Citation7–9 This study is a continuation of the phase 3 study of ZF2001 to assess immune persistence in patients who received three doses of the recombinant novel coronavirus vaccine.
Materials and Methods
Study Design
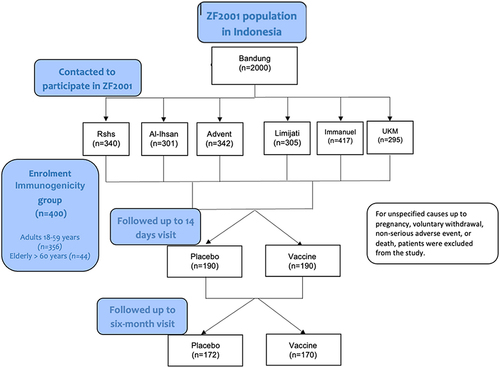
This study monitored and followed up on the immune persistence after 6 months of the phase III (ZF2001) trial in the period from February 2021 to July 2022. In total, 400 participants were enrolled into the immunogenicity subgroups by computerised block randomisation. The participant assigned a number after randomisation was allocated to the immunogenicity subgroup. This study was conducted in six recruitment locations in Bandung, Indonesia: Hasan Sadikin General Hospital, Al-Ihsan Hospital, Limijati Hospital, Immanuel Hospital, Advent Hospital and Unggul Karsa Medika Hospital. Blood samples were collected 14 days and 6 months after the third dose. Data were collected from eCRF of our study. This study is registered with ClinicalTrials.gov NCT04646590. The trial implementation was overseen by Anhui Zhifei Longcom Biopharmaceutical (Zhifei), which manufactures the investigative products and employs contracted clinical research organisations (Child Health Department Faculty of Medicine Universitas Padjadjaran) to coordinate interactions between regulatory authorities and staff at the relevant clinical sites.
Study Vaccine
The participants were divided into two groups: the vaccine group that received the recombinant novel coronavirus vaccine that contains 25 μg of nucleocapsid-recombinant spike RBD and the placebo group that received 0.25 mg aluminium hydroxide adjuvant. The participants received the first three doses of ZF2001 with an interval of 30 days. Blood samples were obtained before vaccination and 14 days and 6 months after the third dose.
Immunogenicity Assessment
Blood samples (5 mL) were collected carefully to avoid hemolysis. The centrifuged serum was subpacked into four freezing tubes (each tube contained approximately 250 μL), including three tubes sent for examination and one tube for reservation. After labelling, these tubes were stored at −20°C. The temperature was monitored and documented on the appropriate form during the trial. The SARS-COV-2 neutralising antibody and/or RBD protein binding antibody IgG were completed by the Eijkman Institute for Molecular Biology. The immunogenicity of the vaccine was determined by measuring the serum IgG antibody levels, which was performed using chemiluminescent microparticle immunoassay from Abbott Laboratories. Antibody titres of ≥50 AU/mL were interpreted as positive according to the manufacturer’s instructions.
Sample Size
The GMT of the anti-SARS-COV-2 neutralising antibody in people in China and outside China are the same, and the standard deviation of the antibody titre after logarithmic conversion is 0.55. In this study, the distribution ratio of the two groups of samples was 1:1. Using PASS 15, the minimum sample size calculated for each investigational vaccine group in China and outside China was 207.
Statistical Analysis
The primary outcome was the GMT, neutralising antibody level and seropositivity of IgG anti-S-RBD 14 days and 6 months after the three doses of ZF2001. Seropositivity was defined as IgG antibody levels of ≥50 AU/mL. The chi-square test/Fisher’s exact test probability method was used for the statistical test of the difference between the vaccine and placebo groups. The Clopper–Pearson method was used to calculate the 95% confidence interval.
Results
Study Population
This study enrolled 400 participants to the immunogenicity group. After 14 days of the primary vaccine administration, the number of participants decreased. Patients were excluded from the study for unspecified reasons. Six months after the primary vaccine administration, the number of participants decreased to 342. The participants were divided into two groups according to whether they had received a placebo vaccine or a control vaccine ().
The demographic characteristics of the participants in each group are shown in . The number of adults enrolled in the study was greater than that of the older adults, with 356 (89.0%) and 44 (11.0%), respectively. The average ages of the adult and older groups were 35.58 and 64.54, respectively. The mean body mass index (BMI) values of the adult and older groups were 25.05 and 24.79, respectively.
Table 1 Summary of the Demographic Characteristics
Immune Persistence 14 Days and 6 Months After the Primary Vaccine Administration
The vaccine group had GMT higher than the placebo group after 14 days, with p < 0.001. In , GMT showed a significant decrease in the vaccine group after 6 months, with p < 0.001. In the placebo group, the GMT increased. The seropositivity rates in the vaccine group 14 days and 6 months after the primary vaccine administration were nearly the same, as presented in .
Table 2 Summary of the Geometric Mean Antibody Concentration
Table 3 Summary of Seropositivity
Total COVID-19 Cases
From January to February 2021, no COVID-19 cases were reported. From March until October 2021, 6, 30, 54, 208, 190, 8, 2 and 1 case of COVID-19 were reported, respectively. No cases of COVID-19 were reported in November and December 2021.
In March 2021, from the 6 total cases of COVID-19, 3 were classified as mild and the other was moderate, and all 3 patients with moderate COVID-19 were hospitalised. In April 2021, 30 cases of COVID-19 were reported, which included 29 mild cases and one moderate case that required hospitalisation. In May 2021, 54 cases of COVID-19 were reported, of which 45 were mild and the other 9 were moderate cases that required hospitalisation. In June 2021, 208 cases were recorded, of which 161 were mild, 39 were moderate, 4 were severe and 4 were critical cases. Moreover, 15 patients were hospitalised and 4 died. In July 2021, 190 cases of COVID-19 were reported, of which 149 were mild, 32 were moderate, 4 were severe and 5 were critical cases. Twelve of them were hospitalised, of which five died. In August 2021, 8 cases were reported, and all of them were mild and did not require hospitalisation. In September 2021, 2 cases, which were all mild, required hospitalisation. In October 2021, one moderate case of COVID-19 was reported, and the patient was hospitalised.
Discussion
Since January 13, 2021, Indonesia implemented a vaccination programme to prevent COVID-19. Until now, the government has implemented a second booster vaccine policy for health workers and older people.Citation1,Citation2,Citation10 COVID-19 is still one of the leading causes of death in both children and adults. Although the symptoms can range from mild to severe, they can also cause severe symptoms of respiratory failure. In Indonesia, the number of COVID-19 cases increased from 2020 to 2021. The peaks were recorded in March 2020 (start of the pandemic), January 2021 and February 2021.Citation9,Citation11 In August 2020, the COVID-19-related mortality rate was the highest in Indonesia compared with those in other countries. Age ≥ 65 years was a risk factor that contributed to the mortality rate.Citation2,Citation12 A COVID-19 test with antigen or swab polymerase chain reaction has been implemented for COVID-19 prevention.Citation1,Citation13 In November 2021, 4732 cases due to the delta variant were reported across Indonesia.Citation10,Citation14
Vaccination is an effective strategy to fight COVID-19.Citation15–17 The efficacy of the vaccine to prevent COVID-19 is 65.30% in Indonesia using inactivated SARS-CoV-2 vaccines, such as CoronaVac®.Citation16,Citation18 The primary vaccine recommended for the three doses for the public has not yet been established; a three-dose recommendation for the primary vaccine is prioritised for immunocompromised participants.Citation16,Citation19 Since November, ZF2001 has been approved for emergency use in Indonesia.Citation7,Citation9 As of April 2022, the vaccination coverage in Indonesia has not yet reached the target of injecting 450 million vaccines. The total coverage rates of the first, second and the first booster or third dose were 95.63%, 79% and 17.68%, respectively. Studies have highlighted the importance of vaccination to fight existing COVID-19 variants.Citation17,Citation20
In this study, ZF2001 was administered in three doses. Data available are still insufficient and difficult to obtain. Likewise, in Australia, the public was recommended to receive two doses of the primary vaccine, whereas those aged >6 months and immunocompromised ones were recommended to receive three doses.Citation17,Citation21 A third dose of the BNT162b2 vaccine provided 95.3% efficacy against COVID-19.Citation17,Citation22 Antibody levels increased after the third dose; the increase in patients with immunocompromised status was very similar to that in healthy patients who were only given two primary doses.Citation17,Citation23
Previous studies of the antibody response to the third dose of inactivated COVID-19 vaccine in healthy blood donors showed that 6–8 months after the last vaccination, nAB seropositivity reached 85% with three vaccine doses.Citation17,Citation24 In the present study, the seropositivity rates in adults aged 18–59 years and older adults aged ≥60 years who received the vaccine decreased from 99.41% to 97.40% and 88.89% to 87.50%, respectively. In the placebo group, the seropositivity rate increased in the adult and older groups because of the existence of natural infections. The seropositive (by natural history) group (26%) had never been diagnosed with COVID-19, representing 4.6% of the total study population, which is much higher than those with mild COVID-19.Citation20,Citation25
The adult (18–59 years) and older groups (≥60 years) have the highest GMT IgG antibody levels 14 days after the primary vaccine administration, but this decreased after 6 months. In a study of different dose regimens of the SARS-CoV-2 recombinant spike protein vaccine NVX-CoV2373 in younger and older adults (a Phase 2 randomised placebo-controlled trial for recipients of the NVX-CoV2373 vaccine), the GMT for the anti-IgG spike protein were higher in the young and old group.Citation18,Citation26,Citation27 GMT were higher in younger than in older participants.Citation18,Citation27 GMT antibodies to SpikoGen® were higher in the vaccine group than in the placebo group.Citation26,Citation27 In a study of the safety and immunogenicity of mRNA-1273 vaccine in older adults at day 57, older adults who received a high dose of mRNA-1273 had a higher GMT than those who received a low dose.Citation26,Citation28 In another study, participants who received primary CoronaVac within 6–9 months before the booster dose with full and half doses of ChAdOx1-S had lower GMT than those who received the vaccine before 3–6 months.Citation22,Citation29 Those who received their first dose of ChAdOx1 nCoV-19 had GMT decreased by half from the 28-day peak seen after 6 months of the administration.Citation22,Citation29 Our study yielded the same results.
The GMT 14 days after the three doses were higher in Phase 1 and 2 ZF2001 study groups than the groups that received two doses. The analysis of immunogenicity in the currently completed Phase II clinical trial for this vaccine showed higher GMT after 14 days in the three-dose group that received a low dose compared with the two-dose group (low dose, high dose and placebo), three-dose group (high dose) and placebo group.Citation8,Citation9 This is because we used three doses and followed up patients up to 6 months after vaccination.
In this study, 490 patients received COVID-19 vaccines and 9 died. Despite the high vaccine efficacy, if the population reaches a high enough level of vaccine coverage, most infections will occur in the vaccinated people.Citation8,Citation30,Citation31 This is called breakthrough infection—a term used to describe infections in fully vaccinated people.Citation31,Citation32 Recording breakthrough infections is challenging because many unvaccinated people are likely to have some immunity from previous infections and the wide spectrum of symptoms and outcomes of COVID-19.Citation32,Citation33 Bergwerk M et al reported a rare breakthrough infection, including infections among healthcare workers who received the BNT162b2 messenger RNA vaccine.Citation33 Thus, more studies are needed to understand how an individual’s stage of protection against breakthrough infections depends on the individual’s prior history, presence of an active infection and history of vaccination against SARS-CoV-2.Citation31,Citation32
Our results shown after the primary vaccine administration, these antibody levels were reduced significantly compared to their peak level. The effectiveness of COVID-19 vaccines against variants such as Omicron and Delta diminishes notably over time, underscoring the significance of booster vaccinations in maintaining robust protection.Citation34,Citation35 Research findings show that the vaccine efficacy may decline to below 20% within six months of primary vaccination against delta and also go down further below 30% regarding booster shots after nine months.Citation34–36 It is more serious compared to the declining immunity towards the delta variant, indicating a fluidity in immunity from one strain to another.Citation34–36 Booster doses have been shown to greatly improve and recover from vaccine efficacy which are important defense for both current and future strains.Citation35,Citation36 In order to achieve optimum vaccination strategies and reduce breakthrough cases during the ongoing control of this pandemic, it is crucial to understand these dynamics of waning immunity coupled with booster roles in COVID-19 management.Citation35–37
This study has several limitations. First, the number of the older participants was lower than that of the younger ones because older participants were immunised before the study started. Second, some participants were lost to follow-up for blood sampling 14 days and 6 months after the primary vaccine administration.
Conclusion
This study highlights a notable decrease in antibody persistence, specifically in the geometric mean titers (GMT) of IgG anti-S-RBD and neutralizing antibodies. These antibody levels were significantly reduced from their peak observed shortly after primary vaccine administration. Despite this decrease, a considerable proportion of individuals remained seropositive for antibodies against SARS-CoV-2 from 14 days up to 6 months post-vaccination. However, given the diminishing antibody levels beyond 6 months, the study underscores the potential benefit and necessity of booster doses to maintain robust immunity against COVID-19.
Institutional Review Board Statement
The investigator obtained approval of the protocol from the Research Ethics Committee Universitas Padjadjaran Bandung (no: LB.02.01/X.6.5/13/2021 dated January 21, 2021) before the start of the trial. Copies of these approvals were forwarded by the investigator to Anhui Zhifei with the composition (names and qualifications of the members) of the Institutional Ethics Committee. The study was conducted in adherence with the Declaration of Helsinki and Good Clinical Practice guidelines.
Informed Consent Statement
Informed consent was obtained from all the study participants.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare no conflicts of interest in this study.
Acknowledgments
The authors would like to thank all the study participants, the Dean of the Faculty of Medicine Universitas Padjadjaran, the Head of Child Health Department Faculty of Medicine Universitas Padjadjaran/Hasan Sadikin Hospital and the staff of the Clinical Research Unit of the Child Health Department Faculty of Medicine Universitas Padjadjaran/Hasan Sadikin Hospital. We also thank Longcom Biopharmaceutical Co., Ltd., for the support and contribution.
Data Sharing Statement
Data will be available on the main site of study. Please contact the correspondence author for future access.
Additional information
Funding
References
- World Health Organization: COVID-19 – china; 2020. Available from: https://www.who.int/emergencies/disease-outbreak-news/item/2020-DON229. Accessed July 25, 2024.
- World Health Organization: COVID-19 – weekly Epidemiological Update, Jan; 2023. Available from: https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19---11-january-2023. Accessed January 5, 2023.
- Centers for Disease Control and Prevention, COVID-19 Data Review: update on COVID-19–Related Mortality; 2022. Available from: https://www.cdc.gov/coronavirus/2019-ncov/science/data-review/index.html. Accessed November 21, 2022.
- Cullinane S, The first public COVID-19 vaccines have been injected just as the US death toll tops 300,000, Dec; 2020. Available from: https://www.kcra.com/article/first-public-covid-19-vaccines-have-been-injected-just-as-The-us-death-toll-nears-300000/34964167. Accessed November 23, 2022.
- World Health Organization, Indonesia received the first batch of COVID-19 vaccines from the COVAX Facility, Indonesia, Mar; 2021. Available from: https://www.who.int/indonesia/news/detail/09-03-2021-indonesia-received-The-first-batch-of-covid-19-vaccines-from-The-covax-facility#:~:text=Paranietharan%20received%20the%20first%20shipment,COVAX%20Facility%20allocated%20to%20Indonesia. Accessed November 23, 2022.
- Kementrian Kesehatan Indonesia, Vaksinasi COVID – 19 Nasional, Indonesia; 2023. Available from: https://vaksin.kemkes.go.id/#/vaccines. Accessed January 30, 2023.
- COVID 19 Vaccine Tracker, 13 Vaccines Approved for Use in Indonesia; 2022. Available from: https://covid19.trackvaccines.org/country/indonesia/. Accessed November 23, 2022.
- Dai L, Gao L, Tao L, et al. Efficacy and safety of the RBD-dimer–based COVID-19 vaccine ZF2001 in adults. N Engl J Med. 2022;386(22):2097–2111. doi:10.1056/NEJMoa2202261
- Yang S, Li Y, Dai L, et al. Safety and immunogenicity of a recombinant tandem-repeat dimeric RBD-based protein subunit vaccine (ZF2001) against COVID-19 in adults: two randomised, double-blind, placebo-controlled, phase 1 and 2 trials. Lancet Infect Dis. 2021;21(8):1107–1119. doi:10.1016/S1473-3099(21)00127-4
- Cabinet Secretariat of The Republic of Indonesia: gov’t Adjust Circular on Booster Vaccination; 2022. Available from: https://setkab.go.id/en/govt-adjusts-circular-on-booster-vaccination/. Accessed April 13, 2023.
- Setiadi W, Rozi IE, Safari D, et al. Prevalence and epidemiological characteristics of COVID-19 after one year of pandemic in Jakarta and neighbouring areas, Indonesia: a single center study. PLoS One. 2022;17(5):e0268241. doi:10.1371/journal.pone.0268241
- Karnadi EB, Kusumahadi TA. Why Does Indonesia Have a High Covid-19 Case-Fatality Rate? JEJAK. 2021;14(2):272–287. doi:10.15294/jejak.v14i2.29580
- Rajan S, Cylus J, Mckee M. What do countries need to do to implement effective ‘find, test, trace, isolate and support’systems? J R Soc Med. 2020;113(7):245–250. doi:10.1177/0141076820939395
- Databoks: RI Records 4732 Cases of Covid-19 Delta Variant, Which Region has the Most?; 2021. Available from: https://databoks.katadata.co.id/datapublish/2021/11/18/ri-catat-4732-kasus-covid-19-varian-delta-daerah-mana-yang-terbanyak. Accessed April 13, 2023.
- Ssentongo P, Ssentongo AE, Voleti N, et al. SARS-CoV-2 vaccine effectiveness against infection, symptomatic and severe COVID-19: a systematic review and meta-analysis. BMC Infect Dis. 2022;22(1):439. doi:10.1186/s12879-022-07418-y
- Centers for Disease Control and Prevention, COVID-19 Vaccines for People Who Are Moderately or Severely Immunocompromised; 2022. Available from: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations/immuno.html#:~:text=EVUSHELDTM%20May%20Also%20be,you%20from%20getting%20COVID%2D19. Accessed January 12, 2023.
- Moreira ED, Kitchin N, Xu X, et al. Safety and efficacy of a third dose of BNT162b2 Covid-19 vaccine. N Engl J Med. 2022;386(20):1910–1921. doi:10.1056/NEJMoa2200674
- Zeng G, Wu Q, Pan H, et al. Immunogenicity and safety of a third dose of CoronaVac, and immune persistence of a two-dose schedule, in healthy adults: interim results from two single-centre, double-blind, randomised, placebo-controlled phase 2 clinical trials. Lancet Infect Dis. 2022;22(4):483–495. doi:10.1016/S1473-3099(21)00681-2
- Fadlyana E, Rusmil K, Tarigan R, et al. A Phase III, observer-blind, randomized, placebo-controlled study of the efficacy, safety, and immunogenicity of SARS-CoV-2 inactivated vaccine in healthy adults aged 18–59 years: an interim analysis in Indonesia. Vaccine. 2021;39(44):6520–6528. doi:10.1016/j.vaccine.2021.09.052
- Sehat Negeriku Sehatlah Bangsaku: COVID-19 Vaccination Coverage Reaches 400.5 Million Doses; 2022. Available from: https://sehatnegeriku.kemkes.go.id/baca/rilis-media/20220428/0639746/cakupan-vaksinasi-covid-19-capai-4005-juta-dosis/. Accessed April 13, 2023.
- The Australian Technical Advisory Group on Immunisation (ATAGI), Clinical recommendations for COVID-19 vaccines; 2022. Available from: https://www.health.gov.au/our-work/covid-19-vaccines/advice-for-providers/clinical-guidance/clinical-recommendations#:~:text=COVID%2D19%20vaccination%20is%20recommended%20for%20children%20aged%206%20months,course%20consists%20of%202%20doses. Accessed January 12, 2023.
- Fadlyana E, Setiabudi D, Kartasasmita CB, et al. Immunogenicity and safety in healthy adults of full dose versus half doses of COVID-19 vaccine (ChAdOx1-S or BNT162b2) or full-dose CoronaVac administered as a booster dose after priming with CoronaVac: a randomised, observer-masked, controlled trial in Indonesia. Lancet Infect Dis. 2023;23(5):545–555. doi:10.1016/S1473-3099(22)00800-3
- Syversen SW, Jyssum I, Tveter AT, et al. Immunogenicity and safety of a three-dose SARS-CoV-2 vaccination strategy in patients with immune-mediated inflammatory diseases on immunosuppressive therapy. RMD Open. 2022;8(2):e002417. doi:10.1136/rmdopen-2022-002417
- Ou S, Su Y, Wu T, Ge S, Cheng T, Yuan Q. The duration and breadth of antibody responses to 3-dose of inactivated COVID-19 vaccinations in healthy blood donors: an observational study. Front Immunol. 2022;13:1027924. doi:10.3389/fimmu.2022.1027924
- Allen N, Brady M, Ni Riain U, et al. Prevalence of antibodies to SARS-CoV-2 following natural infection and vaccination in Irish hospital healthcare workers: changing epidemiology as the pandemic progresses. Front Med. 2022;8:758118. doi:10.3389/fmed.2021.758118
- Formica N, Mallory R, Albert G, et al.; 2019nCoV-101 Study Group. Different dose regimens of a SARS-CoV-2 recombinant spike protein vaccine (NVX-CoV2373) in younger and older adults: a phase 2 randomized placebo-controlled trial. PLoS Med. 2021;18(10):e1003769. doi:10.1371/journal.pmed.1003769
- Tabarsi P, Anjidani N, Shahpari R, et al. Safety and immunogenicity of SpikoGen®, an Advax-CpG55. 2-adjuvanted SARS-CoV-2 spike protein vaccine: a phase 2 randomized placebo-controlled trial in both seropositive and seronegative populations. Clin Microbiol Infect. 2022;28(9):1263e1271. doi:10.1016/j.cmi.2022.04.004
- Anderson EJ, Rouphael NG, Widge AT, et al. Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. N Engl J Med. 2020;383(25):2427–2438. doi:10.1056/NEJMoa2028436
- Flaxman A, Marchevsky N, Jenkin D, et al. Tolerability and immunogenicity after a late second dose or a third dose of ChAdOx1 nCoV-19 (AZD1222. SSRN Electron J. 2021. doi:10.2139/ssrn.3873839
- Lipsitch M, Krammer F, Regev-Yochay G, Lustig Y, Balicer RD. SARS-CoV-2 breakthrough infections in vaccinated individuals: measurement, causes and impact. Nat Rev Immunol. 2022;22(1):57–65. doi:10.1038/s41577-021-00662-4
- Amanatidou E, Gkiouliava A, Pella E, et al. Breakthrough infections after COVID-19 vaccination: insights, perspectives and challenges. Metabol Open. 2022;14:100180. doi:10.1016/j.metop.2022.100180
- Hacisuleyman E, Hale C, Saito Y, et al. Vaccine breakthrough infections with SARS-CoV-2 variants. N Engl J Med. 2021;384(23):2212–2218. doi:10.1056/NEJMoa2105000
- Bergwerk M, Gonen T, Lustig Y, et al. COVID-19 breakthrough infections in vaccinated health care workers. N Engl J Med. 2021;385(16):1474–1484. doi:10.1056/NEJMoa2109072
- Menegale F, Manica M, Zardini A, et al. Evaluation of waning of SARS-CoV-2 vaccine–induced immunity: a systematic review and meta-analysis. JAMA Network Open. 2023;6(5):e2310650. doi:10.1001/jamanetworkopen.2023.10650
- Barnard RC, Davies NG, Jit M, Edmunds WJ. Modelling the medium-term dynamics of SARS-CoV-2 transmission in England in the Omicron era. Nat Commun. 2022;13(1):4879. doi:10.1038/s41467-022-32404-y
- Bates TA, Leier HC, McBride SK, et al. The time between vaccination and infection impacts immunity against SARS-CoV-2 variants. medRxiv. 2023. doi:10.1101/2023.01.02.23284120
- Benotmane I, Gautier G, Perrin P, et al. Antibody Response After a Third Dose of the mRNA-1273 SARS-CoV-2 Vaccine in Kidney Transplant Recipients With Minimal Serologic Response to 2 Doses. JAMA. 2021;326(11):1063. doi:10.1001/jama.2021.12339