Abstract
Muckle–Wells syndrome (MWS) is a rare autoinflammatory disorder. It is due to NLRP3 gene mutations, responsible for excessive caspase-1 activation and interleukin 1β processing. MWS is the intermediate phenotype of severity of cryopyrin-associated periodic syndrome. Urticarial rash, conjunctivitis, recurrent fever, arthralgia, and fatigue are the main clinical manifestations of MWS. Yet, sensorineural hearing loss and renal amyloidosis can occur after long term evolution. Patients’ quality of life has been drastically improved with the advent of IL-1 inhibitors. This review reports recent findings in MWS, particularly genotype/phenotype correlation, and discusses the clinical perspectives of this disease in a time of efficient treatment.
Introduction
Muckle–Wells syndrome (MWS) is one of the three clinical forms of the cryopyrin-associated periodic syndrome (CAPS), a rare hereditary periodic fever syndrome due to genetic mutations in the NOD-like receptor 3 (NLRP3) gene. Chronic Infantile Neurological Cutaneous and Articular syndrome (CINCA) is the most severe form of CAPS with mental and physical disability; familial cold urticaria (FCAS) is the mild form, and MWS is the intermediate form. Urticarial rash, fever, arthralgia, and fatigue are the main clinical signs of CAPS. The NLRP3 gene mutation is responsible for overproduction of proinflammatory interleukin 1β (IL-1β), the driver of CAPS symptoms. Quality of life in MWS patients has been drastically improved since IL-1 inhibitors became available for treatment. This review first recalls the epidemiology, pathophysiology, and clinical presentation of MWS, then discusses the clinical perspectives of this disease now that efficient treatments exist, including the problems linked to their use, especially in very young children.
Epidemiology
The prevalence of CAPS is estimated to be 1–10 cases per million (1/360,000 in France, with 150–200 cases).Citation1 Caucasians seem to be affected more than other races and there is no male/female preponderance.
Genetics and pathogenesis
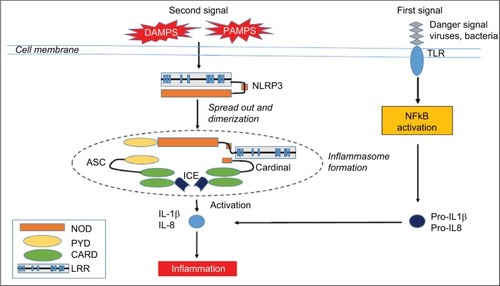
The three phenotypes of CAPS are caused by dominantly inherited or de novo gain-of-function mutations in the NLRP3 (also known as CIAS1) gene located on chromosome 1q44, with a variable penetrance.Citation2 The NLRP3 gene encodes the NALP3 protein (cryopyrin) – a member of the intracellular nucleotide-binding oligomerization domain (NOD)-like receptors (NLRs) family. NLRs belong to the family of pattern- recognition receptors (PRRs). PRRs recognize different danger-associated molecular pattern molecules (DAMPs) and pathogen-associated molecular pattern molecules (PAMPs). All NLRs contain a NACHT domain that enables them to aggregate and oligomerize. Mutations in the NACHT domain of NALP3 may lead to increased inflammation.Citation3 Two signals are necessary for IL-1β production. A first signal, response to the recognition of a PAMP by a PRR induces the production of the inactive form of IL-1β – pro-IL-1β – via the transcription factor NFκB. Upon activation by a second signal, triggered by a further PAMP or a DAMP, NALP3 oligomerizes and recruits other proteins such as Apoptose-associated speck-like protein (ASC) and caspase-1, creating a multi-protein assembly called inflammasome ().Citation4 The protease caspase-1 then cleaves IL-1β, releasing IL-1β from the cell. IL-1β is a proinflammatory cytokine that causes fever, vasodilatation, and systemic inflammation. IL-1β is potent at low concentrations, whereas its natural inhibitor, the IL-1 receptor antagonist (IL-1RA), needs very high concentrations to be effective. In CAPS patients, the inflammasome is activated even in absence of chronic infection. DAMPs may be IL-1 itself, specifically IL-1α, resulting in inflammasome activation.Citation5 Thus, IL-1 induces a vicious IL-1β-producing cycle in an autocrine manner, and the IL-1RA concentrations that are reached in CAPS are not sufficient to inhibit IL-1β. Yet, this IL–1β-producing cycle can be interrupted by anakinra, which blocks both IL-1α and IL-1β.Citation6,Citation7 These different mutations display a strong genotype–phenotype correlation, although a specific mutation may be associated with different phenotypes of variable severity. Few patients with convincing CAPS profile have shown no mutation. Some of them have somatic mosaicism, suggesting a role of somatic mutations.
Figure 1 The NLRP3 inflammasome.
Abbreviations: ASC, apoptosis-associated Speck-like protein containing a CARD; CARD, caspase recruitment domain-containing protein; DAMPS, damage-associated molecular patterns; ICE, interconvertin enzyme; IL-1, interleukin-1; LRR, leucine-repeat rich; NFκB, nuclear factor; NOD, nucleotide-binding oligomerization domain; PAMPS, pathogen-associated molecular pattern molecules; PYD, pyrin domain; TLR, Toll-like receptor.
Clinical manifestations
MWS is characterized by chronic or intermittent episodes of fever, headache, urticarial rash, arthralgia, or arthritis in the absence of a specific trigger.Citation8 The febrile attacks start in early childhood with a median age of onset of 0.8 years.Citation9 Between 40% and 80% of patients experience intense fatigue leading to social isolation.Citation10,Citation11 The attacks can increase nocturnally, accompanied by general malaise with chills, sweating, intense myalgia, and heaviness in lower limbs, sometimes with edema.Citation12 Most attacks resolve within 24 hours (48%), but a substantial portion (36%) last >3 days.Citation9 Half the patients suffer <12 attacks a year, but 40% of patients experience >24 attacks a year.Citation9 Headache and irritability are present in 90% of cases due to migraine, intracranial hypertension, or chronic meningitis, and have a hugely detrimental impact on daily life.Citation9,Citation10 Patients may present a livedoid aspect on the skin, with a non-pruritic urticarial rash appearing during attacks. Histologically, the lesions correspond to neutrophilic urticarial.Citation12,Citation13 More rarely, the patient presents with oral ulcer, folliculitis, or erythema nodosum.Citation13 Osteoarticular symptoms are mainly arthralgia and nail clubbing. Cartilaginous proliferation at growth plates and epiphyses, as well as arthritis are seen only in CINCA patients.Citation14 Patients with MWS can develop progressive sensorineural hearing loss, which becomes apparent after age 10, probably secondary to chronic inflammation of the internal ear.Citation12 Conjunctivitis is frequent while episcleritis and uveitis can also be seen in the severe forms. Amyloidosis leading to proteinuria and renal failure affect up to 25% of cases.Citation9,Citation12
During the attacks, levels of C-reactive protein (CRP) and serum amyloid A (SAA) in the blood, as well as absolute neutrophil count may be increased.Citation12
Differential diagnoses include other periodic inflammatory disorders such as tumor necrosis factor (TNF) receptor-associated periodic syndrome (TRAPS), hyperimmunoglobulinemia D with periodic fever syndrome (HIDS), juvenile systemic granulomatosis, juvenile idiopathic arthritis, familial Mediterranean fever (FMF), and Behçet disease.Citation12 Fever, skin rash, arthralgia, and persistent inflammatory markers were common with Still disease. Other autoimmune diseases could also be evoked as hypocomplementemic vasculitis or systemic lupus.Citation13 CAPS, in general, and MWS, in particular, share common symptoms with other auto-inflammatory diseases such as HIDS, TRAPS, or FMF.Citation12 The hereditary recurrent fever disease which mimics MWS the most is the syndrome associated with NALP 12 mutations, with cold-triggered urticarial during 5–10 days, abdominal pain, oral ulcers, headache, adenomegaly, and, in some patients, deafness.Citation15
A recent study of 287 CAPS patients, including 164 cases of MWS patients, has proposed the following model for the diagnosis of CAPS with a sensitivity of 81% and a specificity of 94%:Citation16
Raised inflammatory markers (CRP/SAA) (mandatory criteria).
In addition to ≥2 of 6 CAPS typical signs/symptoms:
Urticaria-like rash
Cold/stress-triggered episodes
Sensorineural hearing loss
Musculoskeletal symptoms (arthralgia/arthritis/myalgia)
Chronic aseptic meningitis
Skeletal abnormalities (epiphyseal overgrowth/frontal bossing).
A recent survey of a large European registry showed that some NLRP3 mutations correlated with the clinical phenotype and could predict the outcome of CAPS.Citation9 Nine subgroups were defined corresponding to each of the most frequent genetic variants (R260W, E311K, V198M, T348M, D303N, and A439V mutations, and the functional polymorphism Q703K), to rare variants and to the absence of mutation. The patient’s clinical characteristics were compared according to the presence or absence of each of these mutations. The main correlations between clinical characteristics and genotypes are summarized in .
Table 1 NLRP3 mutations and their clinical phenotypes
The R260W variant was associated with cold-triggered symptoms, a positive family history, and a trend toward symptom onset after the age of 6 months. Patients carrying R206W and V198M did not differ in terms of phenotype or disease severity from patients with R260W mutation alone. The T348M variant was associated with disease onset before the age of 6 months, a chronic course, and hearing loss. The V198M, E311K, and A439V alleles were negatively associated with neurological involvement. Patients carrying the functional polymorphism Q703K were characterized by very mild, late-onset disease with no severe neurological or musculoskeletal involvement, no deafness, and no family history. Patients bearing a rare mutation had disease onset before the age of 6 months, neurological manifestations including severe ones, musculoskeletal involvement, and sporadic disease pattern. Furthermore, they had a trend toward a higher risk of sensorineural hearing loss.
In brief, V198M and Q703K alleles are not disease causing, and the A439V and R260W alleles have been variably associated with a less severe phenotype. Patients carrying the T348M and E311K alleles or no mutations may have increased risk for a severe phenotype with hearing loss and neurological disease.
MWS in the age of IL-1 inhibitor treatments
Prior to the discovery of biological treatments, pain killers and antihistamines had been shown to be ineffective for reducing CAPS symptoms, although high-dose corticosteroids were partially efficient.Citation17 Patients had decreased quality of daily life, with fatigue, chronic pain, hearing and visual handicaps, as well as learning difficulties.Citation10 They had to face a lack of understanding in their school or professional milieus, leading to social and affective isolation. Progressively, they could develop various psychological problems such as drug addictions or severe depression leading to suicide.Citation10 Understanding CAPS pathophysiology has allowed efficacious treatment targeting IL-1β.
Three IL-1 inhibitors have shown efficacy in CAPS: anakinra, a recombinant non-glycosylated form of the human IL-1RA; rilonacept, a chimerical protein consisting of the IL-1-binding domains of IL-1RI and IL-1R accessory protein fused to the Fc portion of human IgG1; and canakinumab, a fully human monoclonal IgG1 targeted at IL-1β. Both anakinra and canakinumab are approved for all CAPS phenotypes in Europe. In Japan, only canakinumab is approved for all CAPS phenotypes. In the USA, canakinumab is approved for adults, and children aged >2 years, with FCAS and MWS; anakinra is approved for CINCA; and rilonacept is approved for adults and children aged >12 years with FCAS and MWS.Citation17
Daily subcutaneous injections of anakinra resulted in rapid regression of symptoms and normalization of CRP and SAA levels.Citation18–Citation21 Anakinra has approval in the USA and Europe to be used in infants from 8 months onward at a dose between 1 and 8 mg/kg/day according to clinical severity, and at a dose of up to 300 mg for adults.Citation17 Anakinra has a very short half-life (6 hours); thus, symptoms rapidly reappear if the treatment is interrupted. Anakinra has significant benefits for the treatment of systemic symptoms and as it can penetrate into the cerebrospinal fluid (CSF), it could affect chronic features such as aseptic meningitis, uveitis, and cochlear inflammation.Citation19,Citation22 In a study in CINCA patients, central nervous system inflammation evaluated by CSF analysis and leptomeningeal enhancement on FLAIR MRI were significantly decreased with anakinra, with visual acuity and peripheral vision improvement or stabilization in most patients over age 5.Citation11 Furthermore, anakinra was effective in secondary amyloidosis with stabilization of renal function. However, complete reversal of amyloidosis has only been documented once.Citation19 Canakinumab, with a long half-life of 28 days, has been proven to be efficient and safe versus placebo in a pivotal study published in 2009.Citation23 Thirty-five MWS pediatric and adult patients received 150 mg canakinumab for adults and 2 mg/kg for children every 8 weeks. The effectiveness of canakinumab was rapid and sustained in the long term for all clinical and biological symptoms if the treatment was not discontinued.Citation23,Citation24 Canakinumab is shown to stabilize or decrease hearing loss, but there are no reports on canakinumab concentration in CSF.Citation25,Citation26 In a 2-year long phase III multicenter, single treatment arm, open-label study of canakinumab in patients with FCAS (n=30 patients), MWS (n=103), and CINCA (n=32), repeat neurological assessments of canakinumab treatment showed normalization in 9/20 patients, improvement in two patients, and a new symptom in one patient.Citation24 Abnormal audiograms at baseline (n=63) improved upon treatment in 13/63 patients and were unchanged for 29 patients. Ocular involvement normalized in 1/22 patients, improved in six, and remained unchanged in 15 patients. Three out of four patients with amyloidosis improved and one worsened. The quality of life of patients drastically improved when different assessment tools were used to evaluate it such as the functional assessment of chronic illness therapy fatigue (FACIT-F), the Medical Outcomes Survey 36-item Short Form (SF-36) standard version, the HAQ for adults, and the CHAQ Parent Form 28 (CHQ-PF28) for children.Citation10 Most patients saw an improvement in their physical abilities and social activities.
Rilonacept was demonstrated to be efficient in decreasing the disease activity score compared to placebo at the dose of 160 mg/week administered subcutaneously.Citation27 The effect was maintained during the 96-week follow-up.Citation28
Adverse events due to anti-IL-1 were injection-site reactions, including pain, swelling, redness, and pruritus, for all three IL-1 antagonists; however, this was transient and most patients were able to continue the treatment.Citation23,Citation27–Citation29 Increased incidence of mild infections of the respiratory tract was the main side effect of IL-1 antagonists.Citation23,Citation27–Citation29
Weight gain was reported in 10%–30% of patients with long-term use of anakinra or rilonacept; however, the extent of the weight gain remains to be investigated to determine whether it was excessive.Citation21 Other rare side effects of anakinra included urinary tract infections, leukopenia, hepatitis, and macrophage activation syndrome.Citation29 Anakinra use during pregnancy was safe in a small series of nine women,Citation30 with normal gestational outcome. One of the twin fetus carrying an NLRP3 mutation had renal agenesis and died in utero. Other reports of renal malformation in fetuses carrying CAPS mutations excluded the hypothesis of a causal link with anakinra treatment.Citation11 There are no published data on canakinumab and pregnancy. Thus, a switch from monoclonal anti-IL-1 antibodies to anakinra during pregnancy is recommended and caution is warranted in terms of renal malformations.Citation17 Two large international registries have been established to collect data for anti-IL-1: the Eurofever physician’s registryCitation31 and the β confident Novartis registry on Canakinumab.Citation32
Generally, according to expert opinions, patients presenting with the most severe phenotypes, those at risk of severe complications, and patients presenting continuous biological inflammation (elevated CRP and SAA levels) should receive treatment to avoid organ damage and AA amyloidosis.Citation17 For other patients who do not fulfil these categories, the burden of disease and quality of life should be assessed before a final decision is made. The duration of treatment is theoretically lifelong. Early treatment as soon as the disease is diagnosed may change, at least in part, the natural history, thereby reducing the occurrence of secondary complications.Citation17
In summary, treating patients with IL-1 blockers, regardless of the nature of the drug and the phenotype of CAPS, markedly improved their ability to function in normal society.
Perspectives
To prevent secondary complications and to give children the chance to lead as normal life as possible from birth, IL-1 antagonists should be started as soon as possible, potentially even in very young children and newborns.Citation17 However, for this age group, drug pharmacokinetics and distribution into the tissues are not well studied. Very young children usually need higher doses (3–5 times the recommended doses) to be effective on general symptoms and especially if the aim is to impact neurological manifestations, as well as vision and hearing loss.Citation19,Citation22 For children aged <2 years, the risk of developing encapsulated bacterial infections is high and pneumococcal vaccination prior to starting treatment is strongly recommended.Citation17
Furthermore, the choice of anti-IL-1 monoclonal antibodies in cases of neurological involvement must be further studied as too few data are currently available with regard to diffusion of these drugs into CSF.Citation26 In addition, the long-term outcome of treated patients needs to be followed up to determine the consequences of lifelong blockade of IL-1.
With IL-1 antagonists, the lives of MWS patients normalize, raising the question of reproduction. We have reported a series of nine male MWS patients in whom five had oligozoospermia and three had azoospermia.Citation33 Treatment with IL–1-targeting drugs had a moderate or no effect on spermatozoa counts. The original study of MWS in 1962 reported a case of male infertility in a man with loss of libido and abnormal testicles.Citation8 The extent of infertility in the male MWS population needs to be assessed more precisely in an international study, and the mechanisms leading to infertility need to be explored. Possible mechanisms include inflammation, IL-1β overproduction, altered testosterone level, and/or amyloidosis. Early treatment may be beneficial to preserve normal spermatozoa counts. However, the consequence of complete IL-1 blockade during childhood or adolescence on normal sexual maturation may be problematic, as IL-1α and -1β are known to be required for the regulation of sperm maturation in mice. Sexual health and fertility should be assessed systematically in all male patients to preserve fertility, according to these observations.
Conclusion
Clinical profiles and outcomes of MWS patients have been drastically improved with the increased understanding of CAPS pathophysiology and intervention using anti-IL-1 treatment. Normal life is now possible for patients. Advanced studies are still needed to predict phenotype from genotype for early treatment. Data on pharmacokinetics and diffusion of the drugs into CSF in young children are still needed for better indication and use of anti-IL-1. Consequences on growth, development, and sexual maturation of long-term use of IL–1-antagonists need to be assessed in children treated very early in life.
Acknowledgments
The author thanks Pierre Corbeau and Sarah Kabani for editing the manuscript.
Disclosure
TA Tran has received consulting fees from Novartis, Sobi, and Abbvie. His institution has received research fees from Novartis. The author reports no other conflicts of interest in this work.
References
- CuissetLJeruIDumontBFrench CAPS study groupMutations in the autoinflammatory cryopyrin-associated periodic syndrome gene: epidemiological study and lessons from eight years of genetic analysis in FranceAnn Rheum Dis201170349549921109514
- HoffmanHMMuellerJLBroideDHWandererAAKolodnerRDMutation of a new gene encoding a putative pyrin-like protein causes familial cold autoinflammatory syndrome and Muckle-Wells syndromeNat Genet200129330130511687797
- TschoppJMartinonFBurnsKNALPs: a novel protein family involved in inflammationNat Rev Mol Cell Biol2003429510412563287
- TouitouIKoné-PautIAutoinflammatory diseasesBest Pract Res Clin Rheumatol200822581182919028365
- NeteaMGNold-PetryCANoldMFDifferential requirement for the activation of the inflammasome for processing and release of IL-1beta in monocytes and macrophagesBlood2009113102324233519104081
- DinarelloCAImmunological and inflammatory functions of the interleukin-1 familyAnnu Rev Immunol20092751955019302047
- HaverkampMHvan de VosseEGoldbach-ManskyRHollandSMImpaired cytokine responses in patients with cryopyrin-associated periodic syndrome (CAPS)Clin Exp Immunol2014177372073124773462
- MuckleTJWellsMUrticaria, deafness, and amyloidosis: a new heredofamilial syndromeQ J Med19623123524814476827
- LevyRGérardLKuemmerle-DeschnerJfor PRINTO and Euro-feverPhenotypic and genotypic characteristics of cryopyrin-associated periodic syndrome: a series of 136 patients from the Eurofever RegistryAnn Rheum Dis201574112043204925038238
- Koné-PautILachmannHJKuemmerle-DeschnerJBCanakinumab in CAPS Study GroupSustained remission of symptoms and improved health-related quality of life in patients with cryopyrin-associated periodic syndrome treated with canakinumab: results of a double-blind placebo-controlled randomized withdrawal studyArthritis Res Ther2011136R20222152723
- LeporeLPaloniGCaorsiRFollow-up and quality of life of patients with cryopyrin-associated periodic syndromes treated with AnakinraJ Pediatr2010157231031520472245
- Almeida de JesusAGoldbach-ManskyRMonogenic autoinflammatory diseases: concept and clinical manifestationsClin Immunol2013147315517423711932
- MarzanoAVTavecchioSVenturiniMSalaRCalzavara-PintonPGattornoMUrticarial vasculitis and urticarial autoinflammatory syndromesG Ital Dermatol Venereol20151501415025586657
- HouxLHachullaEKone-PautIMusculoskeletal symptoms in patients with cryopyrin-associated periodic syndromes: a large database studyArthritis Rheumatol201567113027303626245507
- JéruIDuquesnoyPFernandes-AlnemriTMutations in NALP12 cause hereditary periodic fever syndromesProc Natl Acad Sci U S A200810551614161918230725
- Kuemmerle-DeschnerJBOzenSTyrrellPNDiagnostic criteria for cryopyrin-associated periodic syndrome (CAPS)Ann Rheum Dis201776694294727707729
- Koné-PautIGaleottiCCurrent treatment recommendations and considerations for cryopyrin-associated periodic syndromeExpert Rev Clin Immunol201511101083109226312542
- HawkinsPNLachmannHJMcDermottMFInterleukin-1-receptor antagonist in the Muckle-Wells syndromeN Engl J Med2003348252583258412815153
- Kuemmerle-DeschnerJBTyrrellPNKoetterIEfficacy and safety of anakinra therapy in pediatric and adult patients with the autoinflammatory Muckle-Wells syndromeArthritis Rheum201163384084921360513
- Ter HaarNLachmannHÖzenSPaediatric Rheumatology International Trials Organisation (PRINTO) and the Eurofever/Eurotraps ProjectsTreatment of autoinflammatory diseases: results from the Eurofever Registry and a literature reviewAnn Rheum Dis201372567868522753383
- Rossi-SemeranoLFautrelBWendlingDMAIL1 (Maladies Auto-inflammatoires et Anti-IL-1) study Group on behalf of CRI (Club Rhumatisme et Inflammation)Tolerance and efficacy of off-label anti-interleukin-1 treatments in France: a nationwide surveyOrphanet J Rare Dis2015101925758134
- NevenBMarvilletITerradaCLong-term efficacy of the interleukin-1 receptor antagonist anakinra in ten patients with neonatal-onset multisystem inflammatory disease/chronic infantile neurologic, cutaneous, articular syndromeArthritis Rheum201062125826720039428
- LachmannHJKone-PautIKuemmerle-DeschnerJBCanakinumab in CAPS Study GroupUse of canakinumab in the cryopyrin-associated periodic syndromeN Engl J Med2009360232416242519494217
- Kuemmerle-DeschnerJBHachullaECartwrightRTwo-year results from an open label, multicentre, phase III study evaluating the safety and efficacy of canakinumab in patients with cryopyrin-associated periodic syndrome across different severity phenotypesAnn Rheum Dis201170122095210221859692
- Kuemmerle-DeschnerJBKoitschevATyrrellPNEarly detection of sensorineural hearing loss in Muckle-Wells-syndromePediatr Rheumatol Online J20151314326531310
- Rodriguez-SmithJLinYCLi TsaiWCerebrospinal fluid cytokines correlate with aseptic meningitis and blood brain barrier function in neonatal-onset multisystem inflammatory disease: Central Nervous System Biomarkers in Neonatal-Onset Multisystem Inflammatory Disease Correlate With Central Nervous System InflammationArthritis Rheumatol Epub2017124
- HoffmannHMThroneMLAmarNJEfficacy and safety of rilonacept (interleukin-1 Trap) in patients with cryopyrin-associated periodic syndromes: results from two sequential placebo-controlled studiesArthritis Rheum20085882443245218668535
- HoffmannHMThroneMLAmarNJLong-term efficacy and safety profile of rilonacept in the treatment of cryopyrin-associated periodic syndromes: results of a 72-week open-label extension studyClin Ther201234102091210323031624
- KullenbergTLöfqvistMLeinonenMGoldbach-ManskyROlivecronaHLong-term safety profile of anakinra in patients with severe cryopyrin-associated periodic syndromesRheumatology (Oxford)20165581499150627143789
- ChangZSpongCYJesusAAAnakinra use during pregnancy in patients with cryopyrin-associated periodic syndromes (CAPS)Arthritis Rheumatol201466113227323225223501
- Paediatric Rheumatology International Trials OrganisationEurofever project Available from: www.printo.it/eurofever/Accessed June 9, 2017
- ClinicalTrials.govClinical outcomes and safety: a registry study of Ilaris (Canakinumab) patients (B-Confident) Available from: https://clinicaltrials.gov/ct2/show/NCT01213641Accessed June 9, 2017
- TranTAKoné-PautIMarieINinetJCuissetLMeinzerUMuckle-Wells syndrome and male hypofertility: a case seriesSemin Arthritis Rheum201242332733122512814
