Abstract
Rheumatoid arthritis is the most common inflammatory arthritis and continues to have major long-term effects on quality of life. Early and intensive treatment has now become the norm in clinical practice with changes of medication dictated by measuring the presence of continued disease activity. Biologics, particular tumor necrosis factor inhibitors, have a crucial role in the management of very severe disease. Certolizumab is a relatively new tumor necrosis factor inhibitor which uses a novel strategy to neutralize TNF-alpha – the conjugation of tumor necrosis factor specific Fab antibody fragments to polyethylene glycol. Two Phase II and three Phase III randomized controlled trials have evaluated the efficacy and toxicity of certolizumab. More than 2000 patients were enrolled, and followed from 12–52 weeks. The number of patients achieving significant improvements with certolizumab, was indicated by the American College of Rheumatology with a 50% response rate. The risk ratios of achieving this response at 24 weeks was 6.01 (95% confidence interval [CI]: 3.84–9.40). At 52 weeks the risk ratio was 5.27 (95% CI: 3.19–8.71). The number of patients needed to treat, to obtain this benefit at 24 weeks was 4 (95% CI: 3–5). Certolizumab also had important clinical benefits in reducing erosive damage to joints, limiting disability, and enhancing other outcomes of importance to patients such as fatigue. The patient-related benefits were present from the early weeks of treatment. The clinical trials showed serious adverse events, including infections, which were more frequent for certolizumab. The most common adverse events comprised upper respiratory tract infections, hypertension, and nasopharyngitis. The balance of evidence suggests that certolizumab is equivalent to other tumor necrosis factor inhibitors, though no head-to-head trials have been undertaken. Having several effective treatments available, benefits patient choice, because the frequency and route of administration of these treatments varies. Furthermore, as intolerance and antibody development against existing biologics is not uncommon, having several agents allows opportunities to switch from one inhibitor to another.
Keywords:
Introduction
Features of rheumatoid arthritis
Rheumatoid arthritis remains the most common inflammatory arthritis. It affects between 0.5% and 1.0% of the population.Citation1 The incidence of new cases varies from 5 to 50 per 100,000 annually.Citation2 Rheumatoid arthritis can begin at any age, including childhood, but the incidence of new cases increases with age. Rheumatoid arthritis has a marked female predominance. Fifty per cent of the risk for development of rheumatoid arthritis is attributable to genetic factors and smoking is the main environmental risk.Citation2
Rheumatoid arthritis results in chronic pain, disability, fatigue, and loss of productivity both in the workplace and at home. Its impact extends beyond chronic pain and inability to function normally. In particular there are significant economic burdens attributable to the disease, which affects society as a whole, as well as individual patients and their families. Work disability in patients with rheumatoid arthritis occurs early and increases over time, and is a major driver of its economic impact. Finally rheumatoid arthritis is associated with cardiovascular disease and a range of other important comorbidities. These shorten life expectancy.
Managing rheumatoid arthritis
Early and intensive treatment has now become the norm in clinical practice with changes of medication dictated by measuring the presence of continued disease activity. Most patients with rheumatoid arthritis receive treatment with disease-modifying drugs (DMARDs) such as methotrexate and sulfasalazine. These control joint inflammation and are both effective and cost-effective. There is also evidence that combinations of DMARDs are useful in severe, active rheumatoid arthritis.
However, the introduction of biologics has changed the situation. Tumor necrosis factor (TNF) inhibitors are effective at all stages of rheumatoid arthritis. Their benefits extend to early DMARD-naïve rheumatoid arthritis patients.Citation3–Citation5 Some expert groups, such as the American College of Rheumatology (ACR), recommend their use as first-line treatment in patients with high disease activity.Citation6 However, this remains a controversial issue and other groups, such as the National Institute for Health and Clinical Excellence guidance focuses on DMARD combinations for active early disease, based on an economic analysis of benefits.Citation7,Citation8 The combination of adequately dosed methotrexate and a biologic agent, especially a TNF inhibitor, is far more effective in active rheumatoid arthritis than traditional disease modifying drugs used as monotherapy in early and long-standing disease. Biological therapies improve clinical, radiologic, and functional outcomes.
Inevitably, not all patients respond to all medications equally. Some patients respond to one biologic and others respond to another. Some patients may fail a number of medications, either alone or in combination, before responding to an additional treatment. For this reason, the optimal management of rheumatoid arthritis requires access to a range of effective agents. One or two TNF inhibitors are insufficient and there is room for an additional biologic such as certolizumab.
Clinical trials of biologic TNF inhibitors in the late 1990s confirmed the importance of TNF function in the pathogenesis of chronic non-infectious inflammation of joints. Until 2009, more than 2 million patients worldwide had received the first marketed drugs, namely the monoclonal TNF antibodies infliximab and adalimumab and the soluble TNF receptor etanercept.Citation9 All three are equally effective in rheumatoid arthritis. These three drugs targeting TNF are now in common clinical use. Infliximab is a chimeric TNF specific monoclonal antibody with mouse hypervariable domains and human antibody backbone.Citation10 Adalimumab is a recombinant human TNF specific monoclonal antibody.Citation11 Etanercept is a fully human construct comprising the p75 TNF receptor and Fc antibody portion.Citation12 The efficacy of these agents in controlling the symptoms of rheumatoid arthritis provides further clinical evidence that in many patients with rheumatoid arthritis, TNF is a central pathogenic mediator.
Structure and function
Currently, five anti-TNF inhibitors are available to treat rheumatoid arthritis: adalimumab, etanercept, infliximab, golimumab, and certolizumab. Despite having different proposed mechanisms of action,Citation13 all five of the current TNF inhibitors have relatively similar effects on clinical, functional, and radiologic outcomes, although there are no head to head trials to confirm their equivalence. The US Food and Drug Administration approved the use of TNF inhibitors to treat rheumatoid arthritis in the following order: etanercept (1998), infliximab (1999), adalimumab (2002), and certolizumab and golimumab (2009).
More biologics are being introduced; as we know, many patients will respond to these TNF inhibitors but some will not, either due to primary failure or secondary TNF failure. Certolizumab (CDP-870) is a new agent that employs a novel strategy to neutralize TNF-alpha, namely the prokaryotic expression of TNF-alpha-specific Fab antibody fragments, conjugated to polyethylene glycol to produce a drug that is potentially less expensive to manufacture than other anti-TNF-alpha agents and which may be administered by subcutaneous injection once a month.Citation14
Structurally, there are two important regions, the Fab and the Fc portions. The Fab portion contains complementarity-determining regions (CDR), unique sequences of amino acids responsible for binding antigen. The Fc portion is not antigen specific but is necessary for other antibody functions including complement fixation and cell lysis. Monoclonal antibodies have a single identical sequence, in contrast to polyclonal antibodies, which have many different sequences and hence antigen-binding properties. The first generation of monoclonal antibodies was generated in mice, but the immunogenicity of murine proteins in humans precluded their use therapeutically, due to their tendency to induce major immune responses (anaphylaxis). New techniques have been developed to limit the immunogenicity of monoclonal antibodies, such as “humanization”. This involves replacement of murine framework sequences around the CDR with human framework sequences. Certolizumab has been developed using this technique. It consists of only the Fab portion (50 kD) of a monoclonal antibody directed against TNF-α, with humanized framework sequences and a 2 × 20 kD pegol domain. The resulting molecule contains only the smallest effective antigen-binding part of the monoclonal antibody.Citation14
Complement or antibody-dependent cell-mediated cytotoxicity, which has been observed in vitro with adalimumab, etanercept, and infliximab,Citation14 was not seen in certolizumab as it lacks an Fc region. Certolizumab pegol binds to TNF and prevents its interaction with specific receptors, thus neutralizing it. Certolizumab pegol has been demonstrated to be more potent at neutralizing membrane-bound TNF than etanercept and more potent at neutralizing soluble TNF than adalimumab and infliximab.Citation15 It lacks an Fc portion and is therefore unable to fix complement or to lyse cells with surface-bound TNF, in contrast to infliximab and adalimumab.Citation16 Certolizumab pegol does not bind to lymphotoxin (TNFβ), as it is derived from a monoclonal antibody, in contrast to etanercept.Citation17 It is also the only anti-TNF agent that does not kill activated lymphocytes and monocytes by apoptosis or increase levels of degranulation and necrosis of granulocytes in vitro.Citation18
Key studies of certolizumab
Phase II studies
The first Phase II study was published in 2002.Citation18 It was a double-blinded, randomized, placebo-controlled trial; 36 patients were randomized into two groups. One group received a single intravenous infusion of placebo and the other group received a single ascending dose of 1, 5, or 20 mg/kg of certoilizumab.
The trial studied patients with severe active disease who had more than three swollen and six tender joints and an erythrocyte sedimentation rate (ESR) of >28 mm/hour. The response was measured according to ACR response criteria where an ACR20 indicated a 20% clinical improvement from baseline after treatment and an ACR50 and ACR70 indicated a 50% or 70% improvement, respectively. An ACR20 was designed to show difference between drug and placebo, while ACR50 and ACR70 responses were clinically meaningful to patients.Citation19
The study showed a dose dependent response, the 1 mg/kg dose being no better than placebo, but there was a significant response at the higher doses. The 20 mg/kg dose showed no clear benefit over the 5 mg/kg dose in the ACR20 response (75% vs 75%, respectively, at 8 weeks), but did show an increase in the number of patients achieving an ACR50 (50% and 13% respectively at 8 weeks). The treatment was well tolerated, with no infusion-related reactions.Citation19
A second Phase II study was of subcutaneous certolizumab. It was a randomized, double-blind, placebo-controlled trial. Patients were given 50, 100, 200, or 400 mg of certolizumab or placebo subcutaneously every 4 weeks for 12 weeks. The response was measured using the ACR criteria. Patients receiving 400 mg achieved an ACR20 of 60%, ACR50 of 40%, and ACR70 of 29% at 12 weeks which showed clear dose response. Patients receiving 400 mg also had an improvement in their health-related quality of life and the drug was well tolerated.Citation19,Citation20
Key Phase III trials
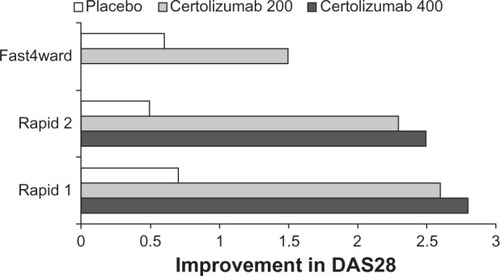
The key Phase III trials comprised RAPID 1,Citation21 RAPID 2,Citation22 and FAST4WARD.Citation23 They explored the use of certolizumab as a monotherapy and also in combination with methotrexate using various dose regimes ().
Table 1 Key trials of certolizumab
RAPID 1 (Rheumatoid Arthritis Prevention of Structural Damage 1) evaluated the efficacy and safety of two doses of certolizumab with methotrexate, or placebo with methotrexate in patients with active rheumatoid arthritis and an inadequate response to methotrexate alone. This 52-week trial involved 982 patients who were randomized to receive subcutaneous certolizumab at an initial dosage of 400 mg given at weeks 0, 2, and 4, with a subsequent dose of 200 mg or 400 mg given every 2 weeks together with methotrexate, or placebo plus methotrexate. The entry criteria comprised nine or more tender joints, nine or more swollen joints, and an ESR of 30 mm/hour or more or C-reactive protein (CRP) of 1.5 mg/dL or more. Disease duration was from 6 months to 15 years ().
At week 24, ACR20 response rates for the certolizumab 200-mg and 400-mg groups were 59% and 61% respectively compared with 14% for the placebo group (P < 0.001; ). Differences in ACR20 response rates compared with placebo were sustained until 52 weeks (P < 0.001).
Table 2 American College of Rheumatology responders in key trials
RAPID 2 was another double-blind, randomized, placebo-controlled study. It enrolled 619 patients with active rheumatoid arthritis who had inadequate responses to methotrexate therapy (). The patients were randomized 2:2:1 to receive certolizumab 200 mg with methotrexate (n = 246), certolizumab 400 mg with methotrexate (n = 246), or placebo with methotrexate (n = 127) every 2 weeks for 24 weeks. The primary outcome measure was ACR 20 response at week 24 (). The ACR20 response at week 24 was achieved in 57%, 58%, and 9% of patients on certolizumab 200 mg, 400 mg, and placebo respectively (P ≤ 0.001; ).
Treatment with certolizumab plus methotrexate was also associated with significant improvement in Disease Activity Score (DAS)28 (ESR) from baseline vs placebo. At week 24 the mean changes were 200 mg −2.27 (SD 1.38), 400 mg, −2.46 (SD 1.31) and placebo −0.50 (SD 1.05). DAS28 remission (with DAS28 scores under 2.6) was seen in 9% of patients treated with certolizumab 200 mg or 400 mg respectively at week 24, compared with 1% of patients in the placebo group (P < 0.05; ).
The final trial, FAST4WARD, was a 24-week, randomized, double-blind, placebo-controlled study evaluating certolizumab as monotherapy in 220 patients who had previously failed one or more DMARDs. Patients were aged 18 to 75 years, and had adult onset rheumatoid arthritis by the 1987 ACR criteriaCitation24 of >6 months duration. Disease duration was from 6 months to 15 years. Disease activity entry criteria were the same as RAPID 1 and RAPID 2 ().
Patients were randomized to receive subcutaneous certolizumab 400 mg (n = 111) or placebo (n = 109) every 4 weeks. The primary end-point was ACR20 response rates at week 24. This was achieved by 46% of the certolizumab group and 9% of the placebo group (P < 0.001). ACR50 and ACR70 at week 24, using non-responder imputation, were significantly higher for certolizumab than placebo (23% vs 4%, P < 0.001 and 6% vs none, P < 0.05 respectively; ).
Effect on function
At week 24 significantly more patients in the certolizumab treatment groups reported improvements in patient reported outcomes (PROs) including fatigue measured by the Fatigue Assessment Scale (FAS), arthritis pain measured on a visual analog scale (VAS) and physical function measured using the Health Assessment Questionnaire (HAQ).Citation25 The beneficial effects of certolizumab were similar between the 200 mg and 400 mg dose levels with no significant difference between treatment groups in any PROsCitation25 ().
Table 3 Changes in fatigue, pain, and disability in key trials
In addition at week 24 63% of certolizumab 200 mg treated patients reported clinically meaningful improvements in one or more PROs compared with 13% in placebo groups. Approximately 23% of all certolizumab-treated patients reported clinically meaningful improvements in all three PROs compared with 3% in placebo groups.Citation23
Significant reductions in fatigue were reported by patients receiving certolizumab monotherapy compared with placebo at Week 1 (P < 0.01) and were sustained to week 24 (P < 0.001; ). Meaningful reductions in fatigue were reported by 46% of certolizumab patients compared with 17% of placebo patients (P < 0.001).
Patients treated with certolizumab 400 mg monotherapy reported statistically significant improvements in physical function assessed by HAQ compared with patients receiving placebo from week 1 (−0.23 vs 0.04, respectively) through week 24 (−0.36 vs 0.13; P < 0.001 for both time points) (). By study end point at week 24, 49% of patients receiving certolizumab reported clinically meaningful improvements in physical function compared with 12% of those receiving placebo (P < 0.001).
Efficacy of certolizumab in systematic reviews
A Cochrane review published in 2009 assessed the effectiveness and safety of certolizumab in patients with rheumatoid arthritis who had not responded well to conventional DMARDs.Citation26 It evaluated randomized controlled trials that compared certolizumab with placebo or methotrexate in adult patients with active rheumatoid arthritis despite current or prior treatment with conventional DMARDs, such as methotrexate.
Five trials were included. The systematic review analyzed 2394 people for effectiveness. The duration of follow-up was from 12 to 52 weeks, and the doses of certolizumab pegol ranged from 50 to 400 mg subcutaneously. In three trials the control was placebo plus methotrexateCitation21–Citation23 and in two trials it was just placebo.Citation19,Citation20
Significant improvements were observed at 24 weeks with the approved dose of 200 mg certolizumab. ACR50 risk ratios (RR) were 6.01 (95% CI: 3.84–9.40) with an absolute benefit of 29% (95% CI: 25%–34%). The number needed to treat to obtain benefit (NNTB) was 4 (95% CI: 3–5) and the HAQ mean difference was −0.39 (95% CI: −0.45– −0.32).
At 52 weeks the results were quite similar: ACR50 RRs were 5.27 (95% CI: 3.19–8.71), HAQ mean difference −0.42 (95% CI: −0.52– −0.32).
A comparative meta-analysis against other anti-cytokine agents has been undertaken by Launois et al.Citation27 In their meta-analysis, using the random-effects model which takes heterogeneity into account, certolizumab showed the highest odds ratio (OR) for ACR20 responses (OR: 11.82, 95% CI 5.98–21.71). Certolizumab also had the highest OR for ACR50 and ACR70 responses. In the main, the differences in ORs between different anti-cytokine treatments did not achieve statistical significance, but are an interesting finding, which might represent either a treatment benefit, trial design, or both.
Effects on erosive damage
Radiographic damage is believed to start early in the disease course of rheumatoid arthritisCitation28 and therefore early intervention is vital.Citation29 The widespread use of biologics has necessitated improved outcome measures. TNF inhibitors have changed the therapeutic goals in rheumatoid arthritis not only by relieving signs and symptoms but also by inducing remission. The value of structural damage and physical function as outcome measures is increasingly recognized, since damage is related to function, which affects quality of life.Citation30 Radiographic damage in patients with rheumatoid arthritis has become an important outcome measure in clinical trials. An association between disease activity and radiographic damage has been demonstrated in several studies.Citation31–Citation33 Radiographs of hands and feet are easy and inexpensive to perform, and standard methodologies are available to measure progression. They are assessed using the Sharp/van der Heijde methodCitation34 or the Larsen method,Citation35 which quantifies erosions and joint space narrowing. RAPID 1 evaluated the efficacy and safety of certolizumab in combination with methotrexate. A decrease in radiographic progression was evident at 52 weeks for both dosage regimens, despite the fact that 60% of the patients in the placebo group withdrew at week 16 and entered the open-label certolizumab treatment.Citation22
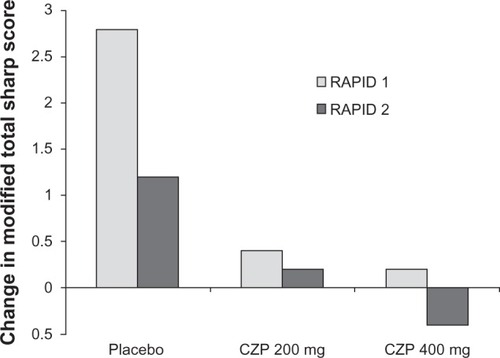
RAPID 2 followed the same dosage regimen as RAPID 1, but the primary end-point was ACR20 at week 24 in a population of 619 patients with rheumatoid arthritis. Radiographic findings in RAPID 2 showed that in patients treated with certolizumab 200 mg the mean changes from baseline in the van der Heijde modified Total Sharpe Score at week 24 in the 200 mg certolizumab, 400 mg certolizumab, and placebo plus methotrexate groups were 0.2, −0.4, and 1.2 respectively. Changes on certolizumab treatment were both significantly less than those with placebo and methotrexate (P < 0.01).
Certolizumab plus methotrexate therapy inhibited the progression of structural damage to a greater extent than placebo plus methotrexate therapy. At week 52, mean radiographic progression from baseline was reduced in patients treated with certolizumab 200 mg (0.4 Sharp units) or 400 mg (0.2 Sharp units) compared with that in placebo-treated patients (2.8 Sharp units) (P < 0.001; ). The trial showed that the drug had slowed mean radiographic progression from baseline by week 52 and improved physical function as early as week 1.
Economic considerations
Kavanaugh et al undertook an evaluation of the economic outcomes in the RAPID 1 and RAPID 2 trials.Citation36 At baseline 40% to 42% of patients were employed outside the home. Certolizumab significantly reduced work absenteeism and presenteeism among patients working outside the home compared with placebo. It also significantly reduced the number of household days lost, household days with productivity reduced by 50% or more, and lost participation in family, social, and leisure activities. Improvements were seen by 4 weeks and continued for up to 12 months.
Detailed cost-effective analyses have been undertaken in the UK in research commissioned by the National Institute For Health and Clinical Excellence.Citation37 As a number of different analytical approaches have been used there is a range of different conclusions, depending on the assumptions made in the various models. The most useful analysis is probably the probabilistic sensitivity analysis. This showed that the probability of certolizumab pegol with methotrexate being cost effective at a willingness-to-pay threshold of £20,000 per quality adjusted life-year gained was 49%. The probability of certolizumab monotherapy being cost effective at £20,000 per quality adjusted life-year gained was 46%.Citation37
Adverse events
The adverse events reported in the key clinical trialsCitation21–Citation23 are summarized in . The majority of adverse events were mild or moderate. Over half the patients in both treatment and placebo groups reported one or more adverse event. However, serious adverse events were seen in about 3% of placebo-treated patients and about 7% of patients receiving different doses of certolizumab.
Table 4 Adverse effects in Phase III trials
Adverse events reported in more than 5% of patients taking certolizumab included headache, nasopharyngitis, upper respiratory tract infections, diarrhea, and sinusitis.Citation20–Citation22 A range of serious adverse events were reported with certolizumab including bacterial arthritis, mastitis, benign parathyroid tumor, postural dizziness, ischemic stroke, and menorrhagia. Overall, within the limited duration of exposure in the trials, the adverse event profile for certolizumab was comparable with other TNF inhibitors.Citation21–Citation23
A systematic review of trials of certolizumab has evaluated safety in 2094 people in five trials.Citation25 Serious adverse events were more frequent for certolizumab pegol 200 mg (Peto OR: 2.02, 95% CI: 1.24–3.30). The most common adverse events with certolizumab 200 mg were upper respiratory tract infections (Peto OR: 2.21, 95% CI: 1.15–4.25); hypertension (Peto OR: 2.81, 95% CI: 1.38–5.75), and nasopharyngitis (Peto OR: 2.71, 95% CI: 1.30–5.66).
Finally, a network meta-analysis by Singh et alCitation38 compared adverse effects with a number of TNF inhibitors (etanercept, adalimumab, infliximab, golimumab, and certolizumab), interleukin (IL)-1 antagonist (anakinra), IL-6 antagonist (tocilizumab), anti-CD28 (abatacept), and anti-B cell (rituximab) therapy in patients with any disease condition except human immunodeficiency disease. This network meta- analysis used mixed-effects logistic regression, and an arm-based, random-effects model within an empirical Bayes framework. This analysis included 163 trials with 50,010 patients and 46 extension studies with a further 11,954 patients. After adjusting for dose, biologics as a group were associated with a statistically significant higher rate of total adverse events (OR: 1.19, 95% CI: 1.09–1.30). The rates of serious adverse events, serious infections, lymphoma, and congestive heart failure were not statistically different between biologics and control treatment. However, certolizumab was associated with significantly higher risk of serious infections compared with control treatment (OR: 3.51, 95% CI: 1.59–7.79).
Conclusions
There is strong evidence from large clinical trials that certolizumab combined with methotrexate or used as a monotherapy improves the signs and symptoms of rheumatoid arthritis within 1–2 weeks of treatment starting. It also inhibits the progression of structural joint damage within 4 months of treatment starting. Finally it improves physical function and reduces pain and fatigue within the first few weeks of treatment. The trials show these benefits are sustained for 12 months and it is likely that they continue beyond 1 year. Certolizumab increases the number of adverse events with statistically significant increases in the number of serious adverse events, infections, and hypertension. There are limited data about its long-term safety but currently there are no causes for concern.
The systematic reviews show some evidence that certolizumab may have both greater efficacy in terms of ACR responders and greater toxicity in terms of serious infections. There are several explanations for these two findings but the most likely one is differences in trial design and patients studied. The differences are small and the balance of evidence suggests that the five available TNF inhibitors are similar with no clear reason to choose one over another.Citation39 However, there are several reasons why it is advantageous to have several TNF inhibitors available. Firstly it increases patient choice, as the frequency and route of administration varies. Secondly, as intolerance and antibody development against existing biologics is not uncommon, it offers opportunity to switch from one TNF inhibitor to another. There is evidence that this is useful when patients have adverse effects or treatment failure.Citation40–Citation42
Certolizumab has structural characteristics which may theoretically confer advantages. Since it consists only of the Fab component of an antibody and lacks the Fc portion, it cannot fix complement or lyse cells with surface-bound TNF-α, unlike infliximab and adalimumab.Citation12 It is also the only TNF inhibitor that does not induce apoptosis of activated lymphocytes and monocytes or degranulation and loss of neutrophil viability in vitro.Citation43,Citation44 The Fab component is bound to a PEG moiety, which increases its half-life and may reduce the likelihood of anti-drug antibody formation compared with conventional chimeric monoclonal antibodies.Citation43,Citation44 Reduced immunogenicity, due to humanization and PEGylation, may result in a decreased likelihood of severe allergic reactions, and also reduced development of neutralizing antibodies.Citation44
It is too early to say how certolizumab will eventually be used to treat rheumatoid arthritis. We believe there is a place for another TNF inhibitor, and have presented the arguments in its favor. However, there is a diversity of opinion with some commentators taking more negative views.Citation45 While there is no evidence that certolizumab is markedly more effective than other TNF inhibitors, the presence of several effective agents is likely to focus attention on their relative costs; and continuing downward pressures on their costs should have substantial positive benefits on their relative cost-effectiveness.
Disclosures
Abdul Khan has received no external grants or personal funding from any industrial sponsor. David L Scott receives grant funding support from Arthritis Research UK and The National Institute for Health Research. In the past 3 years Professor Scott has received no personal funding from any industrial sponsor.
References
- KveinTKEpidemiology and burden and illness of rheumatoid arthritisPharmacoeconomics2004222 Suppl 1112
- ScottDLWolfeFHuizingaTWRheumatoid arthritisLancet20103761094110820870100
- BathonJMMartinRWFleischmannRMA comparison of etanercept and methotrexatein patients with early rheumatoid arthritisN Engl J Med20003431586159311096165
- St ClairEWvan der HeijdeDMSmolenJSCombination of infliximab and methotrexate therapy for early rheumatoid arthritisArthritis Rheum2004503432344315529377
- BreedveldFCWeismanMHKavanaughAFA multicenter, randomised, double-blind clinical trial of combination therapy with adalimumabplus methotrexate versus methotrexate alone or adali-mumab alone in patients with early, aggressive rheumatoid arthritis who had not had previous methotrexate treatmentArthritis Rheum200654263716385520
- SaagKGTengGGPatkarNMAmerican College of Rheumatology 2008 recommendations for the use of nonbiologic and biologic disease-modifying antirheumatic drugs in rheumatoid arthritisArthritis Rheum20085976278418512708
- DeightonCO’MahonyRToshJTurnerCRudolfMGuideline Development GroupManagement of rheumatoid arthritis: summary of NICE guidanceBMJ2009338702
- ToshJCWailooAJScottDLDeightonCMCost-effectiveness of combination nonbiologic disease-modifying antirheumatic drug strategies in patients with early rheumatoid arthritisJ Rheumatol2011381593160021572149
- SfikakisPPThe first decade of biologic TNF antagonists in clinical practice: lessons learned, unresolved issues and future directionsCurr Dir Autoimmun20101118021020173395
- ScallonBJMooreMATrinhHChimeric anti-TNF-alpha monoclonal antibody cA2 binds recombinant transmembrane TNF-alpha and activates immune effector functionsCytokine199572512597640345
- MeasePAdalimumab in the treatment of arthritisTher Clin Risk Manag2007313314818360621
- DanilaMIHughesLBBridgesSLPharmacogenetics of etanercept in rheumatoid arthritisPharmacogenomics200891011101518681777
- WongMZiringDKorinDTNFα blockade in human diseases: mechanisms and future directionsClin Immunol200812612113617916444
- NesbittAFossatiGBerginMMechanism of action of certolizumab pegol (CDP870): in vitro comparison with other anti-tumor necrosis factor alpha agentsInflamm Bowel Dis2007131323133217636564
- GramlickAFossatiGHenryAAssessment of the affinity for soluble TNF and the neutralising potency against soluble and membrane TNF of the anti-TNF agents certolizumab pegol, adalimumab, etanercept and infliximabAnn Rheum Dis200665456
- FossatiGNesbittAIn vitro complement-dependent cytotoxicity and antibody-dependent cellular cytotoxicity by the anti-TNF agents certolizumab pegol, adalimumab, etanercept and infliximabAnn Rheum Dis200665455
- MpofuSFatimaFMootsRJAnti-TNF-alpha therapies: they are all the same (aren’t they?)Rheumatology20054427127315561736
- FossatiGNesbittAEffect of the anti-TNF agents certolizumab pegol, adalimumab, etanercept and infliximab on levels of apoptosis in activated peripheral blood lymphocytes and monocytes and on necrosis and degranulation of peripheral blood granulocytesAnn Rheum Dis200665622
- ChoyEHHazlemanBSmithMEfficacy of a novel PEGylated humanized anti-TNF fragment (CDP870) in patients with rheumatoid arthritis: a phase II double-blinded, randomized, dose-escalating trialRheumatology2002411133113712364632
- KaushikVMootsRCDP-870 (certolizumab) in rheumatoid arthritisExpert Opin Biol Ther2005560160615934837
- KeystoneEHeijdeDMasonDJrCertolizumab pegol plus methotrexate is significantly more effective than placebo plus methotrexate in active rheumatoid arthritis: findings of a fifty-two-week, phase III, multicenter, randomized, double-blind, placebo-controlled, parallel-group studyArthritis Rheum2008583319332918975346
- SmolenJLandeweRBMeasePEfficacy and safety of certolizumab pegol plus methotrexate in active rheumatoid arthritis: the RAPID 2 study. A randomised controlled trialAnn Rheum Dis20096879780419015207
- FleischmannRVencovskyJvan VollenhovenREfficacy and safety of certolizumab pegol monotherapy every 4 weeks in patients with rheumatoid arthritis failing previous disease-modifying antirheumatic therapy: the FAST4WARD studyAnn Rheum Dis20096880581119015206
- ArnettFEdworthySBlochDThe American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritisArthritis Rheum1988313153243358796
- StrandVSmolenJVollenhovenRCertolizumab pegol plus methotrexate provides broad relief from the burden of rheumatoid arthritis: analysis of patient-reported outcomes from the RAPID 2 trialAnn Rheum Dis201170996100221415050
- Ruiz GarciaVJobanputraPBurlsACertolizumab pegol (CDP870) for rheumatoid arthritis in adultsCochrane Database Syst Rev20112CD00764921328299
- LaunoisRAvouacBBerenbaumFComparison of certolizumab pegol with other anticytokine agents for treatment of rheumatoid arthritis: a multiple-treatment Bayesian metaanalysisJ Rheumatol20113883584521239748
- FuchsHAKayeJJCallahanLFNanceEPPincusTEvidence of significant radiographic damage in rheumatoid arthritis within the first 2 years of diseaseJ Rheumatol1989165855912754663
- LindqvistEJonssonKSaxneTEberhardtKCourse of radiographic damage over 10 years in a cohort with early rheumatoid arthritisAnn Rheum Dis20036261161612810421
- KavanaughAHanCBalaMFunctional status and radiographic joint damage are associated with health economic outcomes in patients with rheumatoid arthritisJ Rheumatol20043184985515124242
- JansenLMvan der Horst-BruinsmaIEvan SchaardenburgDPredictors of radiographic joint damage in patients with early rheumatoid arthritisAnn Rheum Dis20016092492711557647
- LandeweRvan der HeijdeDRadiographic progression in rheumatoid arthritisClin Exp Rheumatol200523S63S6816273787
- Van der HeijdeDMvan RielPLNuver-ZwartIHEffects of hydroxychloroquine and sulphasalazine on progression of joint damage in rheumatoid arthritisLancet19891103610382565997
- Van der HeijdeDHow to read radiographs according to the Sharp/van der Heijde methodJ Rheumatol19992674374510090194
- LarsenADaleKEekMRadiographic evaluation of rheumatoid arthritis and related conditions by standard reference filmsActa Radiol Diagn (Stockh)197718481491920239
- KavanaughASmolenJSEmeryPEffect of certolizumab pegol with methotrexate on home and work place productivity and social activities in patients with active rheumatoid arthritisArthritis Rheum2009611592160019877104
- ConnockMTubeufSMalottkiKCertolizumab pegol for the treatment of rheumatoid arthritisHealth Technol Assess201014Suppl 2110
- SinghJAWellsGAChristensenRAdverse effects of biologics: a network meta-analysis and Cochrane overviewCochrane Database Syst Rev20112CD00879421328309
- ScottDLCopeANew tumour necrosis factor inhibitors for rheumatoid arthritis: are there benefits from extending choice?Ann Rheum Dis20096876776919435722
- HyrichKLLuntMWatsonKDOutcomes after switching from one anti-tumor necrosis factor alpha agent to a second anti-tumor necrosis factor alpha agent in patients with rheumatoid arthritis: results from a large UK national cohort studyArthritis Rheum200756132017195186
- HyrichKLLuntMDixonWGEffects of switching between anti-TNF therapies on HAQ response in patients who do not respond to their first anti-TNF drugRheumatology2008471000100518420660
- LloydSBujkiewiczSWailooAJSuttonAJScottDThe effectiveness of anti-TNF-alpha therapies when used sequentially in rheumatoid arthritis patients: a systematic review and meta-analysisRheumatology2010492313232120566736
- NesbittAFossatiGBrownDEffect of structure of conventional anti-TNFs and certolizumab pegol on mode of action in rheumatoid arthritisAnn Rheum Dis200766296
- BarnesTMootsRTargeting nanomedicines in the treatment of rheumatoid arthritis: focus on certolizumab pegolInt J Nanomedicine200723717722505
- Certolizumab pegol and rheumatoid arthritisJust another TNF alpha antagonist, no therapeutic advantagePrescrire Int201019279