Abstract
Certolizumab pegol (CZP) is a pegylated humanized tumor necrosis factor-α inhibitor (TNFi) approved for the treatment of ankylosing spondylitis (AS) in the USA and for AS and non-radiographic axial spondyloarthritis (nr-axSpA) in Europe and in some Latin American countries. CZP lacks Fc region, preventing complement fixation and cytotoxicity mediated by antibody; CZP does not actively cross the placenta, unlike other TNFi. RAPID-axSpA study is a Phase III trial conducted in patients with AS and nr-axSpA as double blind and placebo controlled to week 24, dose blind to week 48 and open label to week 204. Of a total of 325 patients recruited, 107 patients were assigned to placebo and 218 patients to CZP (111 to CZP 200 mg Q2W, 107 to CZP 400 mg Q4W). Improvements in axial involvement, joint involvement, enthesitis and quality of life were reported in patients treated with CZP. Safety profile was like that reported for other TNFi in axSpA patients. In this article, we summarized the pharmacology and we reviewed the efficacy and tolerability of this drug for the treatment of axSpA. Some special considerations of CZP during pregnancy are included. CZP, the latest TNFi to be approved, showed efficacy in all manifestations of AS and nr-axSpA.
Introduction
Axial spondyloarthritis (axSpA) is a chronic disease, characterized by involvement of the sacroiliac (SI) joints and spine, resulting in chronic inflammatory back pain.
The estimated prevalence of axSpA is between 0.2% and 1.2% in white European populations.Citation1 The diagnosis is often delayed by up to 8 years, mainly because sacroiliitis, which is considered a hallmark of ankylosing spondylitis (AS), is not visible on plain radiographs in the early stages of the disease.Citation2,Citation3
For the diagnosis of AS, fulfillment of modified New York classification criteria, which requires the presence of radiographic sacroiliitis, is needed.Citation4 With the use of magnetic resonance imaging (MRI) allowing earlier detection of inflammation in the SI joints, it has been possible to identify patients with clinical characteristics of AS, but who do not fulfill the modified New York criteria. These patients have been classified as having non-radiographic axSpA (nr-axSpA).Citation5,Citation6 New classification criteriaCitation4–Citation7 were developed in an attempt to improve diagnostic delay.Citation8,Citation9 This new criteria allowed the inclusion of nr-axSpA in clinical studies.
It has been proposed that nr-axSpA may represent an early form of AS, as many patients progress to AS over time.Citation10,Citation11 The fact that not all patients progress to AS over time, and the identification of genetic and sex differences between AS and nr-axSpA, has led some to think that they might be distinct diseasesCitation12–Citation14 and have led to the reluctance of FDA to approve tumor necrosis factor inhibitors (TNFis) in nr-axSpA.Citation15 In general, disease activity and burden of symptoms did not differ between both, and both need the same kind of treatment.Citation16–Citation19
The treatment of SpA has changed drastically since the use of biological agents such as TNFi.Citation20–Citation23 Certolizumab pegol (CZP), a novel TNFi, was the last TNFi approved for the treatment of axSpA (European Commission [EMA] and AS (EMA and FDA). We will describe the current evidence for the use of CZP in the treatment of active axSpA in the following text.
Certolizumab pegol
CZP is formed by the combination of a humanized Fab fragment (50 kDa) and a 40-kDa polyethylene glycol moiety (a polymer that is not immunogenic or toxic).Citation24,Citation25 Its molecular structure leads to an increase of plasma half-life of the molecule to about 2 weeks. This allows 2–4 weekly subcutaneous injections. PEGylation is also assumed to be associated with decreased inmmunogenicity.Citation25 The lack of Fc region prevents complement fixation and cytotoxicity mediated by antibody. CZP does not lead to apoptosis of peripheral blood monocytes or lymphocytes or neutrophil necrosis.Citation24,Citation25
CZP has been characterized as producing dose-dependent inhibition of TNF (soluble and membrane-bounded TNF) and inhibition of the production of lipopolysaccharide-induced TNF-α and IL-1β by monocytes. CZP does not induce complement or cytotoxicity mediated by antibody in vitro and induces cell death by a nonapoptotic signaling (probable by transmembrane TNF-α). It has also been shown greater drug distribution into inflamed tissues than that of infliximab (IFX) and adalimumab (ADA).Citation24,Citation26
Clinical efficacy
CZP efficacy in the treatment of both AS and nr-axSpA patients was evaluated by a randomized control trial: RAPID-axSpA trial.Citation27–Citation32
The objective of RAPID-axSpA (NCT01087762), a 4-year, Phase III randomized trial, double blind and placebo controlled to week 24, dose blind to week 48 and open label to week 204, was to study the efficacy and safety of CZP for the treatment of axSpA.Citation27–Citation32
Patients were recruited from 83 different sites in central/Eastern and Western Europe, North America and Latin America. To be included in the study, patients need to fulfill Assessment of SpondyloArthritis international Society (ASAS) criteria for the diagnosis of SpA and to have active disease, defined as a Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) ≥4 and spinal pain ≥4 on a 0–10 Numerical Rating Scale. Intolerance or inadequate response to at least one nonsteroidal anti-inflammatory drug (NSAID) was also needed.Citation28 A total of 325 patients were recruited. One hundred and seven patients were assigned to placebo and 218 patients to CZP (111 to CZP 200 mg Q2W and 107 to CZP 400 mg Q4W). Of the group of patients treated with CZP, 121 had AS and 97 had nr-axSpA ().Citation28 Baseline disease activity among the groups of patients and between AS and nr-axSpA patients was similar. More than half of the patients in the CZP-treated group (63% [199/315]) completed the study at week 204. The completion rates were similar between both CZP groups and between AS and nr-axSpA patients. ASAS20, ASAS40, BASDAI, Ankylosing Spondylitis Disease Activity Score, Bath Ankylosing Spondylitis Metrology Index and Bath Ankylosing Spondylitis Functional Index responses (nonresponder imputation and observed case) at week 204 are shown in .Citation31,Citation32
Figure 1 Chart flow of RAPID-axSpA trialCitation27–Citation32 during the three different phases of the trial.
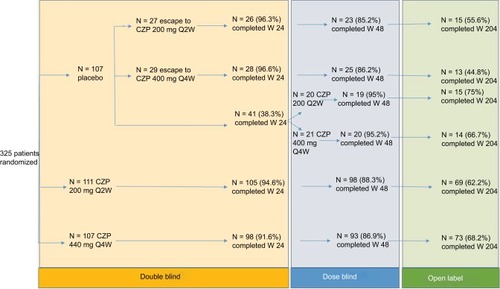
Table 1 Efficacy of subcutaneous certolizumab pegol in adult patients with axial spondyloarthritis in the randomized, double-blind, multicenter RAPID-axSpA trialCitation28
Ankylosing Spondylitis Disease Activity Score-ID sustained remission, and ASAS partial remission was achieved by one-third of patients with similar responses rates among AS and nr-axSpA ().Citation27,Citation32
Patient-reported outcomes
Patients-reported outcomes (PROs) are widely used measurements that allow the collection of information related to specific disease aspects, usually not scored by the physicians, directly from the patient. Among them, improvements in back pain, fatigue, sleep (Medical Outcomes Study Sleep Scale), SF-36 (both physical and mental outcomes) and Ankylosing Spondylitis Quality of Live were seen after 24 weeks of the initiation of treatment with CZP, and these results were maintained until week 204Citation28,Citation31 (). Comparable improvement in total back pain, fatigue and Ankylosing Spondylitis Quality of Live was seen in AS and nr-axSpA groups.Citation28 Clinically relevant improvement in pain response to CZP was observed as rapid as from day 2.Citation30
Table 2 Effect of subcutaneous certolizumab pegol on key patient-reported outcomes in the RAPID-axSpA studyCitation30
Peripheral arthritis and enthesitis
By week 24, similar improvements in arthritis and enthesitis were seen in both AS and nr-axSpA patients,Citation28 which was maintained until week 204 ().Citation27 Enthesitis was assessed by the MASES score. Both AS and nr-axSpA patients had a similar improvement in mean MASES score, although baseline scores were higher in nr-axSpA patients (AS: 4.7 and nr-axSpA: 5.6).Citation28
Table 3 Effect of subcutaneous certolizumab pegol on peripheral arthritis and enthesitis in the RAPID-axSpA studyCitation28
MRI outcomes
MRIs of the sacroiliac (SI) joints and spine were performed at baseline and week 12, 48 and 96.Citation29 For lesions on MRI in the SI joints, the Spondyloarthritis Research Consortium of Canada (SPARCC) scoring methodCitation33 was used and for lesions in the spine the Berlin modification of Ankylosing Spondylitis spine MRI scoring system for disease activity (Berlin) was used.Citation34 Only patients with MRI evidence of inflammation at baseline were analyzed for MRI remission. CZP treatment of patients with axSpA significantly reduced MRI inflammation in the SI joints and spine over 12 weeks.Citation29 Improvements were seen and maintained through week 96 for AS and nr-axSpA patients treated with CZP.Citation29
Nonradiographic axial spondyloarthritis
RAPID-axSpA is the only trial including patients with both r-axSpA and nr-axSpA with either positive C-reactive protein (CRP) or MRI (with stratified randomization for the presence of radiographic sacroiliitis),Citation35 and as mentioned earlier, overlapping results were observed between the two groups for most of the outcomes measured (). The improvement in disability (Bath Ankylosing Spondylitis Functional Index) was greater for patients with nr-axSpA.Citation35 The effect of etanercept, ADA and golimumab in patients with nr-axSpA was tested in three separate trials.Citation36–Citation38 For all three drugs, responses were not statistically significant and smaller in patients with normal CRP and MRI at baseline.Citation35 In patients who had a positive MRI or an increased CRP (ADA and golimumab) and in patients who had both (etanercept), the effect sizes were far greater and statistically significant.Citation35
In summary, for patients with nr-axSpA, there is good evidence for the efficacy of CZP, etanercept, ADA and golimumab, but their use should be restricted to patients with abnormal CRP and/or MRI.
Safety
The safety profile of these TNFi has been shown in different clinical trials performed in patients with psoriatic and rheumatoid arthritis.Citation39–Citation41
The initial RAPID axSpA trial reported that the adverse events (AEs) were mild to moderate in both CZP and placebo groups. During the 24 weeks of the study, any AE occurred in 62.6% of the placebo group and 76% of the CZP group. Serious AEs occurred in 4.7% and 3.6% of patients in the first group and second group, respectively, and serious infections occurred in 0% and 1.8% of patients in the first group and second group, respectively.Citation28 In the placebo group any AE, serious AEs and serious infections occurred in 74.8%, 6.5% and 0%, respectively.Citation28 No deaths, malignancies or tuberculosis events were observed, and no new safety signals for CZP in AS were observed compared to other indications for CZP.Citation28
Sieper et al described the effects of 315 AS patients in the RAPID ax-SPA trial over 96 weeks. AEs occurred in 279 (88.6%) patients (total exposure period 486/patient years). Most of these side effects were mild (74.9%) or moderate (59.4%).Citation32
They reported serious AEs in 41 patients (13.0%), which were predominantly infections and infestations (3.8%); one case of active tuberculosis was identified. Over the 96-week trial period, there were no fatalities, malignancies or drug-induced demyelinating disease. In total, 215 of the 315 patients were tested for anti-CZP antibodies at week 96, and 9 patients tested positive for the antibodies. They did not investigate the efficacy of CZP in patients who developed anti-CZP antibodies because of the small cohort of patients with positive antibodies.Citation32
A boxed warning on the increased risk of serious infection and latent tuberculosis is included in the US prescribing information.Citation42 In the European Union (EU), CZP is contraindicated in active tuberculosis or other severe infections such as sepsis or opportunistic infections and moderate to severe heart failure (NYHA classes III/IV).Citation43
Although there are no data on long-term safety of CZP in AS, data from treatment of other diseases are useful. Loftus et al collected data from five placebo-controlled trials, nine open-label studies and one dose regimen study with Crohn’s disease and found an IR for serious AEs of 31.35/100 patient-years, very similar to that of placebo (24.33/100 patient-years).Citation44 IRs of serious infections or malignancies did not increase with long-term treatment (6.47/100 patient-years and 0.80/100 patient-years, respectively, in the all-studies group). In a comparable way, IRs of psoriasis or psoriasiform dermatitis did not increase with long-term treatment (0.93/100 patient-years and 0.09/100 patient-years, respectively, in the all-studies group).Citation44
Drug survival is a surrogate of efficacy and safety in observational studies. Recently, results from a two-center cohort of AS patients treated with TNFi (ADA: n= 332, etanercept: n = 205, IFX: n = 51, golimumab: n = 40, and CZP: n = 23) in the United Kingdom have been published.Citation45 Median drug survival duration for first TNFi was 10.2 years, which was superior to second TNFi (5.5 years) (P < 0.05). No drug-specific (P = 0.45) differences were observed for TNFi survival, although follow-up for patients with CZP was shorter.Citation45
All data show that CZP safety profile is very similar to the other TNFi that have been in the market for longer time.
Pregnancy
Drugs used to treat women at fertile age, commonly affected with rheumatic diseases, may alter fertility and increase the risk of miscarriages and congenital abnormalities. On the other side, disease activity could be an independent risk factor for adverse pregnancy outcomes.Citation46–Citation48
Concerns related to the safety of biologics during pregnancy existed until few years ago when data from many studies suggested that pregnant women with inflammatory bowel disease or inflammatory arthritis receiving TNF inhibitors was not associated with adverse pregnancy outcomes or any increase in teratogenicity.Citation49–Citation53
CZP differs from other TNF blockers in that it has no Fc region and is not actively transported through the placenta, and as expected, concentrations in the fetus would be lower.Citation54
In a study performed in 2013, authors measured the serum and cord concentrations of drugs in the mother, infant and cord blood.Citation55 They included 31 pregnant women with inflammatory bowel disease receiving IFX (n=11), ADA (n=10) and CZP (n=10). Drug concentrations in infants at birth and cord were compared with those of the mother. IFX and ADA concentrations were higher in infants at birth and their cords than in their mothers. The median level of IFX and ADA in the cord was 160% and 153% higher than that of the mother, respectively. By contrast, the median level of CZP in the cord was 3.9% higher than that of the mother. They detected IFX and ADA in the infants for as long as 6 months, while CZP in the infant plasma and cord was undetectable. No congenital malformations or adverse pregnancy outcomes were reported.Citation24,Citation55
An analysis of 253 pregnancies with known outcomes from a total of 625 pregnancies from UCB Pharma global safety database was published.Citation56 Only one-third of pregnancies continued the treatment through second or third trimester, while most of women were exposed during the first trimester. Most of the women had Crohn’s disease. In total, 75.5% pregnancies resulted in live births. Ten percent of women had elective terminations, and spontaneous miscarriages were reported in 14% of patients.Citation56 Three congenital malformations were reported after maternal exposure, which is comparable to that of the general population.Citation56
The 2016 “EULAR recommendations for use of antirheumatic drugs during pregnancy and lactation” recommended discontinuing monoclonal antibodies IFX, ADA and golimumab around gestational week 20. Etanercept (a fusion protein) may be continued until week 30–32, and CZP has minimal transplacental passage for use throughout pregnancy but needs confirmation from prospective studies.Citation51
Administration and doses
In the United States, CZP has been approved by FDA only for the treatment of adult patients with active AS.Citation42
In the EU, subcutaneous CZP has been approved for the treatment of patients with severe active AS who have had an inadequate response to or are intolerant to NSAIDs and adults with severe active axSpA without radiographic evidence of AS but with objective signs of inflammation by elevated CRP and/or MRI who have had an inadequate response to or are intolerant to NSAIDs.Citation43
The recommended doses and administration schedules are 400 mg (given as 2 subcutaneous injections of 200 mg each) initially and at weeks 2 and 4, followed by 200 mg every other week or 400 mg every 4 weeks.
The purpose of indicating LD is to achieve high concentrations of the drug during early stages of treatment, which accelerates drug response and reduces the production of drug antibodies.Citation24
Place in therapy
TNFis are recommended in all international and local guidelines after NSAID failure in AS and nr-axSpA.Citation57,Citation58 CZP, a new TNFi, is placed at that same level, although some concerns due to less long-term and real-life date might be raised. CZP demonstrated efficacy not only in axial involvement but also in peripheral arthritis and enthesitis and hence should be considered in patients with more than one involvement. Because of its advantages related to low cross-placental and breast milk transfer, CZP may be considered as the TNFi of choice in female patients considering pregnancy and of course in pregnant patients. As always, risks and benefits of therapy should be discussed with the patient taking into account neonatal risks and the risk of disease flare. Also, the course of a pregnancy with a very active disease might have far more consequences to neonatal development. In general, it is not recommended to switch to CZP if a pregnant patient is doing well on other TNFi. Having other options for the treatment of these patients, is there any advantage to use CZP? Some CZP features, apart from placental and breast milk transfer, that make CZP in some way different to other TNFi might be considered at the time of offering the patient a new treatment: a fortnightly or monthly subcutaneous drug regime due to a long half-life; quick and long-lasting efficacy and low generation of anti-TNFi (anti-ADA) antibodies after a loading dose; and proven good distribution into inflamed tissues due to PEGylation. On the other hand, up to now, in spite of these differences, CZP safety profile looks similar to other TNFi.
Conclusion
CZP, the newest original TNFi, has shown very good efficacy and has sustained on the long term in several manifestations of axSpA, including peripheral disease, enthesitis and PROs.
CZP is a new TNFi with a novel composition that is gaining experience around the world and performing according to expectations.
Disclosure
ERS participated in advisory boards, gave conferences or received grants from Abbvie, Bristol-Myers Squibb, Genzyme, GlaxoSmithKline, Janssen, Lilly, Novartis, Pfizer, Roche, Sandoz and UCB. MLAF and JM gave conferences for Novartis. The authors report no other conflicts of interest in this work.
References
- SieperJRudwaleitMKhanMABraunJConcepts and epidemiology of spondyloarthritisBest Pract Res Clin Rheumatol200620340141716777573
- SykesMPDollHSenguptaRGaffneyKDelay to diagnosis in axial spondyloarthritis: are we improving in the UK?Rheumatology (Oxford)201554122283228426283680
- FeldtkellerEKhanMAvan der HeijdeDvan der LindenSBraunJAge at disease onset and diagnosis delay in HLA-B27 negative vs. positive patients with ankylosing spondylitisRheumatol Int2003232616612634937
- van der LindenSValkenburgHACatsAEvaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteriaArthritis Rheum19842743613686231933
- BaraliakosXBraunJNon-radiographic axial spondyloarthritis and ankylosing spondylitis: what are the similarities and differences?RMD Open20151Suppl 1e00005326557375
- KiltzUBaraliakosXKarakostasPThe degree of spinal inflammation is similar in patients with axial spondyloarthritis who report high or low levels of disease activity: a cohort studyAnn Rheum Dis20127171207121122523430
- RudwaleitMvan der HeijdeDLandeweRThe development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part II): validation and final selectionAnn Rheum Dis200968677778319297344
- DeodharAMittalMReillyPAnkylosing spondylitis diagnosis in US patients with back pain: identifying providers involved and factors associated with rheumatology referral delayClin Rheumatol20163571769177626987341
- SalvadoriniGBandinelliFDelle SedieAAnkylosing spondylitis: how diagnostic and therapeutic delay have changed over the last six decadesClin Exp Rheumatol201230456156522510360
- PoddubnyyDRudwaleitMHaibelHRates and predictors of radiographic sacroiliitis progression over 2 years in patients with axial spondyloarthritisAnn Rheum Dis20117081369137421622969
- Sampaio-BarrosPDBertoloMBKraemerMHMarques-NetoJFSamaraAMUndifferentiated spondyloarthropathies: a 2-year followup studyClin Rheumatol200120320120611434474
- Ortega CastroRFont UgaldePCastro VillegasMCDifferent clinical expression of patients with ankylosing spondylitis according to gender in relation to time since onset of disease. Data from REG-ISPONSERReumatol Clin201394221225 Spanish [with English abstract]23474378
- TournadreAPereiraBLhosteADifferences between women and men with recent-onset axial spondyloarthritis: results from a prospective multicenter French cohortArthritis Care Res (Hoboken)20136591482148923463610
- WallisDInmanRDRecognition of preclinical and early disease in axial spondyloarthritisRheum Dis Clin North Am201440468569725437285
- DeodharAReveilleJDvan den BoschFThe concept of axial spondyloarthritis: joint statement of the spondyloarthritis research and treatment network and the Assessment of SpondyloArthritis International Society in response to the US Food and Drug Administration’s comments and concernsArthritis Rheumatol201466102649265625154344
- RumyantsevaDGDubininaTVDeminaABAnkylosing spondylitis and non-radiographic axial spondyloarthritis: two stages of disease?Ter Arkh20178953337 Russian [with English abstract]
- de WinterJJvan MensLJvan der HeijdeDLandeweRBaetenDLPrevalence of peripheral and extra-articular disease in ankylosing spondylitis versus non-radiographic axial spondyloarthritis: a meta-analysisArthritis Res Ther20161819627586785
- WallmanJKKapetanovicMCPeterssonIFGeborekPKristensenLEComparison of non-radiographic axial spondyloarthritis and ankylosing spondylitis patients—baseline characteristics, treatment adherence, and development of clinical variables during three years of anti-TNF therapy in clinical practiceArthritis Res Ther20151737826703005
- SongIHWeissAHermannKGSimilar response rates in patients with ankylosing spondylitis and non-radiographic axial spondyloarthritis after 1 year of treatment with etanercept: results from the ESTHER trialAnn Rheum Dis201372682382523172749
- SongIHHermannKGHaibelHConsistently good clinical response in patients with early axial spondyloarthritis after 3 years of continuous treatment with etanercept: longterm data of the ESTHER trialJ Rheumatol201441102034204025028375
- RudwaleitMClaudepierrePWordsworthPEffectiveness, safety, and predictors of good clinical response in 1250 patients treated with adalimumab for active ankylosing spondylitisJ Rheumatol200936480180819273449
- RudwaleitMSchwarzloseSHilgertESListingJBraunJSieperJMRI in predicting a major clinical response to anti-tumour necrosis factor treatment in ankylosing spondylitisAnn Rheum Dis20086791276128118006539
- RudwaleitMListingJBrandtJBraunJSieperJPrediction of a major clinical response (BASDAI 50) to tumour necrosis factor alpha blockers in ankylosing spondylitisAnn Rheum Dis200463666567015037444
- Acosta-FelquerMLRosaJSorianoERAn evidence-based review of certolizumab pegol in the treatment of active psoriatic arthritis: place in therapyOpen Access Rheumatol20168374427843368
- GoelNStephensSCertolizumab pegolMAbs20102213714720190560
- NesbittAFossatiGBerginMMechanism of action of certolizumab pegol (CDP870): in vitro comparison with other anti-tumor necrosis factor alpha agentsInflamm Bowel Dis200713111323133217636564
- van der HeijdeDDougadosMLandeweRSustained efficacy, safety and patient-reported outcomes of certolizumab pegol in axial spondyloarthritis: 4-year outcomes from RAPID-axSpARheumatology (Oxford)20175691498150928498975
- LandewéRBraunJDeodharAEfficacy of certolizumab pegol on signs and symptoms of axial spondyloarthritis including ankylosing spondylitis: 24-week results of a double-blind randomised placebo-controlled Phase 3 studyAnn Rheum Dis2014731394724013647
- BraunJBaraliakosXHermannKGEffect of certolizumab pegol over 96 weeks of treatment on inflammation of the spine and sacroiliac joints, as measured by MRI, and the association between clinical and MRI outcomes in patients with axial spondyloarthritisRMD Open201731e00043028848654
- SieperJKivitzAvan TubergenAImpact of certolizumab pegol on patient-reported outcomes in patients with axial spondyloarthritisArthritis Care Res (Hoboken)201567101475148025832312
- SieperJKivitzAvan TubergenALong-term maintenance of improvements in patient-reported outcomes with certolizumab pegol in patients with axial spondyloarthritis, including ankylosing spondylitis and non-radiographic axial spondyloarthritis: 96-week results of the RAPID-axSpA StudyValue Health2014177A385A386
- SieperJLandeweRRudwaleitMEffect of certolizumab pegol over ninety-six weeks in patients with axial spondyloarthritis: results from a phase III randomized trialArthritis Rheumatol201567366867725470228
- MaksymowychWPInmanRDSalonenDSpondyloarthritis research Consortium of Canada magnetic resonance imaging index for assessment of sacroiliac joint inflammation in ankylosing spondylitisArthritis Rheum200553570370916208659
- LukasCBraunJvan der HeijdeDScoring inflammatory activity of the spine by magnetic resonance imaging in ankylosing spondylitis: a multireader experimentJ Rheumatol200734486287017407241
- SeprianoARegelAvan der HeijdeDEfficacy and safety of biological and targeted-synthetic DMARDs: a systematic literature review informing the 2016 update of the ASAS/EULAR recommendations for the management of axial spondyloarthritisRMD Open201731e00039628176964
- DougadosMvan der HeijdeDSieperJSymptomatic efficacy of etanercept and its effects on objective signs of inflammation in early nonradiographic axial spondyloarthritis: a multicenter, randomized, double-blind, placebo-controlled trialArthritis Rheumatol20146682091210224891317
- SieperJvan der HeijdeDDougadosMEfficacy and safety of adalimumab in patients with non-radiographic axial spondyloarthritis: results of a randomised placebo-controlled trial (ABILITY-1)Ann Rheum Dis201372681582222772328
- SieperJvan der HeijdeDDougadosMA randomized, double-blind, placebo-controlled, sixteen-week study of subcutaneous golimumab in patients with active nonradiographic axial spondyloarthritisArthritis Rheumatol201567102702271226139307
- KamedaHNishidaKNannkiTWatanabeAOshimaYMomoharaSSafety and effectiveness of certolizumab pegol in patients with rheumatoid arthritis: interim analysis of post-marketing surveillanceNihon Rinsho Meneki Gakkai Kaishi2017403196205 Japanese [with English abstract]28747607
- ZhouQZhouYChenHWangZTangZLiuJThe efficacy and safety of certolizumab pegol (CZP) in the treatment of active rheumatoid arthritis (RA): a meta-analysis from nine randomized controlled trialsInt J Clin Exp Med20147113870388025550895
- FerranteMVermeireSRutgeertsPJDrug safety evaluation of certolizumab pegolExpert Opin Drug Saf201413225526624156537
- Cimzia® (certolizumab pegol) [prescribing information]BrusselsUCB Inc2013 Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/125160s215lbl.pdfAccessed November 18, 2017
- European Medicines AgencyCimzia (certolizumab pegol): summary of product characteristicsLondonEuropean Medicines Agency2014 Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/001037/WC500069763.pdfAccessed November 18, 2017
- LoftusEVJrColombelJFSchreiberSSafety of long-term treatment with certolizumab pegol in patients with Crohn’s disease, based on a pooled analysis of data from clinical trialsClin Gastroenterol Hepatol201614121753176227464588
- YahyaFGaffneyKHamiltonLTumour necrosis factor inhibitor survival and predictors of response in axial spondyloarthritis-findings from a United Kingdom cohortRheumatology (Oxford)20185761962429272541
- ElliottABChakravartyEFImmunosuppressive medications during pregnancy and lactation in women with autoimmune diseasesWomens Health (Lond)20106343144020426608
- OstensenMBrucatoACarpHPregnancy and reproduction in autoimmune rheumatic diseasesRheumatology (Oxford)201150465766421097449
- LevyRAde JesusGRde JesusNRKlumbEMCritical review of the current recommendations for the treatment of systemic inflammatory rheumatic diseases during pregnancy and lactationAutoimmun Rev2016151095596327490204
- CalligaroAHoxhaARuffattiAPunziLAre biological drugs safe in pregnancy?Reumatismo201566430431725829190
- BazzaniCScrivoRAndreoliLProspectively-followed pregnancies in patients with inflammatory arthritis taking biological drugs: an Italian multicentre studyClin Exp Rheumatol201533568869326311348
- Götestam SkorpenCHoeltzenbeinMTincaniAThe EULAR points to consider for use of antirheumatic drugs before pregnancy, and during pregnancy and lactationAnn Rheum Dis201675579581026888948
- Weber-SchoendorferCOppermannMWackerEPregnancy outcome after TNF-α inhibitor therapy during the first trimester: a prospective multicentre cohort studyBr J Clin Pharmacol201580472773925808588
- BrömsGGranathFEkbomALow risk of birth defects for infants whose mothers are treated with anti-tumor necrosis factor agents during pregnancyClin Gastroenterol Hepatol2016142234234.e1-e5241.e1-e526375613
- PorterCArmstrong-FisherSKopotshaTCertolizumab pegol does not bind the neonatal Fc receptor (FcRn): consequences for FcRn-mediated in vitro transcytosis and ex vivo human placental transferJ Reprod Immunol201611671227123565
- MahadevanUWolfDCDubinskyMPlacental transfer of anti-tumor necrosis factor agents in pregnant patients with inflammatory bowel diseaseClin Gastroenterol Hepatol2013113286292 quiz e2423200982
- ClowseMEWolfDCFörgerFPregnancy outcomes in subjects exposed to certolizumab pegolJ Rheumatol201542122270227826523031
- WardMMDeodharAAklEAAmerican College of Rheumatology/Spondylitis Association of America/Spondyloarthritis Research and Treatment Network 2015 recommendations for the treatment of ankylosing spondylitis and nonradiographic axial spondyloarthritisArthritis Rheumatol201668228229826401991
- van der HeijdeDRamiroSLandeweR2016 update of the ASAS-EULAR management recommendations for axial spondyloarthritisAnn Rheum Dis201776697899128087505
