Abstract
Giant cell arteritis (GCA) is a systemic autoimmune disease that affects medium- and large-sized arteries. The diagnostic gold standard is the temporal artery biopsy, but it has limited sensitivity and some difficulties in reproducibility. Color duplex ultrasonography is a noninvasive, reproducible, and inexpensive method for diagnosis of temporal arteries involvement (temporal arteritis [TA]) in GCA with high sensitivity and specificity. We present the ultrasound findings at baseline and during follow-up in a case of TA in a patient with GCA.
Introduction
Giant cell arteritis (GCA) is a medium/large vessels vasculitis that predominantly affects temporal arteries (temporal arteritis [TA]). It represents the most common form of systemic vasculitis in adults.Citation1,Citation2 The temporal artery biopsy (TAB) is the latest diagnostic gold standard but it suffers a low sensitivity because of the segmental nature of the vasculitis process in TA and a negative biopsy does not rule out the diagnosis. As stated by several studies, the ultrasonography has a key role in the diagnosis and follow-up of TA. The ultrasonographic evaluation can assess stenosis, occlusions, and Doppler flow abnormalities of temporal artery segments and the dark halo around the vessel.Citation3–Citation7
We present a case of TA with pathologic findings in color duplex ultrasonography (CDU) that disappeared after 7 days of steroid treatment.
Case report
On February 24, 2016, a 77 year old male was admitted to our Rheumatology Unit for a frontal temporal non-steroidal anti-inflammatory drugs resistant headache. The patient provided his written consent to have his data used for this case report. He had no scalp tenderness, jaw claudication, or systemic manifestations (fever, weight loss, anorexia, and malaise). Patient had no myalgia or ocular symptoms (such as monocular, binocular vision loss, diplopia, or ocular pain). The temporal arteries were painful and thickened. The acute-phase reactants were raised with an erythrocyte sedimentation rate value of 71 mm/h (normal range 0–10) and a C-reactive protein value of 11.1 mg/dL (normal range 0–5). A normocytic anemia (hemoglobin 12.7 g/dL, normal range of 14–18) and an abnormal liver function with raised a1 and a2 globulins on serum electrophoresis were present. Laboratory tests did not show thrombocytosis. Brightness-mode (B-mode) ultrasound of shoulders did not show ultrasound subacromial/subdeltoid bursitis or long head biceps tenosynovitis. The CDU assessment was performed by an experienced rheumatologist, using an Esaote MyLab Seven ultrasound system with a high-resolution linear probe VFX 18-6 MHz (Esaote SpA, Genoa, Italy). Doppler frequency was >6.5 MHz, dynamic range between 45 and 50 dB, other system settings were set to meet the high standards defined in the literature.Citation8,Citation9 Examination of the superficial temporal arteries (the common, frontal, and parietal branches) was performed in longitudinal and transverse planes. We found a hypoechogenic halo of the left temporal artery >0.5 mm in thickness (). Moreover, left temporal artery Doppler signal showed the presence of turbulent flow ().
Figure 1 (A) Before treatment: left temporal artery ultrasonography features before steroid therapy showed a hypoechogenic halo of the temporal artery >0.5 mm in thickness. (B) Before treatment: left temporal artery ultrasonography features before steroid therapy showed a hypoechogenic halo of the temporal artery in longitudinal (left) and transverse (right) view (B-mode ultrasound).
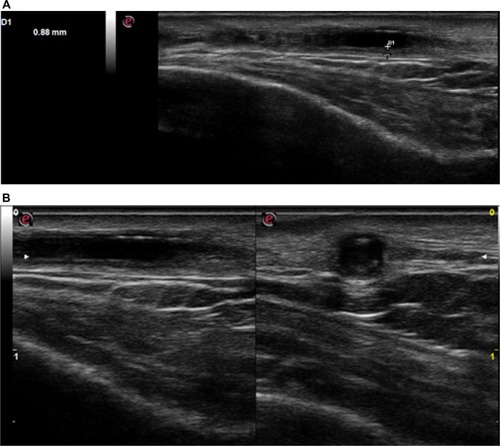
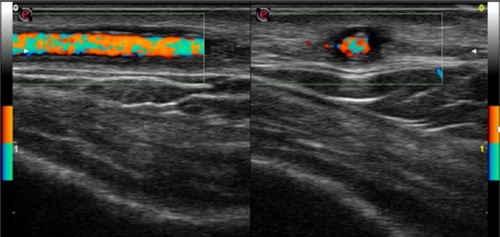
Figure 2 Before treatment, left temporal artery color duplex ultrasonography features before steroid therapy showed a hypoechogenic halo of the temporal artery and the presence of turbulent flow; longitudinal (left) and transverse (right) view.
The follow-up CDU examination was performed 7 days later. On clinical, laboratory, and ultrasound findings, we formulated the diagnosis of GCA and we immediately started therapy to avoid retinal damage with blindness. We administered oral prednisone 1 mg/kg and introduced low-dose aspirin.
As seen in , the halo sign and the turbulent flow disappeared (left temporal artery). The right temporal artery showed a marked reduction of halo sign, as seen in (before treatment) and (after 7 days of treatment). TAB was not performed because of difficulties in reproducibility and because of the presence of bilateral halo sign that has a high specificity in the diagnosis of TA, suggesting that TAB can be spared by CDUs.
Figure 3 Posttreatment left temporal artery color duplex ultrasonography, transverse (left) and longitudinal (right) view, showed disappearance of hypoechogenic halo of the temporal artery and of turbulent flow; longitudinal and transverse view, respectively.
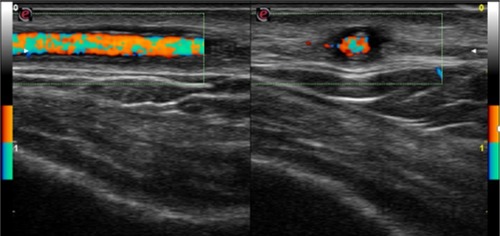
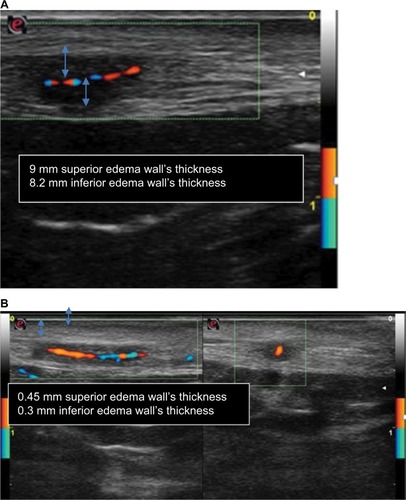
Figure 4 (A) In the left side: before treatment, longitudinal view of right temporal artery CDU features before steroid therapy showed a hypoechogenic halo of the temporal artery and the presence of turbulent flow and weak flow because of the presence of halo sign corresponding to edema of artery wall (measured ~9 mm superior edema wall’s thickness and 8.2 mm inferior edema wall’s thickness). (B) Posttreatment, longitudinal and transverse view, respectively, of right temporal artery CDU showed reduction of halo sign and turbulent flow and a stronger flow than before treatment that corresponds to a significant reduction of edema of artery wall (measured ~0.45 mm superior edema wall’s thickness and 0.3 mm inferior edema wall’s thickness).
Discussion
GCA, first described by Horton et al,Citation10 is a systemic immune-mediated vasculitis affecting medium- and large-sized arteries.Citation9–Citation11 The pathophysiology of GCA includes both innate and adaptative immune response dysregulation. The characteristic histologic lesion is a granulomatous inflammation nearby the internal elastic lamina.Citation11 The inflammatory infiltrate (constituted of lymphocytes, macrophages eventually as multinucleated giant cells and fibroblasts) can affect all the three arterial layers. Chew et al found an association between the presence of giant cells and visual loss.Citation12 The clinical manifestations of GCA are related to both systemic inflammation (malaise, fever, and weight loss) and vascular insufficiency/ischemia (headache, scalp tenderness, jaw claudication, and monocular or binocular vision loss).Citation13 The serological findings are representative of an acute-phase response with high levels of erythrocyte sedimentation rate, C-reactive protein, and anemia.Citation14,Citation15 Arteritic anterior ischemic optic neuropathy is the most common ophthalmologic manifestation and typically presents with sudden, painless, and profound unilateral or bilateral vision loss (78%–99%).Citation11 The mainstay of management includes high-dose corticosteroids, and additional cytotoxic drugs, antitumor necrosis factor monoclonal antibody, and antiplatelet aggregation therapy may be used. The goal of treatment is to prevent ischemic damage and halt progression of visual loss in the affected eye and prevent involvement of the fellow eye. Till date, TAB remains the gold standard diagnostic test, with a temporal artery specimen of at least 2 cm should be sought. If a TAB is negative and clinical suspicion remains high, biopsy on the contralateral side should be considered, providing a positive specimen in 4%–15% of patients when the first biopsy is negative.Citation16,Citation17 Various imaging techniques have been recommended for diagnosis and follow-up of GCA and GCA-related complications. CDU can visualize the restricted arterial flow, wall edema, appearing as hypoechoic halos immediately adjacent to the arterial wall, with a sensitivity and specificity of 69% and 82%, respectively.Citation18,Citation19 Salvarani et al reported that only halos >1 mm in thickness increased the probability of positive result of TA.Citation20 On the contrary, Habib et al showed that smaller halos of ≥0.5 mm thickness can predict TA, a finding reported by others.Citation7,Citation21,Citation22 In addition, bilateral halo sign yields a sensitivity of 37% and specificity of 100% in the diagnosis of TA, suggesting that the presence of bilateral halo sign in patients with clinically suspected TA can replace TAB in their final diagnosis, a suggestion reported by other studies.Citation7,Citation20 The goals of GCA treatment are to avoid visual loss in the affected eye. Glucocorticoids exert multiple anti-inflammatory effects.Citation7,Citation22 There is still no consensus on the correct dose or route of administration of corticosteroids. The starting steroid treatment dosage ranges between 1 mg/kg and 1 g/day for a minimum of 3 days.Citation11,Citation19 The clinical and laboratoristic responses are usually rapid within 24–72 h after initiation of therapy. Regardless of the initial starting dose, most patients remain on oral prednisone 1 mg/kg for a minimum of 4 weeks after which time steroids should be tapered on the basis of symptoms and serial monitoring of inflammatory markers. Other drugs, such as methotrexate, azathioprine, cyclophosphamide, dapsone, and cyclosporine, have been used as adjuvant steroid-sparing therapy. Antitumor necrosis factor monoclonal antibody (eg, infliximab) has been proposed as either first-line therapy or a steroid-sparing agent in GCA.Citation23–Citation26 Antiplatelet aggregation therapy with low-dose aspirin before or at the time of diagnosis has been associated with a lower risk for developing acute vision loss and cerebrovascular accidents.Citation27,Citation28 In our case, steroids induced early disappearance of the halo sign, leading to the normalization of CDU, after 7 days from beginning the therapy. We can conclude that CDU has many advantages: first of all, it has an easy reproducibility (more reproducible than high-resolution computed tomography/positron emission tomography-computed tomography/magnetic resonance imaging) because of bedside and radiation-free hallmarks; it is sensitive to both cranial and extracranial disease. CDU is not only useful in helping in the diagnosis of TA but also useful in following up of patients after initiation of therapy. However, ultrasonography is highly operator and equipment dependent, and it is unable to easily visualize thoracic aorta. CDU has high sensitivity and specificity when it is performed by an experienced operator; however, in literature, there are very heterogeneous results regarding this technique, with test sensitivities ranging from 10% to 87%.Citation29–Citation33 Hence, larger studies focusing on technical standardization of equipment, probe settings, image acquisition, and examination are required.
Disclosure
The authors report no conflicts of interest in this work.
References
- HunderGGThe early history of giant cell arteritis and polymyalgia rheumatica. First descriptions to 1970Mayo Clin Proc20068181071108316901030
- HellmannDBGiant cell arteritis and polymyalgia rheumaticaImbodenJBHellmannDBStoneJHCurrent Rheumatology Diagnosis and TreatmentNew York, NYMc Graw Hill2004235241
- WeyandCMGoronzyJJGiant-cell arteritis and polymalgia rheumaticaAnn Intern Med2003139650551513679329
- HallJKVolpeNJGalettaSLLiuGTSyedNABalcerLJThe role of unilateral temporal artery biopsyOphthalmology20031103543548 discussion 54812623818
- BallELWalshSRTangTYGohilRClarkeJMRole of ultrasonography in the diagnosis of temporal arteritisBr J Surg201097121765177120799290
- NesherGShemeshDMatesMSonnenblickMAbrmowitzHBThe predictive value of the halo sign in color Doppler ultrasonography of the temporal arteries for diagnosing giant cell arteritisJ Rheumatol20022961224122612064840
- KarahaliouMVaiopoulosGPapaspyrouSKankisMARevenasKSfikakisPPColour duplex sonography of temporal arteries before decision for biopsy: a prospective study in 55 patients with suspected giant cell arteritisArthritis Res Ther200684R11616859533
- SchmidtWADoppler ultrasonography in the diagnosis of giant cell arteritisClin Exp Rheumatol2000184 Suppl 20S40S4210948760
- SchmidtWAGromnica-IhleEWhat is the best approach to diagnosing large-vessel vasculitis?Best Pract Res Clin Rheumatol200519222324215857793
- HortonBTMagathBTBrownGEArteritis of temporal vessels: report of 7 casesProc Staff Meet Mayo Clin193712548553
- JenningsGHArteritis of the temporal vesselsLancet19381424
- ChewSSKerrNMDanesh-MeyerHVGiant cell arteritisJ Clin Neurosci200916101263126819586772
- ChatelainDDuhautPSchmidtJGroupe de Recherche sur l’Artérite à Cellules GéantesPathological features of temporal arteries in patients with giant cell arteritis presenting with permanent visual lossAnn Rheum Dis2009681848818252763
- StoneJHPapaliodisGNDunbarMRStoneJRCase records of the Massachusetts General Hospital. Case 4-2010. A 53-year-old man with arthralgias, oral ulcers, vision loss, and vocal-cord paralysisN Engl J Med2010362653754620147720
- HunderGGBlochDAMichelBAThe American College of Rheumatology 1990 criteria for the classification of giant cell arteritisArthritis Rheum1990338112211282202311
- DasguptaBBorgFAHassanNBSR and BHPR Standards, Guidelines and Audit Working GroupBSR and BHPR guidelines for the management of giant cell arteritisRheumatology (Oxford)20104981594159720371504
- BreuerGSNesherGNesherRRate of discordant findings in bilateral temporal artery biopsy to diagnose giant cell arteritisJ Rheumatol200936479479619228656
- Danesh-MeyerHVSavinoPJEagleRCJrKubisKCSergottRCLow diagnostic yield with second biopsies in suspected giant cell arteritisJ Neuroophthalmol200020321321511001197
- CarrollSCGaskinBJDanesh-MeyerHVGiant cell arteritisClin Exp Ophthalmol200634215917316626432
- SalvaraniCSilingardiMGhirarduzziAIs duplex ultrasonography useful for the diagnosis of giant-cell arteritis?Ann Intern Med2002137423223812186513
- HabibHMEssaAAHassanAAColor duplex ultrasonography of temporal arteries: role in diagnosis and follow-up of suspected cases of temporal arteritisClin Rheumatol201231223123721743987
- ReinhardMSchmidtDHetzelAColor coded sonography in suspected temporal arteritis – experience after 83 casesRheumatol Int200424634034614600785
- HoffmanGSCidMCHellmannDBInternational Network for the Study of Systemic VasculitidesA multicenter, randomized, double-blind, placebo-controlled trial of adjuvant methotrexate treatment for giant cell arteritisArthritis Rheum20024651309131812115238
- SpieraRFMitnickHJKupersmithMA prospective, double-blind, randomized, placebo controlled trial of methotrexate in the treatment of giant cell arteritis (GCA)Clin Exp Rheumatol200119549550111579707
- Devauchelle-PensecVJousseSDestombeCSarauxAEpidemiology, imaging, and treatment of giant cell arteritisJoint Bone Spine200875326727218378179
- HoffmanGSCidMCRendt-ZagarKEInfliximab-GCA Study GroupInfliximab for maintenance of glucocorticosteroid-induced remission of giant cell arteritis: a randomized trialAnn Intern Med2007146962163017470830
- NesherGBerkunYMatesMBarasMRubinowASonnenblickMLow-dose aspirin and prevention of cranial ischemic complications in giant cell arteritisArthritis Rheum20045041332213715077317
- LeeMSSmithSDGalorAHoffmanGSAntiplatelet and anticoagulant therapy in patients with giant cell arteritisArthritis Rheum200654103306330917009265
- DiamantopoulosAPHaugebergGHetlandHSoldalDMBieRMyklebustGDiagnostic value of color Doppler ultrasonography of temporal arteries and large vessels in giant cell arteritis: a consecutive case seriesArthritis Care Res (Hoboken)201466111311924106211
- MaldiniCDepinay-DhellemmesCTraTTLimited value of temporal artery ultrasonography examinations for diagnosis of giant cell arteritis: analysis of 77 subjectsJ Rheumatol201037112326233020810501
- SchmidtWAKraftHEVorpahlKVolkerLGromnica-IhleEJColor duplex ultrasonography in the diagnosis of temporal arteritisN Engl J Med199733719133613429358127
- KarassaFBMatsagasMISchmidtWAIoannidisJPMeta-analysis: test performance of ultrasonography for giant-cell arteritisAnn Intern Med2005142535936915738455
- AridaAKyprianouMKanakisMSfikakisPPThe diagnostic value of ultrasonography-derived edema of the temporal artery wall in giant cell arteritis: a second meta-analysisBMC Musculoskelet Disord2010114420210989
