Abstract
Rituximab (RTX) is established for the treatment of rheumatoid arthritis. This systematic review of the literature since 2006 summarizes evidence for the use of RTX in the treatment of additional rheumatological diseases: antineutrophil cytoplasmic autoantibody-associated vasculitis (AAV), hepatitis C virus-related cryoglobulinemic vasculitis, Henoch–Schönlein purpura, ankylosing spondylitis, and Raynaud’s phenomenon. Data from randomized controlled trials are available only for AAV, confirming efficacy for remission induction, including in disease resistant to conventional treatment, and maintenance of remission. Further studies are required to confirm optimal maintenance regimens in AAV, important questions needing to be addressed including protocol administration versus treatment in response to clinical relapse and the importance of maintaining B-cell depletion. Sufficient data are available in other diseases to suggest RTX to be useful and that randomized controlled trials should be conducted.
Introduction
Antineutrophil cytoplasmic autoantibody (ANCA)-associated vasculitis (AAV), hepatitis C virus (HCV)-related cryoglobulinemic vasculitis (HCVrCV), Henoch–Schönlein purpura (HSP), ankylosing spondylitis (AS), and Raynaud’s phenomenon (RP) are all inflammatory conditions that may present challenges in management, due to failure/intolerance of or contraindications to first-line treatment.
The introduction of cyclophosphamide (CYC) for induction of AAV remission improved remission rates to >90% and reduced mortality dramatically.Citation1–Citation3 CYC-, azathioprine (AZA)-, and glucocorticoid (GC)-based regimens have become standard treatment for AAV. However, nearly half of patients relapse using such regimens.Citation4,Citation5 Similarly, HCVrCV, HSP, and RP commonly present with severe refractory exacerbations that require one or more cytotoxic medications to control disease activity, with significant associated side effects.
Rituximab (RTX) is a cytotoxic monoclonal antibody that depletes B cells following binding to the B-cell-specific CD20 molecule. This reduces both antibody production and presentation of T-cell epitopes to class II major histocompatibility complex (MHC)-restricted T-helper (TH) lymphocytes, inhibiting humoral and TH-cell-dependent autoimmune responses. However, additional mechanisms of action have been suggested.Citation6 RTX has proven efficacy in the treatment of non-Hodgkin’s lymphoma, chronic lymphocytic leukemia, and rheumatoid arthritis (RA).Citation7,Citation8 RTX may have a role in the management of a wide range of chronic inflammatory conditions, in which effects on disease progression are suggested when other treatment modalities fail or are contraindicated, and it may have a role in the management of relapses.Citation9–Citation11 This systematic review addresses the use of RTX in AAV, HCVrCV, HSP, AS, and RP.
Materials and methods
Search strategy
The Medline (using PubMed), Ovid, EBSCO, Scopus, CINAHL, Trip, and Google Scholar databases were searched in October 2016, restricted to studies published in the English language. Search terms used were rituximab, treatment, remission, update, refractory, relapsing, failure, severe, AAV, AS, HCVrCV, HSP, and RP. References cited in studies identified were also retrieved, and clinical experts were consulted to identify any additional studies.
Eligibility criteria
Inclusion criteria were: patients with AAV, HCVrCV, AS, HSP, or RP; RTX as the intervention being studied; outcomes of treatment described clearly; randomized controlled trial, cohort study, case series, case report, or systematic review; articles published from 2006 to 2016 (inclusive); and studies published in the English language. Articles related to HCVrCV associated with “other” viral infections (eg, HIV or HBV) were excluded. Patients with destructive AS or who received nonsteroidal anti-inflammatory drugs (NSAIDs) at the time of the study were also excluded. Study characteristics, including RTX-treatment regimens, are summarized in .
Table 1 Summary of publications describing use of RTX in vasculitides
Screening and data extraction
Two independent reviewers screened retrieved reports by review of titles and abstracts. The full texts of potentially relevant reports were retrieved for detailed review. After the second screening, the reviewers considered additional references cited in the retrieved studies. Once a complete list of included studies had been established, the reviewers extracted study-level characteristics (authors, year of publication, geographical location, and number of patients), patient-level characteristics (type of condition, previous treatment, age, sex ratio, number of relapsed cases, duration of relapse, possible factors leading to relapse), intervention/exposure-level characteristics (regimen, dose, duration), and outcome-level characteristics (response, adverse events, and reported side effects).
Rituximab in other rheumatological diseases
Additional rheumatological diseases that RTX may have shown efficacy against include rheumatological diseases resistant to known therapies, primary Sjögren’s syndrome, scleroderma with myositis, and Behçet’s disease. The authors believe that many of these review areas merit separate research. To this extent, the authors are conducting a systematic review and meta-analysis of RTX in resistant lupus cases, as well as another separate analysis of RTX in scleroderma with myositis along with Behçet’s disease, and the authors believe that no new evidence has emerged since a review was conducted in 2017 on the safety of biological disease-modifying antirheumatic drugs modulating B cells in primary Sjögren’s syndrome.Citation12
Assessment of the quality of included studies
Reviewers documented the type of study, the journal, whether data collection was prospective, sequential patient enrolment, the selected intervention, and the duration of follow-up. Assessors were blinded to study author, journal, publication date, and main results. Each study was assessed for overall quality, comprehensiveness, and possible sources of bias. Independent adjudication resolved disagreements.
Statistical analysis
A narrative systematic review was prepared. Meta-analysis was not undertaken, due to heterogeneity in the design and populations of included studies.
Results
Description of included studies
A total of 875 reports were retrieved from the literature search, manual search of cited references, and suggestions by clinical experts. After the first screening process, a total of 145 studies were deemed potentially relevant. Of these, 50 publications were included in this systematic review. There were 21 studies recruiting patients with AAV (including two long-term follow-up studies of patients previously reported)Citation10,Citation19 and two additional studies reporting organ-specific outcomes in AAV.Citation20,Citation21 Three additional studies reported use of RTX in eosinophilic granulomatosis with polyangiitis (EGPA), and two reported use in HSP. We identified ten studies describing RTX use in HCVrCV, seven in AS, and four in RP. A Cochrane review of treatment of AAV published in 2015Citation4 was also considered. A flowchart describing study selection is shown in . Study design for selected studies is documented in and .
Figure 1 Search strategy and articles excluded and selected.
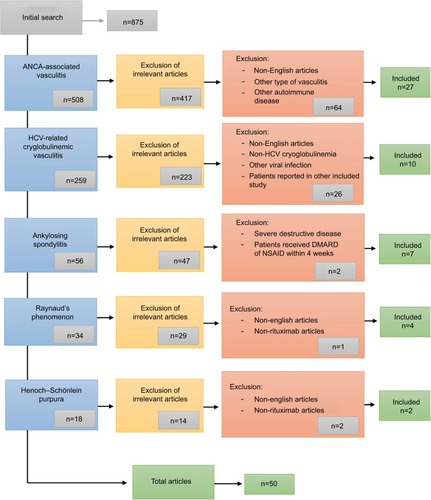
Table 2 Summary of publications describing use of RTX in nonvasculitic musculoskeletal disease
ANCA-associated vasculitis
Between 2001 and 2006, there were a number of case reports and case series and three prospective open-label studies suggesting RTX to be effective in induction of remission in AAV (predominantly AAV with granuloma formation). These cases were largely patients with treatment failure on conventional treatment, and the open-label studies reported conflicting results.Citation22 However, RTX was suggested to be effective in the treatment of AAV. The published literature since these initial studies has been reviewed, seeking clarity with regard to the efficacy of RTX in AAV, in particular given the publication of results from three prospective randomized controlled trials. We identified 22 articles addressing the role of RTX in three different clinical settings: the induction of remission, the maintenance of remission, and the use of RTX for induction and maintenance of remission in the same patient, including one Cochrane review. Two of these articles described long-term follow-up of patients already described and subgroup analyses of these studies. Two additional studies described the effect of RTX treatment on specific manifestations of AAV (scleritis and pulmonary granulomatous disease). Three articles were identified describing the use of RTX for treatment of EGPA. Finally, one case series and one case report described the use of RTX in four patients with HSP.
These publications described RTX use for de novo induction of remission and as “rescue therapy” in patients in whom conventional treatment had failed to induce remission or in whom relapse was seen. RTX was also used in small numbers of patients in whom conventional therapy (most often CYC) was contraindicated. Overall, RTX was shown to be effective in all settings in AAV. These studies and the RTX regimens used are summarized in .
Induction
The RAVECitation11 and RITUXVASCitation19,Citation23 studies demonstrated the efficacy of RTX in remission induction in AAV.Citation4 RAVE confirmed RTX (375 mg/m2 weekly ×4) remission induction to be noninferior to conventional CYC treatment in a multicenter randomized double-blind placebo-controlled study (remission achieved in 64% vs 53%, P=0.09). In this study, 48% of RTX and 49% of control patients were newly diagnosed. Of patients treated for relapse, 82% receiving RTX and 74% of controls had previously received CYC. RITUXVAS demonstrated RTX (375 mg/m2 weekly ×4) plus two pulses of intravenous CYC not to be superior to conventional 3- to 6-month intravenous CYC for remission induction in AAV, with outcomes as good following RTX induction through to 2 years’ follow-up.Citation19 In contrast to the RAVE study, all patients in this study were newly diagnosed, and all had renal involvement. In RAVE, RTX induction was followed by withdrawal of GCs by ~6 months, with no additional maintenance immunosuppression. This was central to study design.Citation10,Citation11,Citation24
In RITUXVAS, patients receiving RTX (plus two doses of CYC) induction continued with a low-dose GC (5 mg prednisolone). In RAVE and RITUXVAS control arms, patients received conventional CYC continued with oral AZA maintenance. Subgroup analysis of the RAVE cohort indicated that RTX appeared to be equally effective as CYC in those patients with glomerulonephritis or pulmonary hemorrhage.Citation24 Longer follow-up (18 months) of the RAVE cohort confirmed that the initial single course of RTX was as effective for maintenance of remission as CYC induction plus AZA maintenance.Citation10 Subgroup analysis demonstrated treatment specifically for renal involvement to be associated with equivalent outcomes in the RTX and CYC arms.Citation25 Comparison of these studies, in addition to differences in induction regimen, shows that RAVE and RITUXVAS recruited different patient groups, with RITUXVAS confined to de novo diseaseCitation23 and RAVE including slightly over 50% of patients treated for relapse following “conventional” de novo disease induction.Citation4,Citation11
A number of studies have reported specifically on treatment of refractory disease with RTX. A report of seven Japanese patients with AAV refractory to treatment with CYC demonstrated the induction of remission using RTX in six, with one death.Citation26 These patients did not receive planned maintenance, with relapse being seen in five of the six surviving patients. Wendt et alCitation27 similarly reported good outcomes using RTX for treatment of patients with “difficult” refractory or relapsing disease, although one patient died in this series. Also, de Menthon et alCitation28 reported RTX to be effective at achieving remission in five of eight patients with disease refractory to conventional treatment in a study comparing infliximab and RTX. RTX achieved remission in 14 of 15 patients with refractory disease reported by Lovric et al.Citation29 These authors sounded a note of caution with regard to complications, as one patient died of pneumonia and a further patient experienced reactivation of hepatitis B and died following refusal of dialysis. Subgroup analysis in the RAVE study confirmed RTX to be as effective in de novo as in refractory/relapsing disease.Citation11,Citation24 Cartin-Ceba et alCitation30 reported retrospectively on the use of RTX for induction of remission in a cohort of 53 patients with refractory AAV at the Mayo Clinic. In contrast to conventional findings, this group reported increasing PR3 titers to be predictive of relapse, suggesting this to be an indication for retreatment. Furthermore, they only saw relapse following return of peripheral blood B cells.
Maintenance
The efficacy of RTX for maintenance has also been studied. In keeping with the report from the Mayo Clinic,Citation30 the RITUXVAS study team commented that relapses seen following RTX induction all occurred in patients in whom B-cell recovery had occurred.Citation19 Numbers were small (n=2), and as 85% of patients had B-cell recovery by study end, this may not be a significant observation. However, given that relapse is seen in ~20% of patients receiving both conventional immunosuppressive regimens and RTX induction regimens for AAV, investigation of optimal maintenance therapy is of interest. Many of the studies reporting use of RTX for induction also comment on treatment of relapses. A number of studies have focused specifically on maintenance treatment. Rhee et alCitation31 reported successful use of RTX for maintenance of remission in 39 patients with AAV (1 g 4-monthly in 33 of 39 patients, minor variations to dosing in remaining six) in a retrospective study. Roubaud-Baudron et alCitation32 reported retrospectively specifically on the efficacy of a variety of RTX maintenance regimens () in AAV (GPA, n=24; microscopic PA, n=4). A total of 21 of these patients had received RTX for initial disease treatment also and received RTX maintenance as part of a continuing RTX treatment protocol. Six of the remaining seven patients received RTX maintenance due to intolerance or lack of efficacy of AZA/mycophenolate mofetil regimens, and one received RTX maintenance due to renal insufficiency. Of 28 patients, 17 maintained complete remission and 11 partial remission at a median follow-up of 38 months. Of the 17 patients achieving complete remission, 11 had irreversible organ damage, including 6 with end-stage renal disease.
The MAINRITSAN study subsequently confirmed RTX to be superior to AZA for maintenance of remission in AAV.Citation33 In this randomized, unblinded, controlled trial, 118 patients received either AZA or RTX after remission induction with CYC and GC. Major relapse occurred in 29% of patients in the AZA group compared with 5% in the RTX group. The frequency of severe adverse events was similar in the two groups. Smith et alCitation34 compared three different RTX maintenance regimens in a retrospective cohort study recruiting 73 patients. Group A received induction followed by RTX treatment only in response to relapses. Following induction of remission, group B were given planned maintenance RTX every 6 months. Group C included relapsed patients who were then given regular maintenance treatment. Complete/partial remission occurred in 93% of group A, 96% of group B, and 95% of group C. Relapse rates after 2 years of treatment were 73%, 12%, and 11% in each group, respectively, and at last follow-up (median 44 months) relapse rates were 85%, 26%, and 56%, respectively, for the three groups.
Induction and maintenance
With demonstrated efficacy of RTX at induction of remission and superiority compared to AZA in remission maintenance in patients receiving conventional CYC-based induction, the remaining question is whether RTX used for induction and maintenance in the same patient is superior to either of these two strategies. Awad et al reported successful use of RTX for induction and maintenance of remission in a 57-year-old female patient.Citation35 Further retrospective studies reported outcomes in 17Citation36 and 66Citation37 patients, demonstrating RTX to be effective and safe for use in both remission induction and maintenance therapy in GPA. In the first study, Moog et alCitation36 administered relatively low-dose RTX (375 mg/m2) for induction and maintenance. In the second study,Citation37 59 of the patients reported had received previous CYC. The maintenance dose of RTX was relatively low in this study also (500 mg every 6 months). At the 18-month follow-up, the incidence of severe infections was 13.6%. In a multicenter retrospective study of RTX treatment for AAV, low-dose RTX was shown to induce and maintain remission effectively, with a significantly lower relapse rate than conventional treatment.Citation38 This study sounded a note of caution also with regard to complication rates, with 22 documented side effects (predominantly infectious) and 4 deaths. A long-term follow-up of 69 patients who received repeated RTX as maintenance therapy following RTX induction supported the efficacy and safety of a fixed-interval regimen. A total of 67 of these patients were failing other therapies. Patients received maintenance for 2 years, with 29 patients relapsing a median of 34 months after the last RTX infusion and 9% of patients needing additional immunosuppression.Citation37 A single-center retrospective analysis of 172 patients with AAV who underwent RTX induction and maintenance therapy demonstrated major relapse in only 5%, associated with weaning of additional immunosuppressive agents.Citation39 This was significantly lower than the 20% remission reported using conventional CYC- and AZA-based regimens or RTX induction, and is equivalent to the 5% relapse reported with RTX use for maintenance following CYC remission induction in the MAINRITSAN study.Citation33 Pendergraft et alCitation39 administered higher doses of RTX with routine 4-monthly 1 g maintenance doses for up to 7 years, specifically aiming to maintain B-cell depletion throughout, in part addressing the comment in the RITUXVAS study that B-cell return was associated with relapse. The dose interval was shortened to 3 months if B-cell return was seen. A total of 25 serious infections were reported, and 17 patients developed late-onset neutropenia, 13 requiring treatment with granulocyte colony-stimulating factor. Neutropenia recovered, and RTX was continued in all patients.
A report of complication rates in 35 patients receiving RTX induction and maintenance for a median of 44 months (2–88 months) is of note.Citation40 Following induction with RTX 1 g ×2 2 weeks apart, further maintenance doses of 2 g were given annually: 37% of patients discontinued RTX, mainly due to hypogammaglobulinemia, and 26% of patients developed a severe infection.Citation40 A high dose of concomitant CYC treatment was suggested to be a significant risk factor for severe infection. This study used higher doses of RTX than other studies already discussed, where regular maintenance doses were administered.
Considering specific organ involvement, as discussed, the RAVE and RITUXVAS studies confirmed RTX to be effective in patients with renal disease.Citation11,Citation19,Citation23 In a retrospective analysis of eight patients, RTX (1 g ×2 2 weeks apart) was useful in the treatment of refractory necrotizing scleritis in patients with GPA.Citation21 A case report of a patient with AAV of high disease activity demonstrated the efficacy of RTX in lung disorders, but no significant remission of granulomatous infiltration in the orbits was seen.Citation41 The Japanese study already referred toCitation26 demonstrated resolution of granulomatous orbital involvement in one patient. A further report of five cases of GPA with persistent pulmonary lesionsCitation20 demonstrated RTX to be effective at improving radiological appearances of pulmonary disease. These authors reported readministration of RTX if necessary to maintain B-cell depletion up to 18 months’ follow-up.
RTX has also been used successfully for treatment of EGPA refractory to GC, CYC, and intravenous Ig.Citation42 A retrospective study of nine patients treated with RTX for relapsing or refractory disease further suggested the efficacy in EGPA.Citation43 Mohammad et al reported retrospectively on 41 patients (36 with relapsing or refractory disease and five with new presentations) from four centers treated effectively with RTX.Citation44
HSP is a further clinical variant of small-vessel vasculitis, in which RTX has been suggested to be effective. The vast majority of patients with HSP recover spontaneously, but about a third have recurrent disease episodes.Citation45 The recurrence rate is higher in those with severe disease and renal involvement. Conventional treatment for recurrent disease is GCs and CYC.Citation46 RTX has been noted to be a successful treatment for severe refractory chronic HSP in three pediatric patients. All three patients responded to one or two courses of RTX, with no serious adverse events reported.Citation47 RTX has also been used successfully as de novo treatment in an adult with HSP with renal involvement ().Citation48
HCV-related cryoglobulinemic vasculitis
Ten publications describing RTX for treatment of HCVrCV were identified, describing treatment of 294 patients (). One publication described a larger studyCitation49 incorporating data from 16 patients described previously.Citation50 A total of 97 patients received RTX only, 110 patients received RTX in combination with other drugs, and 87 patients received either control or conventional treatment.
Sneller et alCitation9 randomized 24 patients with HCVrCV in whom antiviral therapy had failed to receive RTX versus “best available therapy”. Ten of 12 (83.3%) patients who received RTX achieved remission at 6 months compared to one of 12 in the control group. Of the remaining two patients, one withdrew from the study at month 5 because of a severe febrile illness and the other achieved remission at month 4, then relapsed at month 6. After 6 months of follow-up, six of ten patients in whom remission was achieved were still in remission, while three of the remaining four patients experienced relapse and were treated with a second course of RTX. These patients achieved and remained in remission for more than 6 months after the second course. One patient was lost to follow-up at month 7.
In a similar study, De Vita et alCitation51 randomized 59 patients with CV to receive RTX versus conventional treatment, data on 57 patients being available for analysis. Although HCV infection was not an inclusion criterion, 53 of 57 patients had HCV infection, in keeping with our understanding of CV. This study primarily showed good compliance with RTX treatment, confirming also noninferiority of RTX.
RTX has been shown to be more effective when combined with antiviral drugs. Terrier et alCitation52 demonstrated 80% complete remission of HCVrCV with RTX combined with polyethylene glycol (PEG)-IFNα and ribavirin compared to 57% complete remission with RTX alone. Dammacco et alCitation53 compared PEGylated IFNα + ribavirin + RTX (n=22) with PEGylated IFNα + ribavirin (n=15). In the group receiving RTX, 54.5% achieved a complete response compared to 33.3% in PEG-IFNα + ribavirin group. Five patients from the RTX group (22.7%) and five from the PEG-IFNα + ribavirin group (33.3%) achieved a partial response.Citation53 Two separate prospective studies by the same group reported complete responses to RTX treatment in 13 patients at six months with two partial responders and four patients relapsing,Citation54 and in the second publication, complete clinical response was achieved in four of five patients (80%).Citation55
RTX has also been shown to have a useful therapeutic effect in patients with liver cirrhosis due to HCV infection. Petrarca et alCitation56 reported two cases of HCVrCV with liver cirrhosis. Both cases had increased portal vein diameter on ultrasound, splenomegaly, and ascites. After RTX infusion, both patients experienced improvement in their symptoms. At the end of follow-up, their portal vein diameter had improved, close to normal, with reduction in maximum longitudinal spleen diameter, no ascites in the first case, and persistence of a very small amount of ascites in the second case. Finally, RTX in combination with PEGylated IFNα has been demonstrated to induce remission of HCVrCV in a patient with non-Hodgkin’s lymphoma following failure of conventional treatment.Citation57
Ankylosing spondylitis
Seven publications reported use of RTX in the management of AS: five case reports, one case series, and one open-label trial (). Nocturne et alCitation58 reported data from the French autoimmunity and RTX registry on outcomes in ten patients with spondyloarthritides treated with RTX. Two were excluded because of concomitant infliximab treatment in one and treatment for vasculitis with no effect on concomitant AS in the other. Of the remaining eight patients, three had AS, two had undifferentiated spondyloarthritides, and three had psoriatic arthritis. Seven of the eight patients had previously received anti-TNF treatment. All received RTX 1 g ×2 2 weeks apart. Five patients also received methotrexate. Clinicians reported a response to RTX in only two of these patients. A further open-label study reported greater efficacy of RTX.Citation59 In this study, 20 patients received RTX (1 g ×2 2 weeks apart), ten of whom were anti-TNF-naïve. All patients had active disease, as defined by a Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) score ≥4, with response defined as a 20% reduction in disease activity. No patients had received immunosuppression within 4 weeks of RTX administration. A clinical response to RTX occurred as early as 4–8 weeks after the first RTX infusion; 90% of the naïve group responded to treatment. In contrast, at best only a partial response was seen in 30% of the group that had failed anti-TNF treatment.
The five case reports supported the efficacy of RTX in AS. The commonest regimen used was 1 g ×2 2 weeks apart. Kobak et al reported this regimen to achieve a partial response in a patient who had failed with disease-modifying antirheumatic drugs and anti-TNF treatment, as evidenced by improvement in acute-phase reactants and magnetic resonance imaging. No BASDAI score was recorded.Citation60 A further case report documented a 28-year-old female HBV-positive patient with AS and peripheral arthritis who responded to RTX. She had failed on NSAID and methotrexate treatment and refused anti-TNF treatment, fearing TB reactivation. Lamivudine was given with RTX. There was a dramatic improvement in her arthritis, without reactivation of her HBV infection.Citation61 A further case of axial AS that had failed to improve with NSAID improved significantly with RTX, with resolution of sacroiliac joint edema.Citation62 A lower RTX dose (500 mg ×2) was used to treat this patient. Finally, a case of AS complicated by demyelinating disease due to anti-TNF therapy showed partial improvement after RTX administration, with resolution of neurological symptoms ().Citation63
A further case reportCitation64 documented a 38-year-old male patient with AS who initially responded to infliximab and was then given etanercept in response to a disease flare. He subsequently developed papillary thyroid cancer treated with thyroidectomy and radioiodine. RTX was given for a further disease flare not responsive to sulfasalazine and an NSAID, with good response (BASDAI score improved from 7.1 to 2.3).
Raynaud’s phenomenon
Four cases of use of RTX use in RP have been reported (). All patients were female and had mixed connective-tissue disease (MCTD). Three of four responded to RTX after having failed on a first-line immunosuppressant. In the first case, there were very high titers of ribonucleoprotein antibody that became negative after treatment, correlating with clinical improvement.Citation65 In the second case also, there was severe RP due to MCTD that started to fail to respond to regular immunosuppressive treatment. The patient began to improve after plasmapheresis with intravenous Ig, but there was complete remission after RTX administration. The patient was followed up for 1 year with no relapse.Citation66 A third case of RP with MCTD and high titers of ANA received immunosuppression and iloprost infusion, but achieved further significant clinical improvement following RTX. This patient relapsed after 10 months, despite further depletion of B cells at 2- and 6-month intervals.Citation67 The fourth caseCitation68 suffered from primary RP and had responded poorly to aspirin, nifedipine, sildenafil, GC, intravenous heparin, and intravenous iloprost. RTX resulted in an excellent clinical response. Four and a half years after initial treatment, the patient experienced a relapse, which again responded to RTX.
Discussion
Most patients with autoimmune musculoskeletal disease require immunosuppression and/or anti-TNF treatment to control disease activity and slow progression, with a wide range of adverse effects. It is thus important to address the role of alternative medication that has efficacy against these diseases, in particular if alternatives are associated with a safer side-effect profile and can be used in cases where conventional immunosuppression is contraindicated. RTX is suggested to have such benefits. RTX is an established treatment for RA. For other rheumatological diseases, its use is best supported in AAV. The earliest clinical evidence supporting RTX use in AAV came from a case study in 2001,Citation69 in which a 66-year-old man with GPA developed severe CYC-induced bone-marrow toxicity. He was thus treated with a combination of GC and RTX. After four doses of RTX, he experienced clinical remission, allowing discontinuation of GC. RTX alone was later used to treat a relapse in the same patient, thus fulfilling the requirement of efficacy with reduced side-effect profile, introducing a new line of treatment for AAV.
RTX is now established as an effective alternative to standard CYC-based treatment in induction of remission of AAV, with RCT evidence supporting use of 375 mg/m2 weekly ×4, but there is evidence for 1 g ×2 2 weeks apart, as used in RA, to be effective also (). RTX is effective in remission induction in both de novo disease and following relapse. Cases refractory to conventional treatment may respond to RTX.
The strongest evidence for remission maintenance in AAV with RTX comes from the MAINRITSAN study using 500 mg 6-monthly to month 18 (). However, uncertainty remains with regard to the optimal dose, dosing regimen, and whether long-term maintenance is needed. The role of sustained B-cell depletion also remains to be clarified. Importantly, the main benefit of RTX would seem to be avoidance of toxicity, and if prolonged B-cell depletion is needed to maintain remission, then the role of RTX in maintenance of long-term remission will need to be studied carefully in appropriately powered RCTs specifically balancing efficacy against adverse events.
RTX has also been shown to be useful in two other vasculitic diseases: EGPA and HCVrCV. In EGPA, RTX 375 mg/m2 weekly ×4 and 1 g ×2 2 weeks apart have been suggested to be effective. Although no RCTs are available, the efficacy in AAV suggests that the evidence of efficacy in EGPA (from more limited reports) is probably reliable. The use of RTX in HCVrCV is of particular interest. A dosing regimen of 1 g ×2 2 weeks apart has typically been used in this setting. Importantly, even when used alone, RTX is effective and not associated with exacerbations of viral infection. However, combination therapy of RTX with antiviral therapy would seem to be optimal and is associated with better outcomes than monotherapy with antiviral agents. Two case reports have demonstrated reduction in portal vein diameter and reduction in maximum longitudinal spleen size in patients with associated liver cirrhosis.Citation56
RTX also shows efficacy in anti-TNF-naïve patients with AS. The suggestion of a better response to RTX in anti-TNFα-naïve patients is of interest.Citation64 However, care must be taken in interpreting these data, due to the potential for selection bias. In general, patients who receive RTX having received anti-TNF treatment have failed the anti-TNF treatment. It is thus possible that the better response in anti-TNF-naïve patients is because those who have failed anti-TNF treatment are a selected population enriched with patients with more “difficult” disease. A report of a patient who had responded to anti-TNF treatment, but in whom RTX was chosen for further treatment due to the development of cancer is of interest. A good response to RTX was seen, despite this patient having received anti-TNF treatment. Therefore, anti-TNF-naïve versus anti-TNF-treated may not be the important discriminator, anti-TNF responder versus nonresponder perhaps better defining those who will and those who will not subsequently respond to RTX.
There were insufficient data in primary RP to give a conclusive result. However, a significant reduction in autoantibody titer was reported in patients who responded clinically to RTX with an increase in antibody titers in relapse. The report of a patient with primary RP responding to RTXCitation68 is of interest, although the authors noted a number of risk factors for progression to secondary RP, making long-term follow-up necessary to confirm that this is a case of primary RP.
Mechanisms of action and predictors of response
RTX binds CD20, depleting B lymphocytes, thus most obviously having an effect on antibody production. However, B lymphocytes have multiple roles in immunoresponses,Citation70 including a role as antigen-presenting cells, priming class II MHC-restricted CD4+ TH lymphocytes, in particular at low antigen levels.Citation71 These cells in turn are essential in CD8+ T-cell and macrophage-mediated (delayed-type hypersensitivity) autoimmune diseases. In the context of AAV, the relative contribution of cellular versus humoral responses in disease pathogenesis continues to be debated. However, RTX would impact on both arms of the immunoresponse through direct B-cell depletion and as a consequence an indirect effect on antigen presentation to T cells. Similar considerations may be relevant in EGPA, HCVrCV, and HSP. AS is a disease in which no role has been demonstrated for antibodies, it being mainly an inflammatory process due to CD4+ and CD8+ T lymphocytes and macrophages. Based on histopathological studies, CD3+ T cells (both CD4+ and CD8+), as well as CD20+ B cells, are seen to infiltrate cartilage and subchondral bone.Citation72 Interestingly, the number of infiltrating B cells was higher than that of infiltrating T cells.Citation73,Citation74 Therefore, the efficacy of RTX in this disease is understandable, due to its impact on T-cell priming. Inflammatory cytokines also play an important role in immunomediated disease.Citation61 RTX has also been shown to reduce the production of proinflammatory cytokines significantly, including TNFα, IL1, and IL6.Citation75
In further consideration of the role of B-cell depletion in the efficacy of RTX, whatever the role of B cells in the disease process, a number of studies have demonstrated differential depletion of B cells in different B-cell compartments. Peripheral blood B cells are very effectively depleted by RTX, as are synovial infiltrates in RA. However, depletion of germinal center B cells is less effective. It is not clear whether this is due to pharmacokinetic or biological reasons. However, immunosuppressive monoclonal antibodies (anti-MHC class II, anti-CD4, anti-CD52) penetrate germinal centers poorly in animal models (Richard Smith, personal communication), supporting a pharmacokinetic explanation.
The median half-life of RTX after completion of the first infusion is about 21 days. The infusion can be repeated after 6–12 months if the patient relapses.Citation70 Minimal data from uncontrolled trials, particularly in RA, show that the clinical effect of RTX is first evident 4–8 weeks after infusion and lasts longer than 1 month.Citation76,Citation77 Differences in the reported response to RTX may be due to differences in concomitant GC adminstration.Citation78,Citation79
RTX is effective in AAV, as reported herein. In a large European cohort with relapsing–refractory AAV, RTX was an effective treatment. Favorable prognostic factors in this cohort included kidney involvement, age >50 years, and achievement of negative ANCA following 6 months of treatment.Citation80 In HCVrCV, the combination of RTX with antiviral treatment (PEGylated IFN/ribavirin) was associated with better outcomes than the use of RTX alone.Citation50 In AS it is of note that TNFα blocker-naïve patients respond better than patients who have failed with anti-TNF therapy. The reason for this is not clear. A lack of response to anti-TNF may mark a subgroup of patients who for whatever reason are destined to respond poorly to RTX.
In RP associated with MCTD and high titers of RNP, RTX treatment was successful in patients with negative ANA, but failed in those who had positive ANA.Citation66 Some cases relapsed after weaning of other immunosuppressive agents.Citation39 Penetration of RTX into affected tissues might also play a role, as there was a case reportCitation41 showing dramatic improvement in lung symptoms, but limited improvement in orbital manifestations of disease.
Adverse reactions
RTX has been shown to be effective, with a good margin of safety in many rheumatological diseases. However, it should be noted that its safety is not yet definitively established, in particular in AAV, because of the relatively short follow-up of patients so far. Discontinuation of RTX due to toxicity has been reported. Adverse effects associated with RTX use include infusion reactions, hypogammaglobulinemia, especially after repeated courses, infections, reactivation of HBV, and late-onset neutropenia, which may manifest months after therapy and is mostly asymptomatic and reversible. In patients with AAV, infections are a major cause of morbidity and mortality, particularly during remission induction, when the most intensive immunosuppressive regimens are administered. It is likely that total immunosuppressive exposure is an important determinant of infection risk.
In AS, HBV reactivation has been reported following treatment with RTX, despite the administration of lamivudine. No hepatocyte damage was observed in this case. Upper respiratory tract infection and allergic reactions have also been reported in AS following RTX treatment, although no serious events were attributed to RTX.Citation59 Clear guidelines that minimize the use of immunosuppression may minimize toxicities. In patients who are HBV- or HCV-positive, concomitant use of antiviral agents is most probably of benefit. Finally, some studies reported hypotension during infusion, it being recommended to infuse RTX slowly and withhold antihypertensive medication at the time of RTX administration.Citation81
Suggested studies
We suggest that further randomized trials are required, in particular: 1) to define clear guidelines for use of RTX in rheumatological disease, including in particular definition of optimal maintenance regimens and whether sustained B-cell depletion is necessary; 2) to address how RTX may be used to minimize the use of anti-TNF therapies and overall immunosuppressive load; and 3) to define more clearly toxicities associated with RTX use.
Limitations of the study
Differences in inclusion and exclusion criteria among studies is a minor limitation of this study. More significant is the quality of the studies identified. Case reports have been included, as they define possible therapeutic uses of RTX. However, definitive studies to address the questions posed by these case reports are largely lacking. We feel that this makes this review particularly relevant as a documentation of these areas of interest and a stimulus to conduct definitive studies.
Also, we have included evidence from low-quality studies with regard to RP. We felt that this was appropriate to report, as higher quality studies are not available. However, this should be recognized as a research gap, and results from these studies should be interpreted with caution.
Conclusion
RTX might be revolutionary in rheumatological disease therapy, but its use is limited by lack of high-quality studies. Efficacy is suggested in AAV, HCVrCV, anti-TNF-naïve AS, and RP, but prospective RCTs have only addressed induction of remission in AAV. In particular, a role replacing conventional immunosuppression due to reduced toxicity is suggested. However, further studies are needed to address this. Finally, although RTX is an expensive drug, if it reduces remission rates (reducing hospital admissions) and avoids adverse events, it might overall be a cost-effective treatment.
Disclosure
The authors report no conflicts of interest in this work.
References
- FauciASHaynesBFKatzPWolffSMWegener’s granulomatosis: prospective clinical and therapeutic experience with 85 patients for 21 yearsAnn Intern Med198398176856336643
- FauciASWolffSMWegener’s granulomatosis: studies in eighteen patients and a review of the literatureMedicine (Baltimore)19735265355614748591
- HoffmanGSKerrGSLeavittRYWegener granulomatosis: an analysis of 158 patientsAnn Intern Med199211664884981739240
- WaltersGWillisNSCraigJCInterventions for renal vasculitis in adultsCochrane Database Syst Rev20159CD00323226400765
- WestmanKWBygrenPGOlssonHRanstamJWieslanderJRelapse rate, renal survival, and cancer morbidity in patients with Wegener’s granulomatosis or microscopic polyangiitis with renal involvementJ Am Soc Nephrol1998958428529596082
- TaylorRPLindorferMADrug insight: the mechanism of action of rituximab in autoimmune disease – the immune complex decoy hypothesisNat Clin Pract Rheumatol200732869517299446
- ChatzidionysiouKLieENasonovEEffectiveness of two different doses of rituximab for the treatment of rheumatoid arthritis in an international cohort: data from the CERERRA collaborationArthritis Res Ther2016185026883119
- RandallKLRituximab in autoimmune diseasesAust Prescr201639413113427756976
- SnellerMCHuZLangfordCAA randomized controlled trial of rituximab following failure of antiviral therapy for hepatitis C virus-associated cryoglobulinemic vasculitisArthritis Rheum201264383584222147444
- SpecksUMerkelPASeoPEfficacy of remission-induction regimens for ANCA-associated vasculitisN Engl J Med2013369541742723902481
- StoneJHMerkelPASpieraRRituximab versus cyclophosphamide for ANCA-associated vasculitisN Engl J Med2010363322123220647199
- LetaiefHLukasCBarnetcheTGaujoux-VialaCCombeBMorelJEfficacy and safety of biological DMARDs modulating B cells in primary Sjögren’s syndrome: systematic review and meta-analysisJoint Bone Spine Epub2017630
- InnamiKMukaiTKodamaSMoritaYSuccessful treatment using rituximab in a patient with refractory polymyositis complicated by scleroderma renal crisisBMJ Case Rep20172017221205
- MelsensKVandecasteeleEDeschepperETwo years follow-up of an open-label pilot study of treatment with rituximab in patients with early diffuse cutaneous systemic sclerosisActa Clin Belg Epub2017911
- OddisCVReedAMAggarwalRRituximab in the treatment of refractory adult and juvenile dermatomyositis and adult polymyositis: a randomized, placebo-phase trialArthritis Rheum201365231432423124935
- DavatchiFShamsHRezaipoorMRituximab in intractable ocular lesions of Behçet’s disease; randomized single-blind control study (pilot study)Int J Rheum Dis201013324625220704622
- SadreddiniSNoshadHMolaeefardMNoshadRTreatment of retinal vasculitis in Behçet’s disease with rituximabMod Rheumatol200818330630818438602
- ZhaoBHOswaldAEImproved clinical control of a challenging case of Behçet’s disease with rituximab therapyClin Rheumatol201433114915024305944
- JonesRBFurutaSTervaertJWRituximab versus cyclophosphamide in ANCA-associated renal vasculitis: 2-year results of a randomised trialAnn Rheum Dis20157461178118225739829
- HendersonSRCopleySJPuseyCDIndPWSalamaADProlonged B cell depletion with rituximab is effective in treating refractory pulmonary granulomatous inflammation in granulomatosis with polyangiitis (GPA)Medicine (Baltimore)20149327e22925501085
- Recillas-GispertCSerna-OjedaJCFlores-SuarezLFRituximab in the treatment of refractory scleritis in patients with granulomatosis with polyangiitis (Wegener’s)Graefes Arch Clin Exp Ophthalmol2015253122279228426507398
- WangCHFinkelRSBertiniESConsensus statement for standard of care in spinal muscular atrophyJ Child Neurol20072281027104917761659
- JonesRBTervaertJWHauserTRituximab versus cyclophosphamide in ANCA-associated renal vasculitisN Engl J Med2010363321122020647198
- SpecksUMerkelPAHoffmanGSDesign of the Rituximab in ANCA-Associated Vasculitis (RAVE) TrialOpen Arthritis J20114118
- GeethaDSpecksUStoneJHRituximab versus cyclophosphamide for ANCA-associated vasculitis with renal involvementJ Am Soc Nephrol201526497698525381429
- NagafuchiHAtsumiTHattaKLong-term safety and efficacy of rituximab in 7 Japanese patients with ANCA-associated vasculitisMod Rheumatol201525460360825496405
- WendtMGunnarssonIBrattJBruchfeldARituximab in relapsing or refractory ANCA-associated vasculitis: a case series of 16 patientsScand J Rheumatol201241211611922118245
- de MenthonMCohenPPagnouxCInfliximab or rituximab for refractory Wegener’s granulomatosis – long-term follow up: a prospective randomised multicentre study on 17 patientsClin Exp Rheumatol2011291 Suppl 64S63S71
- LovricSErdbrueggerUKümpersPRituximab as rescue therapy in anti-neutrophil cytoplasmic antibody-associated vasculitis: a single-centre experience with 15 patientsNephrol Dial Transplant200924117918518685144
- Cartin-CebaRGolbinJMKeoghKARituximab for remission induction and maintenance in refractory granulomatosis with polyangiitis (Wegener’s): ten-year experience at a single centerArthritis Rheum201264113770377822730028
- RheeEPLaliberteKANilesJLRituximab as maintenance therapy for anti-neutrophil cytoplasmic antibody-associated vasculitisClin J Am Soc Nephrol2010581394140020498238
- Roubaud-BaudronCPagnouxCMeaux-RuaultNRituximab maintenance therapy for granulomatosis with polyangiitis and microscopic polyangiitisJ Rheumatol201239112513022089465
- GuillevinLPagnouxCKarrasARituximab versus azathioprine for maintenance in ANCA-associated vasculitisN Engl J Med2014371191771178025372085
- SmithRMJonesRBGuerryMJRituximab for remission maintenance in relapsing antineutrophil cytoplasmic antibody-associated vasculitisArthritis Rheum201264113760376922729997
- AwadNHafizSAlbeityAAlmoallimHA case of rituximab use as an induction and maintenance of remission in ANCA-associated vasculitisCase Rep Rheumatol20162016727586027006851
- MoogPProbstMKuechleCHauserCHeemannUThuermelKSingle-dose rituximab for remission induction and maintenance therapy in ANCA-associated vasculitis: a retrospective analysis of 17 patientsScand J Rheumatol201443651952325179776
- CalichALPuechalXPugnetGRituximab for induction and maintenance therapy in granulomatosis with polyangiitis (Wegener’s): results of a single-center cohort study on 66 patientsJ Autoimmun20145013514124703438
- CharlesPNéelATieuliéNRituximab for induction and maintenance treatment of ANCA-associated vasculitides: a multicentre retrospective study on 80 patientsRheumatology (Oxford)201453353253924282319
- PendergraftWFCortazarFBWengerJLong-term maintenance therapy using rituximab-induced continuous B-Cell depletion in patients with ANCA vasculitisClin J Am Soc Nephrol20149473674424626432
- BesadaEKoldingsnesWNossentJCLong-term efficacy and safety of pre-emptive maintenance therapy with rituximab in granulomatosis with polyangiitis: results from a single centreRheumatology (Oxford)201352112041204723934313
- Brodowska-KaniaDRymarzASaracynMGeislerPNiemczykSZastosowanie rituksimabu w indukcji remisji ciężkiej, opornej na leczenie postaci zapalenia naczyń z obecnością przeciwciał c-ANCA – opis przypadku [Use of rituximab in the induction of remission of severe, resistant and recurrent form of polyangiitis associated with c-ANCA antibodies: case report]Pol Merkur Lekarski201538226216218 Polish25938389
- UmezawaNKohsakaHNankiTSuccessful treatment of eosinophilic granulomatosis with polyangiitis (EGPA; formerly Churg-Strauss syndrome) with rituximab in a case refractory to glucocorticoids, cyclophosphamide, and IVIgMod Rheumatol201424468568724517553
- ThielJHasslerFSalzerUVollREVenhoffNRituximab in the treatment of refractory or relapsing eosinophilic granulomatosis with polyangiitis (Churg-Strauss syndrome)Arthritis Res Ther2013155R13324286362
- MohammadAJHotAArndtFRituximab for the treatment of eosinophilic granulomatosis with polyangiitis (Churg-Strauss)Ann Rheum Dis201675239640125467294
- PraisDAmirJNussinovitchMRecurrent Henoch-Schönlein purpura in childrenJ Clin Rheumatol2007131252817278945
- CoppoRAndrulliSAmoreAPredictors of outcome in Henoch-Schönlein nephritis in children and adultsAm J Kidney Dis2006476993100316731294
- DonnithorneKJAtkinsonTPHinzeCHRituximab therapy for severe refractory chronic Henoch-Schönlein purpuraJ Pediatr2009155113613919559299
- PilleboutERochaFFardetLRybojadMVerineJGlotzDSuccessful outcome using rituximab as the only immunomodulation in Henoch-Schönlein purpura: case reportNephrol Dial Transplant20112662044204621436378
- SaadounDRigonMRSeneDRituximab plus PEG-interferon-α/ribavirin compared with PEG-interferon-α/ribavirin in hepatitis C-related mixed cryoglobulinemiaBlood2010116332633420439619
- SaadounDResche-RigonMSeneDPerardLKarrasACacoubPRituximab combined with PEG-interferon-ribavirin in refractory hepatitis C virus-associated cryoglobulinaemia vasculitisAnn Rheum Dis200867101431143618178690
- De VitaSQuartuccioLIsolaMA randomized controlled trial of rituximab for the treatment of severe cryoglobulinemic vasculitisArthritis Rheum201264384385322147661
- TerrierBSaadounDSèneDEfficacy and tolerability of rituximab with or without PEGylated interferon alfa-2b plus ribavirin in severe hepatitis C virus-related vasculitis: a long-term followup [sic] study of thirty-two patientsArthritis Rheum20096082531254019644879
- DammaccoFTucciFALaulettaGPEGylated interferon-α, ribavirin, and rituximab combined therapy of hepatitis C virus-related mixed cryoglobulinemia: a long-term studyBlood2010116334335320308602
- VisentiniMLudovisiSPetrarcaAA phase II, single-arm multicenter study of low-dose rituximab for refractory mixed cryoglobulinemia secondary to hepatitis C virus infectionAutoimmun Rev2011101171471921570494
- VisentiniMTinelliCColantuonoSEfficacy of low-dose rituximab for the treatment of mixed cryoglobulinemia vasculitis: phase II clinical trial and systematic reviewAutoimmun Rev2015141088989626031898
- PetrarcaARigacciLMontiMImprovement in liver cirrhosis after treatment of HCV-related mixed cryoglobulinemia with rituximabDig Liver Dis200739Suppl 1S129S13317936214
- LamprechtPLerin-LozanoCMerzHRituximab induces remission in refractory HCV associated cryoglobulinaemic vasculitisAnn Rheum Dis200362121230123314644867
- NocturneGDougadosMConstantinARituximab in the spondyloarthropathies: data of eight patients followed up in the French autoimmunity and rituximab (AIR) registryAnn Rheum Dis201069247147220107035
- SongIHHeldmannFRudwaleitMDifferent response to rituximab in tumor necrosis factor blocker-naive patients with active ankylosing spondylitis and in patients in whom tumor necrosis factor blockers have failed: a twenty-four-week clinical trialArthritis Rheum20106251290129720461780
- KobakSKaraarslanAOkselFThe efficacy and safety of rituximab in a patient with rheumatoid spondylitisCase Rep Rheumatol2013201379252624324911
- Rodríguez-EscaleraCFernández-NebroAThe use of rituximab to treat a patient with ankylosing spondylitis and hepatitis BRheumatology (Oxford)200847111732173318786966
- HuangYChengFZhangXTangJMarked reduction of sacroiliac joint inflammation on magnetic resonance imaging in a patient with ankylosing spondylitis after rituximab treatmentJ Rheumatol20113892083208421885528
- OmairMAAlnaqbiKALeePRituximab in a patient with ankylosing spondylitis with demyelinating disease: a case report and review of the literatureClin Rheumatol20123181259126122644089
- AldhaheriFAlmteriTDwidNMajdaliAJanoudiNAlmoallimHRituximab can induce remission in a patient with ankylosing spondylitis who failed anti-TNF-α agentAm J Case Rep20171814314728179619
- HaroonMO’GradaighDFoley-NolanDA case of Raynaud’s phenomenon in mixed connective tissue disease responding to rituximab therapyRheumatology (Oxford)200746471871917289791
- DunkleyLGreenMGoughAComment on: a case of Raynaud’s phenomenon in mixed connective tissue disease responding to rituximab therapy responseRheumatology (Oxford)200746101628162917766999
- RechJKallertSHueberAJRequadtCKaldenJRSchulze-KoopsHCombination of immunoadsorption and CD20 antibody therapy in a patient with mixed connective tissue diseaseRheumatology (Oxford)200645449049116461439
- ShabrawishiMAlbeityAAlmoallimHSevere primary Raynaud’s disease treated with rituximabCase Rep Rheumatol20162016205380427651971
- SpecksUFervenzaFCMcDonaldTJHoganMCResponse of Wegener’s granulomatosis to anti-CD20 chimeric monoclonal antibody therapyArthritis Rheum200144122836284011762944
- DalakasMCB cells as therapeutic targets in autoimmune neurological disordersNat Clin Pract Neurol200841055756718813230
- RiveraAChenCCRonNDoughertyJPRonYRole of B cells as antigen-presenting cells in vivo revisited: antigen-specific B cells are essential for T cell expansion in lymph nodes and for systemic T cell responses to low antigen concentrationsInt Immunol200113121583159311717199
- BraunJBollowMNeureLUse of immunohistologic and in situ hybridization techniques in the examination of sacroiliac joint biopsy specimens from patients with ankylosing spondylitisArthritis Rheum19953844995057718003
- AppelHKuhneMSpiekermannSImmunohistologic analysis of zygapophyseal joints in patients with ankylosing spondylitisArthritis Rheum20065492845285116947385
- AppelHLoddenkemperCGrozdanovicZCorrelation of histopathological findings and magnetic resonance imaging in the spine of patients with ankylosing spondylitisArthritis Res Ther200685R14316925803
- DornerTCrossroads of B cell activation in autoimmunity: rationale of targeting B cellsJ Rheumatol Suppl20067731116652439
- MintzGEnriquezRDMercadoURoblesEJJimenezFJGutierrezGIntravenous methylprednisolone pulse therapy in severe ankylosing spondylitisArthritis Rheum19812457347367236329
- RichterMBWooPPanayiGSTrullAUngerAShepherdPThe effects of intravenous pulse methylprednisolone on immunological and inflammatory processes in ankylosing spondylitisClin Exp Immunol198353151596223736
- CohenSBEmeryPGreenwaldMWRituximab for rheumatoid arthritis refractory to anti-tumor necrosis factor therapy: results of a multicenter, randomized, double-blind, placebo-controlled, phase III trial evaluating primary efficacy and safety at twenty-four weeksArthritis Rheum20065492793280616947627
- PrevooMLvan ‘t HofMAKuperHHvan LeeuwenMAvan de PutteLBvan RielPLModified disease activity scores that include twenty-eight-joint counts: development and validation in a prospective longitudinal study of patients with rheumatoid arthritisArthritis Rheum199538144487818570
- AlbericiFSmithRCassiaMClinical predictors of response to rituximab in ANCA-associated vasculitis: a European cohortPoster presented at: 53rd European Renal Association–European Dialysis and Transplant Association CongressMay 21–24, 2016Vienna, Austria
- KosmidisMLDalakasMCPractical considerations on the use of rituximab in autoimmune neurological disordersTher Adv Neurol Disord2010329310521179602
