Abstract
Objective
In 2016, ASAS and EULAR made joint recommendations for the management of patients with spondyloarthritis. Although Global and European perspectives are important, they cannot accurately reflect the situation for all patients in all countries and regions. As such, the group worked to tailor the existing international recommendations to suit the specific demographic needs of local populations in the Gulf region, with a specific focus on Kuwait.
Methods
Recommendations drafted following a PubMed search for relevant literature were reviewed and then underwent Delphi vote to reach consensus on those to be included. Advice for newly approved agents, including targeted synthetic disease-modifying anti-rheumatic drugs, was included based on the group’s clinical experience.
Results
The resulting 41 recommendations are grouped into five categories covering key definitions and principles for the management and treatment of both axial and peripheral forms of spondyloarthritis.
Conclusion
Through adaptation of existing guidelines and incorporating the current evidence and clinical experience of the members of the group, these recommendations have been developed to reflect the unique situation in Kuwait with regard to differing patient profiles, local culture and approved therapeutic approaches, and are designed to aid in clinical decision-making.
Introduction
Spondyloarthritis (SpA) is an umbrella term that encompasses several related inflammatory rheumatic conditionsCitation1 and has a heterogeneous presentation.Citation2 SpA may present in an axial form, predominantly involving the sacroiliac joints and/or the spine, or peripheral form, with arthritis, enthesitis and dactylitis.Citation1 Axial SpA may be further divided into radiographic axial SpA, with radiographic signs of damage in the sacroiliac joints, or non-radiographic without sacroiliac joint damage.Citation3 Only recently has it been properly acknowledged that radiographic sacroiliitis is a late finding in the disease course for many patients, and that magnetic resonance imaging (MRI) may show signs of inflammation much earlier than X-ray radiographs show structural damage.Citation2
SpA also features extra-articular manifestations, such as inflammatory bowel disease (IBD), anterior uveitis and psoriasis.Citation1,Citation3 In the past decade, major progress has been made in the recognition, classification and management of SpA.Citation3 To provide guidance on the clinical picture of SpA, classification criteria have been developed by the Assessment of SpondyloArthritis international Society (ASAS).Citation4,Citation5 Also, recommendations on general treatment targets in SpA and the strategy to treat SpA to these targets were developed in 2012 by an international task force.Citation6
In recent years, the rheumatology treatment landscape has benefited from key developments that modulate the inflammatory response through diverse molecular pathways. The successful introduction of tumor necrosis factor (TNF)-blocking therapies in SpA over 15 years ago demonstrated clinical efficacy for axial disease, arthritis and enthesitis, as well as extra-articular manifestations.Citation7 While TNF inhibitors (TNFi), categorized as biological disease-modifying anti-rheumatic drugs (bDMARDs), have dramatically improved the treatment and care of SpA patients, there is still a high unmet need for additional therapeutic compounds.Citation7 A number of novel treatment strategies have recently yielded promising results in SpA, including non-TNFi bDMARDs, such as interleukin-17inhibitors (IL-17i), and the introduction of the class of targeted synthetic DMARDs (tsDMARDS), such as Janus kinase inhibitors (JAKi).Citation7 The first-in-class JAKi, tofacitinib, has been approved in the US for rheumatoid arthritis (RA) since 2012, and in Kuwait since 2013.Citation8 This license has now been extended to include the SpA subtype psoriatic arthritis (PsA) in the US and Europe, and recently in Kuwait.Citation9–Citation11
International Guidelines and Recommendations
In 2017, ASAS and the European League Against Rheumatism (EULAR) jointly published an update of their evidence-based recommendations for the management of people with axial SpA.Citation2
Recommendations from the American College of Rheumatology/Spondylitis Association of America/Spondyloarthritis Research and Treatment Network (ARC/SAA/SPARTAN) were also released in the US for ankylosing spondylitis (AS) and non-radiographic axial SpA (nrAxSpA) in 2015.Citation12 These recommendations gave guidance on the most frequent and meaningful points related to clinical decision-making in the care of people with AS and nrAxSpA.Citation12 Also in 2015, the Canadian Rheumatology Association (CRA) and the Spondyloarthritis Research Consortium of Canada (SPARCC) collaborated to update the recommendations for the management of SpA.Citation13
In North Africa and the Middle East, experts tend to use the ASAS/EULAR recommendations for the management of axial SpA.Citation14 The ASAS/EULAR international recommendations state that bDMARDs should be considered in patients with persistently high disease activity despite conventional treatments, with TNFi therapy being the current treatment standard.Citation2
In Kuwait, approved treatments for SpA include non-steroidal anti-inflammatory drugs (NSAIDs) essentially for pain relief, conventional DMARDs (csDMARDs), bDMARDs such as TNFis, and tsDMARDs such as JAKi, for SpA subtypes such as PsA (). At present, there are no current guidelines available from the Ministry of Health in Kuwait for any class of drugs to treat patients with SpA.
Table 1 Approved Treatments for Symptom Relief and Disease Modification of SpA in Kuwait
Why are Regional Considerations Important?
Global and European perspectives for patient care are essential, but they may not reflect the personal backgrounds and situations of patients in different countries and regions.Citation15 For example, for some countries in North Africa and the Middle East, access to agents to treat SpA and convincing some patients to accept long-term treatment can be challenging.Citation14 A recent Kuwait registry study demonstrated that accessibility to biologic treatments in Kuwait affects the rate of prescription and the impact on disease activity and quality of life in patients with RA.Citation16 Management practices vary widely and can be affected by cultural variance, socioeconomics and inadequate local infrastructure, but there is indication that strategies for disease management are evolving in the Middle East, particularly in the Gulf region.Citation15,Citation17
Kuwait, situated at the north eastern edge of the Arabian Peninsula, occupies an area of nearly 18,000 square kilometres and, in 2018, has an estimated population of over 4.2 million, with high expatriate numbers (approximately 70%) and immigration rates.Citation18 Survey data from Kuwait found that 6.6% of the general population have musculoskeletal pain.Citation19 This figure increases to 15.7% in people over the age of 50, and 13.5% in people with a body mass index of over 40.Citation19 Information about the burden of SpA in the North Africa and Middle East region is scarce.Citation14 Data from Iran give prevalence of 0.23% and 0.12% for SpA and AS, respectively.Citation20
Because patient profiles, local considerations and clinical practice in Kuwait may vary from those in Europe or US, a group of experts comprising 14 rheumatologists – including members of the Kuwaiti Association of Rheumatology (KAR) – met in Kuwait City in March 2018, in order to develop adapted recommendations of relevance to local patients. The expert group undertook a modified Delphi process to gain consensus on the applicability of the existing ASAS/EULAR international recommendations for local patients in Kuwait, and to make suggestions for amends and updates. The resulting recommendations are based on the opinions and clinical experience of the authors.
Methods
Delphi Process
PubMed searches using Medical Subject Headings terms for spondyloarthritis, axial spondyloarthritis, psoriatic arthritis and ankylosing spondylitis were made from January 2000 to December 2017 to identify any literature that could inform the development of amended recommendations, including updated guidelines and latest studies in the field. Only articles in the English language and those about patients aged over 18 years were included. Other papers considered relevant could be added by the authors at their discretion.
Thirty-eight drafted recommendations were formulated after the initial searches. In a closed online Delphi exercise, the experts voted on a scale of 0 to 9 to indicate their agreement with each recommendation statement. The calculated means and standard deviations demonstrated that a positive consensus was achieved on 27 recommendations and neutral consensus on the remainder. After discussion and consultation at the March 2018 meeting, 8 recommendations were unchanged and 3 were amended. Also, during the meeting, the expert panel added 4 more recommendations to be considered for the post-meeting Delphi vote. Thus, a final vote incorporated 42 recommendations to reach consensus on. At that time, the experts suggested to combine 2 recommendations into 1, which gives the consensus of 41 recommendations in total that are presented here (Supplement Table 1).
Results
Recommendations
The expert group recommendations are grouped into five categories (). The first group encompasses the definition of diseases, treatment response and medications, followed by the categories around general management recommendations, and the recommendations for treatment choice, including considerations in special populations ().
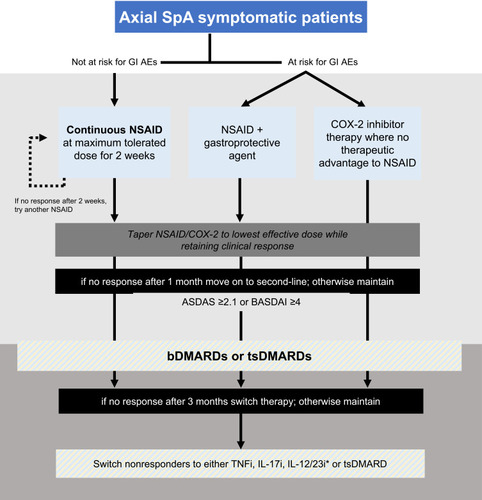
Figure 1 Recommendations for the management of spondyloarthritis patients in Kuwait. *Consider if the patient has concomitant moderate to severe psoriasis. †Poor prognostic factors include five or more tender or swollen joints; radiographic joint destruction, especially if there is inflammation; elevated acute phase reactants (serum values of inflammatory indicators above the upper limit of normal); and extra-articular manifestations, particularly dactylitis. ‡Consider if the patient has concomitant inflammatory bowel disease or uveitis.
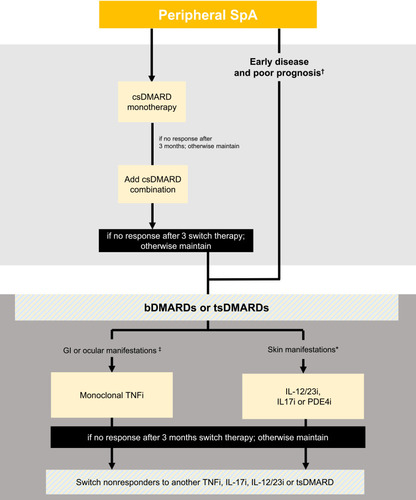
Table 2 Definitions and Recommendations for the Management of SpA in Kuwait
Definition of Diseases, Treatment Response and Medications
Alongside recommendations, the experts agreed on a standardized definition of diseases, treatment response and medications as stated below.
Definition of Diseases and Treatment Response
1. In axial SpA, a clinical response is defined as an improvement equal or more than 1.1 in AS Disease Activity Score (ASDAS), or an absolute reduction of the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) by 2 (0−10 scale), or a relative reduction of 50% within 3 months of treatment initiation.
There are several tools for measuring clinical response to treatment in axial SpA, including the ASAS-defined improvement criteria, BASDAI response criteria, ASDAS improvement criteria and ASDAS disease activity states.Citation21 The ASDAS improvement criteria define a change in score of at least 1.1 units is equivalent to a “clinically important improvement”, and a change of at least 2.0 units is considered as “major improvement”.Citation22 Any ASDAS score less than 1.3 is defined as inactive disease.Citation22
2. In peripheral SpA, a clinical response is defined as a reduction in the active joint count of at least 30% within 3 months of treatment initiation.
Once treatment is started, the SpA patient should be monitored to investigate if the target is obtained.Citation2 While in principle the ultimate goal is to achieve an inactive disease state, this may be unreasonable depending on disease phase and previous treatments.Citation2
Definition of Medication
3. csDMARDs for drugs such as sulfasalazine, leflunomide and methotrexate (MTX).
4. bDMARDs for drugs such as TNFi, IL-17i, and IL-12/23 inhibitors.
5. tsDMARDs for drugs such as JAKi and PDE-4 inhibitors.
General Principles
SpA Recommendations
6. The primary goals of managing patients with SpA are control of symptoms and inflammation, prevention of progressive structural damage, and preservation of function.
This recommendation was an amendment of the ASAS/EULAR recommendation for axial SpA.Citation2 SpA is an inflammatory disease; as such, pharmacological suppression of inflammation may be needed in order to relieve symptoms and preserve physical function.Citation2 The 2014 recommendations of an international task force endorsed a treat-to-target approach for SpACitation6 and were updated in 2017.Citation23 The treatment target for SpA was defined as remission or low disease activity.Citation6,Citation23 The optimal management of patients with SpA requires a combination of non-pharmacological and pharmacological approaches.Citation2 In order to measure the efficacy of a management strategy, it is important to have a measure of clinical response.
There is evidence to support a treat-to-target approach in SpA.Citation23–Citation25 Disease activity has been found to contribute longitudinally to radiographic progression in the spine of patients with ASCitation24 and, used early in the disease, therapeutic interventions that target systemic inflammation may effectively modify the disease course.Citation25
7. The treatment strategy for SpA usually requires a multidisciplinary team coordinated by a rheumatologist.
SpA disorders do not exclusively involve musculoskeletal inflammatory disease but also systemic disease, with clinical features occurring at sites other than the axial skeleton and peripheral joints.Citation26 Extra-articular manifestations in SpA are common, estimated to occur in 50% of patients.Citation27 Extra-articular manifestations may affect the eyes, skin or bowels, triggering uveitis, psoriasis, and inflammatory colitis, respectively.Citation26,Citation27 Patients with SpA may also suffer from other less common manifestations such as cardiovascular or pulmonary complications.Citation26 These manifestations can occur at any time during the disease course, or be the presenting SpA symptom in 20–60% of patients.Citation2,Citation27 Uveitis occurs in 25–40% of patients with SpA;Citation27 psoriasis was found to be present in 32.4%, 82.7% and 1.5% of SpA, PsA and AS patients, respectively;Citation28 and 5–10% of SpA patients will also have IBD.Citation26
Because of the diverse manifestations of SpA, a multidisciplinary approach is often needed. Each area of Kuwait has primary-care polyclinics, and patients seen in primary care are referred to specialist rheumatology clinics. Referrals to rheumatology clinics may also come from other speciality clinics such as dermatology, gastroenterology, ophthalmology, or internal medicine clinics. On the other hand, the rheumatologist may refer patients with SpA to ophthalmology, gastroenterology or dermatology clinics to treat specific extra-articular manifestations. Clinics usually cooperate in sharing the patient’s full file.
We believe that the rheumatologist is best placed to coordinate other specialties and should retain overall management of the patient, especially if the patient is prescribed bDMARDs or tsDMARDs, which may require additional monitoring. Such coordination in rheumatology can be facilitated by the treating rheumatologist possessing extensive knowledge of the entire SpA spectrum of disease.Citation2
8. Disease monitoring of patients with SpA should include patient-reported outcomes, clinical findings, laboratory tests and imaging, if needed.
This recommendation is amended from the ASAS/EULAR recommendation for axial SpA.Citation2
Due to the heterogeneity of SpA clinical features, patient monitoring should be at the heart of clinical practice using a broad range of assessments toolsCitation2 and following the ASAS core set,Citation29 as well as other more recent relevant measures such as the ASDAS.Citation2 The ASAS core set covers symptom-modifying anti-rheumatic drugs and physiotherapy, clinical record keeping, and disease-controlling anti-rheumatic treatment, with level and frequency of monitoring based on disease severity and treatment.Citation21 Monitoring in clinical practice will depend on the available resources to perform such regulatory requirements.
Individual patient-reported outcomes alone may result in high variability,Citation30 but are essential to monitor a patient’s pain. Serum C-reactive protein (CRP) levels predict syndesmophyte progression,Citation31 which in turn correlates with functional impairment;Citation32 regular laboratory tests can therefore be useful in monitoring. There is also a relationship between disease activity and radiographic progression,Citation24 which can be monitored using non-invasive imaging techniques. The current practice in Kuwait is to use X-ray and MRI to provide information on inflammation in SpA.
Axial SpA Recommendations
9. The treatment of patients with axial SpA should be individualized according to the current signs and symptoms of the disease (axial, peripheral, extra-articular manifestations), and patient characteristics, including comorbidities and psychosocial factors.
SpA is a heterogeneous disease, indicating the importance of personalized patient management. Both patient fluctuations over time and heterogeneity, respectively, are found at the individual patient level in clinical practice.Citation2,Citation30 As such, rheumatologists should consider that not all available treatments may be effective in all disease domains in each patient.Citation2
Non-Pharmacological Treatment
SpA Recommendations
10. Treatment of a SpA patient should include education, exercise and physical therapy.
This recommendation is amended from the ASAS/EULAR recommendation for axial SpA.Citation2 Every patient should receive education to help them understand their disease and make informed decisions about their treatment.Citation2,Citation33–Citation35 Non-pharmacological approaches can be used in mild cases, and as an adjunct to support pharmacological treatment in moderate-to-severe disease. Patients should be encouraged to take appropriate exercise to help strengthen their joints and to keep them mobile.Citation2,Citation33 Physical therapy may be beneficial and thus advised in some cases.Citation2 Physical therapy is supported by the Ministry of Health in Kuwait, and also widely available in private practice.
11. We recommend smoking cessation or reduction for a patient with SpA.
In Kuwait, 39% of adult men and 3% of adult women are smokers.Citation36 Smoking is an important public health concern and increases the disease burden in SpA.Citation37 There is an association between smoking and radiographic progression, disease activity, inflammation and syndesmophyte formation.Citation38–Citation40 Smoking is a modifiable lifestyle factor. Smoking cessation has a potential benefit for patients with axial SpA over those for the general population and should be recommended to all patients.Citation40
Axial SpA Recommendations
12. We recommend active physical therapy interventions (supervised exercise) over passive physical therapy interventions (massage, ultrasound, heat) in the treatment of axial SpA.
The optimal management of axial SpA requires a combination of both non-pharmacological and pharmacological approaches.Citation2 Both active and passive physical therapy options are available in Kuwait. Physiotherapy is considered to play a role in patient management as it helps to maintain function and an adequate quality of life.Citation41 Tailored to the individual patient, regular physical activity and interruption of sedentary occupations should be encouraged to promote general health and well-being and improve function.Citation42 Passive physical therapy, such as massage, ultrasound or the application of heat, may help to alleviate symptoms of axial SpA such as pain and stiffness.Citation41,Citation43 However, it should be used as an adjunct to regular physical activity,Citation12 as some forms of deep tissue massage and spinal manipulation can cause disease flare-ups in people with axial SpA and should therefore be avoided.Citation43
Our preference is to recommend active physical therapy and supervised exercise programmes,Citation12 which may help to improve mobility, strength, balance and cardio-respiratory function.Citation42 Exercise programmes should take into consideration the individual’s physical changes, particularly for those with more severe or later disease. Most types of exercise are safe for the majority of patients, although some high-impact sports and activities (eg, contact sports) may be contraindicated in people with advanced disease.Citation42 Individual exercise programmes should place an emphasis on spinal mobility, although maintaining peripheral joint mobility is also important.Citation42
Pharmacological Treatment
NSAIDs
SpA Recommendations
13. NSAIDs are considered to be first-line therapy for patients with axial SpA without any preferred choice.
NSAIDs are considered to be first-line therapy for patients with axial SpA.Citation44 Most patients with axial SpA show improvement after the recommended 4 weeks of NSAID therapy, although for many patients the disease is still active.Citation44 The inflammatory nature of SpA results in the characteristic signs and symptoms of inflammation and leads to functional impairment and structural changes.Citation6 Arresting inflammation is therefore essential to improve patient outcomes, and use of NSAIDs can alleviate signs and symptoms, improve physical function, and potentially inhibit structural spinal damage.Citation6 There is evidence that continuous NSAID use in patients with an elevated CRP can reduce radiographic progression in AS,Citation45,Citation46 although the evidence is equivocalCitation2 and they do not improve levels of acute phase reactants, suggesting no abrogation of the underlying inflammation.Citation44
NSAIDs available in Kuwait for the treatment of SpA are listed in . We do not recommend one specific NSAID as the preferred choice; physicians should exercise their judgement to optimize safety and efficacy in line with individual patient factors.
14. We conditionally recommend continuous treatment over on-demand treatment with NSAIDs. The individual risk profile of each patient should be assessed where appropriate and indicated, considering also the individual patient profile.
Compared to on-demand use only, continuous use of NSAIDs may reduce progression of structural damage in the spine.Citation2,Citation45,Citation46 However, both strategies appear to have a similar effect on inflammatory signs and symptoms.Citation45 NSAIDs should be maintained at the maximum tolerated dose for 2 weeks and then tapered to the lowest effective dose while retaining clinical response.
15. When there is no therapeutic advantage of a traditional NSAID, selective COX-2 inhibitor therapy should be used in patients at an increased risk for gastrointestinal (GI) adverse events. In at-risk patients who respond best to a traditional NSAID, a gastroprotective agent should be used.
The potential side-effects of NSAIDs must be considered for each patient before treatment, with continuous risk-benefit assessment during treatment.Citation2 In general, traditional non-selective NSAIDs are associated with GI adverse events, while the more GI-sparing selective cyclooxygenase-2 inhibitors (COX-2i) are associated with a reduction in GI risk in most patients (who do not take aspirin concomitantly).Citation47 Evidence suggests that the risk reduction for GI adverse events with COX-2i is approximately equivalent to that achieved by co-prescribing a proton-pump inhibitor with traditional NSAIDs.Citation47 However, COX-2i are not superior to traditional NSAIDs, and individual drugs in the NSAID class (including COX-2i) vary in their ability to potentiate cardiovascular effects.Citation47
Axial SpA Recommendations
16. NSAIDs can be used for symptomatic patients with axial SpA. An appropriate trial consists of at least one NSAID, administered over a minimum of 2 weeks at a maximum tolerated dosage, unless contraindicated.
Risks and benefits of NSAID therapy should be considered on a case-by-case basis. In contrast to the potential safety issues associated with NSAIDs, there may also be risks associated with not using NSAIDs.Citation2
We recommend to use NSAIDs in symptomatic patients only.Citation2 A trial of the NSAIDs should be given at the maximum tolerated dosage.Citation2 If the initiating NSAID elicits a clinical response, then it should be tapered to the lowest dose that maintains the clinical response. However, in cases of treatment failure after administering for at least 2 weeks, rheumatologists have the option to try a different NSAID agent.
Corticosteroids
SpA Recommendations
17. Consider corticosteroid injections at the inflammation sites, including sacroiliac joints, peripheral joints and entheses.
Local injections of glucocorticoid into the site of musculoskeletal inflammation may be an option to treat arthritis and enthesitis in SpA patients.Citation2
18. Consider a short course of systemic corticosteroids for specific manifestations of SpA, such as active peripheral arthritis.
Systemic steroid treatment should not be continued in the long-term because they are associated with serious adverse events and reduction in quality of life.Citation48
Axial SpA Recommendations
19. In adults with active axial SpA, we strongly recommend against long-term treatment with systemic glucocorticoids. However, there might be situations where systemic glucocorticoids are the only treatment option available.
This recommendation is amended from the ASAS/EULAR recommendation for axial SpA.Citation2 The ASAS/EULAR task force suggest that patients with axial SpA should not receive long-term treatment with systemic glucocorticoids at any dose.Citation2 Short-term high-dose courses of glucocorticoids (<50 mg/day) may achieve a modest improvement in the signs and symptoms of axial SpA.Citation2,Citation49
csDMARDs
Axial SpA Recommendations
20. Do not use csDMARDs in the treatment of axial SpA.
csDMARDs available in Kuwait for the treatment of axial SpA are MTX, leflunomide and sulfasalazine (). However, these disease-modifying therapies may not be effective in treating axial symptoms.Citation2
Peripheral SpA Recommendations
21. csDMARDs can be used in the treatment of patients with chronic peripheral SpA.
Sulfasalazine may be a treatment option in patients with peripheral arthritis.Citation2
22. Consider combination therapy with csDMARDs in peripheral SpA, particularly in patients with poor prognostic factors, greater disease activity, recent-onset disease and monotherapy resistance.
There is limited evidence on the efficacy of combinations of csDMARDs, which focus on peripheral arthritis and PsA.Citation50,Citation51 However, csDMARDs may be suitable for patients with peripheral arthritis, especially people with early disease, or with a poor prognosis. Poor prognostic factors include five or more tender or swollen joints; radiographic joint destruction (especially if there is inflammation); elevated acute phase reactants (serum values of inflammatory indicators above the upper limit of normal); and extra-articular manifestations, particularly dactylitis.Citation51
bDMARDs and tsDMARDS
SpA Recommendations
23. bDMARDs and tsDMARDs (according to the local approved indications) should be given only under supervision by a trained physician with experience in monitoring such treatments.
Advanced therapies available in Kuwait for the treatment of SpA are listed in . bDMARDs should be considered in patients with persistently high disease activity following treatment with conventional therapies, including non-pharmacological management and NSAIDs.Citation2 bDMARDs have been shown to be effective in improving signs and symptoms and reducing spinal inflammation.Citation52 Achieving early treatment response with bDMARDs is predictive of better long-term treatment outcomes, with high rates of partial remission or low disease activity.Citation52 Available data on tsDMARDS indicates their effectiveness as a treatment option for axSpA.Citation53–Citation55 However, the risk–benefit of treatment must be assessed by a specialist with experience in the use of bDMARDs or tsDMARDs on an individual case-by-case basis,Citation2 and both bDMARDs and tsDMARDs require continued monitoring for adverse events.
24. Several TNFi are available for the treatment of SpA, including infliximab, etanercept, adalimumab, golimumab and certolizumab. The choice of TNFi should be determined during a consultation between the physician and patient.
TNFi therapies available in Kuwait for the treatment of SpA are listed in . The evidence for the use of TNFi in the treatment of SpA is very high,Citation54,Citation56 and they can provide sustained long-term efficacy.Citation57 Overall, all TNFi have proven efficacy in AS and SpA.Citation56 Large treatment effects have been reported for both radiographic and non-radiographic axial SpA for all TNFi.Citation54 TNFi also show beneficial effects on extra-articular manifestations.Citation56 Additionally, treatment with a second TNFi can be effective in cases of treatment failure with the first TNFi.Citation2
25. The choice of TNFi should incorporate the presence or absence of extra-articular manifestations.
Not all TNFi appear to be effective in all disease domains and, as noted in the 2016 update of the ASAS-EULAR management recommendations for axSpA, differences in efficacy exist regarding the effect on extra-articular manifestations between the TNFi.Citation2
26. We do not recommend a specific TNFi as the preferred choice, except for patients with concomitant IBD or recurrent uveitis, we recommend monoclonal TNFi.
The beneficial effects of TNFi treatment in patients with SpA extend to extra-articular manifestations including uveitis and IBD.Citation56 A meta-analysis of TNFi demonstrated their efficacy in preventing uveitis flares or new onset of uveitis in AS patients.Citation58 For patients with uveitis, the monoclonal antibodies adalimumab, certolizumab pegol, golimumab or infliximab are effective therapies.Citation2,Citation59 These monoclonal antibodies are also efficacious in the treatment of IBD.Citation60
27. The decision to maintain a patient on bDMARDs or tsDMARDs should be based on achieving clinical response at least 3 months after initiating treatment.
There is evidence to suggest advantages of targeting disease activity due to the formation of new syndesmophytes in patients with axial SpA with active disease.Citation2 Treat-to-target strategies with DMARDs in the rheumatic diseases aim for clinical response at 3 months.Citation23 If patients are non-responders to treatment at this point, they should be switched to another therapy.Citation2 Achieving early treatment responses and controlling disease activity leads to better long-term outcomes.Citation24 Disease activity has been shown to have an effect on radiographic damage and to be significantly associated with radiographic progression in the spine of patients with AS over time.Citation24
28. Non-responders to TNFi may benefit from switching to another TNFi, IL-17i, IL-12/23i therapy or tsDMARD.
This recommendation is amended from the ASAS/EULAR recommendation for axial SpA.Citation2 There is a potential choice of treatment after failure with the first TNFi; patients may switch to an alternative TNFi or to a bDMARD with an alternative mode of action, such as interleukin inhibitors.Citation2
29. Consider a monoclonal antibody against IL-12/23 or IL-17i for SpA patients with concomitant moderate-to-severe psoriasis.
The cytokines IL-17, IL-12, and IL-23 play a crucial role in the pathogenesis of psoriasisCitation61 and the IL-17i secukinumabCitation62 and IL-12/23 monoclonal antibodies have proven efficacy in psoriasis.Citation63 To date, trial data on IL-17i are available in radiographic axial SpACitation2
30. In patients with active enthesitis and/or dactylitis and insufficient response to NSAIDs or local glucocorticoid injections, therapy with a bDMARD should be considered.
Primary characteristics of SpA include enthesitis and dactylitis.Citation64,Citation65 Clinical studies in patients with AS have demonstrated the efficacy of TNFi in treating enthesitis.Citation64,Citation65 bDMARDs should be considered for patients with refractory enthesitis or dactylitis. TNFi have been shown to deliver high levels of response, and to have good drug adherence in patients with axial SpA.Citation66
31. For patients with refractory enthesitis or dactylitis, bDMARDs or tsDMARDs should be considered.
Enthesitis and dactylitis are primary characteristics of SpA.Citation51,Citation64,Citation65 Clinical studies have demonstrated the efficacy of TNFi in treating enthesitis in patients with AS.Citation65,Citation66
Axial SpA Recommendations
32. bDMARDs should be offered to patients who show signs of active axial SpA, defined by at least two of the following: BASDAI >4, elevated CRP or erythrocyte sedimentation rate (ESR), inflammatory lesions in the sacroiliac joints and/or spine on MRI.
TNFi are approved for patients with radiographic axial SpA without further limitations, and in patients with non-radiographic axial SpA only when their CRP is elevated and/or when signs of inflammation are found on MRI.Citation2 However, there is evidence that patients with radiographic axSpA who also have an elevated CRP have an increased chance of treatment success, and that radiographic sacroiliitis alone is not a predictor of response.Citation2 In both patients with radiographic and non-radiographic axial SpA, predictors of a good response to TNFi are considered to be firstly elevated CRP and secondly inflammation on MRI.Citation2 Therefore, there is suggestion that these factors should also be considered when initiating bDMARD therapy regardless of the presence of radiographic sacroiliitis.Citation2 Also, a recent Phase II placebo-controlled dose-ranging study demonstrated the efficacy and safety of a JAKi, tofacitinib, in reducing signs and symptoms of adults with active AS.Citation2
Peripheral SpA Recommendations
33. In patients with peripheral arthritis (eg PsA) and an inadequate response to at least one csDMARD, a bDMARD or tsDMARD (according to the local approved indications) should be considered.
Patients with peripheral arthritis who have an inadequate response to at least one csDMARD should be started on bDMARD therapy.Citation51 A tsDMARD may also be considered, including in patients in whom bDMARDs are inappropriate, such as those with comorbidities or an infection history contraindicating bDMARDs.Citation51
Special Populations
SpA Recommendations
34. Prior to initiating therapy with csDMARDs, bDMARDs or tsDMARDs, we recommend testing for hepatitis B and C virus infections, and screening for tuberculosis (TB) either by tuberculin skin test or interferon-release assay. A chest X-ray should be done as an initial test in all patients with SpA, regardless of patient risk factors for latent TB.
Hepatitis B virus and TB may be reactivated during anti-rheumatic therapy.Citation67,Citation68 All SpA patients considered for a treatment with csDMARDs, bDMARDs or tsDMARDs should receive screening for HBV infection, followed by antiviral prophylaxis with oral nucleoside analogue as appropriate.Citation14,Citation69 A chest X-ray should be done as an initial test for latent TBCitation70 in all patients with SpA. Chest radiography prior to initiating treatment is required because of the high importance of excluding active TB disease and latent TB infection. Testing for latent TB infection before initiating TNFi is advised due to the increased risk of progression to active TB disease.Citation71
35. We recommend a pre-evaluation of the TB exposure risk in sero-negative patients receiving bDMARDs or JAKi in whom the TB exposure is likely.
The majority of people infected with TB have no signs or symptoms of disease, although they are at risk for developing active TB disease depending on their immunological status.Citation71 In sero-negative patients in whom TB exposure is likely, risk evaluation is recommended before treating with bDMARDs or JAKi.
36. Following the initiation of csDMARDs, or when the dose of these drugs is significantly increased, complete blood counts, liver function tests and serum creatinine should be measured, within appropriate intervals.
Recommended blood monitoring strategies for DMARDs include assessments of full blood counts, creatinine or estimated glomerular filtration rate, alanine aminotransferase and/or aspartate aminotransferase, and albumin.Citation72 These assessments are recommended at treatment initiation and every 2 weeks thereafter until the dose has been stable for 6 weeks; once the dose is stable, every month for 3 months; and thereafter, every 12 weeks.Citation72 In cases of dose increases, these assessments should be carried out every 2 weeks until the dose has been stable for 6 weeks; then every month for 3 months; and thereafter, every 12 weeks.Citation72 Patients at high risk of toxicity should be monitored more frequently.Citation72
37. In adults with AS and recurrent iritis, we conditionally recommend treatment with infliximab or adalimumab over treatment with etanercept to decrease recurrences of iritis.
While the evidence is inadequate to support a recommendation for use of any particular TNFi over another in patients with AS, the preferred choice of TNFi for patients with AS and recurrent iritis is infliximab or adalimumab over etanercept.Citation12
38. In patients with AS and IBD, we strongly recommend treatment with TNFi monoclonal antibodies over treatment with etanercept.
IBD is associated with AS and axial SpA.Citation73 In patients with AS and IBD, the recommended treatment is with TNFi monoclonal antibodies over treatment with etanercept.Citation2,Citation12 Trials have demonstrated the efficacy of TNFi monoclonal antibodies, including infliximab, adalimumab, certolizumab, and golimumab, but inefficacy of the fusion protein etanercept in treating IBD.Citation2,Citation12 Available evidence has indicated patients are at lower risks of either flare or new onset of IBD with infliximab or adalimumab than with etanercept.Citation12
Axial SpA Recommendations
39. In adults with axial SpA and recurrent uveitis or iritis, we recommend management is coordinated with an ophthalmologist.
Patients with axial SpA have an increased risk of uveitis.Citation74 Rheumatologists should be alerted to a disease flare and or inadequate treatment when observing cases of acute anterior uveitis in patients with axial SpA. Ophthalmologists should consider SpA as a possible differential diagnosis in cases of acute anterior uveitis.Citation74 Ophthalmologists are best placed for monitoring and manage uveitis or iritis under treatment.
40. In adults with active non-radiographic axial SpA despite treatment with NSAIDs, we recommend treatment with bDMARDs.
This recommendation is in line with ARC/SAA/SPARTAN recommendations for patients with non-radiographic axial SpA whose disease is active and not responding to NSAIDs.Citation12 They particularly recommend TNFi in those patients with sacroiliitis on MRI and/or a raised CRP level.
Peripheral SpA Recommendations
41. When managing patients with PsA, extra-articular manifestations, metabolic syndrome, and cardiovascular disease should be taken into account.
PsA is associated with a number of extra-articular manifestations and comorbidities.Citation75–Citation77 A number of studies have suggested that patients with psoriatic disease have an increased risk of vascular inflammation, atherosclerotic conditions, myocardial infarction, and stroke, independent of traditional risk factors for cardiovascular disease.Citation78–Citation82 Additionally, an increased prevalence of metabolic syndrome has been observed in PsA, over and above that in other inflammatory arthropathies.Citation78,Citation83 Overlapping inflammatory pathways and genetic susceptibility may be potential biologic links underlying this association.Citation78
Discussion
The listed 41 recommendations cover the core aspects of treating SpA, including general principles, non-pharmacological treatment, pharmacological treatment, and special populations.
These recommendations are based on the adaptations of existing guidelines, particularly the ASAS/EULAR international recommendations, the current evidence and clinical experience of the members of the group, as well as the unique situation in Kuwait around differing patient profiles, local culture and approved therapeutic approaches. However, these are not designed to be full treatment guidelines and should be used appropriately to aid in clinical decision-making rather than guiding it.
Author Contributions
Yaser Ali was project supervisor and contributed to the conception and design of the work, the data analysis and interpretation, and drafting the article; Xenofon Baraliakos contributed to the design of the work and drafting the article. All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
Yaser Ali, Fatemah Abutiban, Adel Alawadhi, Ali AlDei, Ahmad Alenizi, Hebah Alhajeri, Adeeba Al-Herz, Waleed Alkandari, Ahmad Dehrab, Eman Hasan, Sawsan Hayat, Aqeel Ghanem and Khulood Saleh are members of the Kuwaiti Association for Rheumatology. Yaser Ali is employed by the Ministry of Health Kuwait and is a member of KAR. Xenofon Baraliakos has received honoraria from Abbvie, Janssen, Novartis and UCB. He is a Consultant Rheumatology, Clinical Immunology and Allergy, FRCPC, MsC, ABIM. Adel Alawadhi is a Professor and a Consultant Rheumatologist, MD, FRCPC, FACP, FACR. Ali AlDei is a Rheumatology Senior Specialist, MD, FACP. Ahmad Alenizi is a Rheumatologist and the President of the Kuwait Medical Association Kuwait Association of Rheumatology, MD, FRCPC, FACR, MACP. Hebah Alhajeri is a Senior Specialist in Rheumatology, MD, FRCP. Adeeba Al-Herz is a Consultant Rheumatologist and the President of Kuwait Association for Rheumatology, MD, FRCPC, FACP. Waleed Alkandari is a Consultant of internal medicine and rheumatology, MD, FRCP. Fatemah Abutiban is a Consultant medicine and rheumatology, MD, FRCP. Ahmad Dehrab, MBBCh BAO, FACP. Eman Hasan is a Consultant Physician and Rheumatologist, MD, FRCP (UK). Sawson Hayat is a Consultant rheumatologist, MBBCh, MRCP (UK), KBIM, CESR (UK). Aqeel Ghanem is a Rheumatology Consultant and the Head of the Rheumatology Unit, MD, FRCPC. Khulood Saleh is a Consultant rheumatologist, FRCPc. Xenofon Baraliakos is an Associate Professor, MD. The authors report no conflicts of interest in this work.
Additional information
Funding
References
- Proft F, Poddubnyy D. Ankylosing spondylitis and axial spondyloarthritis: recent insights and impact of new classification criteria. Ther Adv Musculoskel Dis. 2018;10(5–6):129–139. doi:10.1177/1759720X18773726
- van der Heijde D, Ramiro S, Landewé R, et al. 2016 update of the ASAS-EULAR management recommendations for axial spondyloarthritis. Ann Rheum Dis. 2017;76(6):978–991. doi:10.1136/annrheumdis-2016-21077028087505
- van Tubergen A. The changing clinical picture and epidemiology of spondyloarthritis. Nat Rev Rheumatol. 2015;11(2):110–118. doi:10.1038/nrrheum.2014.18125385414
- Rudwaleit M, van der Heijde D, Landewé R, et al. The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part II): validation and final selection. Ann Rheum Dis. 2009;68(6):777–783. doi:10.1136/ard.2009.10823319297344
- Rudwaleit M, van der Heijde D, Landewé R, et al. The Assessment of SpondyloArthritis International Society classification criteria for peripheral spondyloarthritis and for spondyloarthritis in general. Ann Rheum Dis. 2011;70(1):25–31. doi:10.1136/ard.2010.13364521109520
- Smolen JS, Braun J, Dougados M, et al. Treating spondyloarthritis, including ankylosing spondylitis and psoriatic arthritis, to target: recommendations of an international task force. Ann Rheum Dis. 2014;73(1):6–16. doi:10.1136/annrheumdis-2013-20341923749611
- Paramarta JE, Baeten D. Spondyloarthritis: from unifying concepts to improved treatment. Rheumatology. 2014;53(9):1547–1559. doi:10.1093/rheumatology/ket40724369419
- Pfizer. Press release. [Internet. Accessed 813, 2018]. Available from: https://www.pfizer.com/news/press-release/press-release-detail/pfizer_provides_update_on_global_regulatory_approvals_and_launches_of_xeljanz_tofacitinib_citrate_for_the_treatment_of_rheumatoid_arthritis.
- Pfizer. Press release. [Internet]. 2018 Available from: https://press.pfizer.com/press-release/pfizer-announces-fda-approval-xeljanz-tofacitinib-and-xeljanz-xr-treatment-active-psor.
- European Medicines Agency. Summary of opinion. Xeljanz. Available from: www.ema.europa.eu/docs/en_GB/document_library/Summary_of_opinion/human/004214/WC500247974.pdf. Accessed 7 2018
- Xeljanz® (tofacitinib) Summary of Product Characteristics (Gulf Levant), Pfizer; 2019
- Ward MM, Deodhar A, Akl EA, et al. American College of Rheumatology/Spondylitis Association of America/Spondyloarthritis Research and Treatment Network 2015 recommendations for the treatment of ankylosing spondylitis and nonradiographic axial spondyloarthritis. Arth Rheumatol. 2016;68:282–298. doi:10.1002/art.3929826401991
- Rohekar S, Chan J, Tse SM, et al. 2014 Update of the Canadian Rheumatology Association/spondyloarthritis research consortium of Canada treatment recommendations for the management of spondyloarthritis. Part I: principles of the management of spondyloarthritis in Canada. J Rheumatol. 2015;42:654–664. doi:10.3899/jrheum.14100025684770
- Hammoudeh M, Abdulaziz S, Alosaimi H, et al. Challenges of diagnosis and management of axial spondyloarthritis in North Africa and the Middle East: an expert consensus. J Int Med Res. 2016;44(2):216–230. doi:10.1177/030006051561153626811411
- Al Enizi A, AlSaeid K, Alawadhi A, et al. Kuwait recommendations on vaccine use in people with inflammatory rheumatic disease. Int J Rheumatol. 2018;5217461.29861734
- AL-Herz A, Saleh K, Al-Awadhi A, et al. Easy accessibility of biologics and its impact on disease activity and quality of life in Kuwaiti patients with rheumatoid arthritis [abstract]. Arthritis Rheumatol. 2018;70(suppl):10.
- Al Emadi S, Hammoudeh M, Mounir M, Mueller RB, Wells AF, Sarakbi HA. An assessment of the current treatment landscape for rheumatology patients in Qatar: recognising unmet needs and moving towards solutions. J Int Med Res. 2017;45(2):733–743. doi:10.1177/030006051668687228415924
- World population review. Available from: http://worldpopulationreview.com/countries/kuwait-population/. Accessed 138 2018.
- Alawadhi A, Olusi SO, Al-Saeid K, et al. Incidence of musculoskeletal pain in adult Kuwaitis using the validated Arabic version of the WHO-ILAR COPCORD Core Questionnaire. Ann Saudi Med. 2005;25(6):459–462. doi:10.5144/0256-4947.2005.45916438453
- Oswald AE, Bell MJ, Snell L, et al. The current state of musculoskeletal clinical skills teaching for preclerkship medical students. J Rheumatol. 2008;35(12):1384. doi:10.3899/jrheum.08030818464299
- Landewé R, van Tubergen A. Clinical tools to assess and monitor spondyloarthritis. Curr Rheumatol Rep. 2015;17(7):47. doi:10.1007/s11926-015-0522-326063534
- Machado P, Landewé R, Lie E, et al. Ankylosing Spondylitis Disease Activity Score (ASDAS): defining cut-off values for disease activity states and improvement scores. Ann Rheum Dis. 2011;70(1):47–53. doi:10.1136/ard.2010.13859421068095
- Smolen JS, Schöls M, Braun J, et al. Treating axial spondyloarthritis and peripheral spondyloarthritis, especially psoriatic arthritis, to target: 2017 update of recommendations by an international task force. Ann Rheum Dis. 2018;77(1):3–17. doi:10.1136/annrheumdis-2017-21173428684559
- Ramiro S, van der Heijde D, van Tubergen A, et al. Higher disease activity leads to more structural damage in the spine in ankylosing spondylitis: 12-year longitudinal data from the OASIS cohort. Ann Rheum Dis. 2014;73(8):1455–1461. doi:10.1136/annrheumdis-2014-20517824812292
- Poddubnyy D, Protopopov M, Haibel H, Braun J, Rudwaleit M, Sieper J. High disease activity according to the Ankylosing Spondylitis Disease Activity Score is associated with accelerated radiographic spinal progression in patients with early axial spondyloarthritis: results from the GErman SPondyloarthritis Inception Cohort. Ann Rheum Dis. 2016;75(12):2114–2118. doi:10.1136/annrheumdis-2016-20920927125522
- Pereira IA, Neves FS, Castro GRW. Extra-articular manifestations in spondyloarthritis are common and should be screened. Rheumatol Curr Res. 2012;2:3.
- Gupta N, Agarwal A. Management of uveitis in spondyloarthropathy: current trends. Perm J. 2018;22:17–041.
- Przepiera-Będzak H, Fischer K, Brzosko M. Extra-articular symptoms in constellation with selected serum cytokines and disease activity in spondyloarthritis. Mediators Inflamma. 2016;7617954.
- Sieper J, Rudwaleit M, Baraliakos X, et al. The Assessment of SpondyloArthritis international Society (ASAS) handbook: a guide to assess spondyloarthritis. Ann Rheum Dis. 2009;68(Suppl 2):ii1–44. doi:10.1136/ard.2008.10401819433414
- Essers I, Boonen A, Busch M, et al. Fluctuations in patient reported disease activity, pain and global being in patients with ankylosing spondylitis. Rheumatology (Oxford). 2016;55(11):2014–2022. doi:10.1093/rheumatology/kew30327520798
- Tan S, Wang R, Ward MM. Syndesmophyte Growth in Ankylosing Spondylitis. Curr Opin Rheumatol. 2015;27(4):326–332. doi:10.1097/BOR.000000000000017926002023
- Landewé R, Dougados M, Mielants H, van der Tempel H, van der Heijde D. Physical function in ankylosing spondylitis is independently determined by both disease activity and radiographic damage of the spine. Ann Rheum Dis. 2009;68(6):863–867. doi:10.1136/ard.2008.09179318628283
- Zangi HA, Ndosi M, Adams J, et al. EULAR recommendations for patient education for people with inflammatory arthritis. Ann Rheum Dis. 2015;74(6):954–962. doi:10.1136/annrheumdis-2014-20680725735643
- Sudre A, Figuereido IT, Lukas C, Combe B, Morel J. On the impact of a dedicated educational program for ankylosing spondylitis: effect on patient satisfaction, disease knowledge and spinal mobility, a pilot study. Joint Bone Spine. 2012;79(1):99–100. doi:10.1016/j.jbspin.2011.06.00521852173
- Candelas G, Villaverde V, García S, Guerra M, León MJ, Cañete JD. Benefit of health education by a training nurse in patients with axial and/or peripheral psoriatic arthritis: a systematic literature review. Rheumatol Int. 2016;36(11):1493–1506. doi:10.1007/s00296-016-3549-527544392
- World Health Organization. WHO report on the global tobacco epidemic, 2017 Country profile: Kuwait Available from: http://www.who.int/tobacco/surveillance/policy/country_profile/kwt.pdf. Accessed 7 2018.
- Kaut IK, Abourazzak FE, Jamila E, Sènami FA, Diketa D, Taoufik H. Axial spondyloarthritis and cigarette smoking. Open Rheumatol J. 2017;11(1):53–61. doi:10.2174/187431290171101005328659998
- Poddubnyy D, Haibel H, Listing J, et al. Baseline radiographic damage, elevated acute-phase reactant levels, and cigarette smoking status predict spinal radiographic progression in early axial spondyloarthritis. Arthritis Rheum. 2012;64:1388–1398. doi:10.1002/art.3346522127957
- Ramiro S, Landewé R, van Tubergen A, et al. Lifestyle factors may modify the effect of disease activity on radiographic progression in patients with ankylosing spondylitis: a longitudinal analysis. RMD Open. 2015;1(1):e000153. doi:10.1136/rmdopen-2015-00015326535153
- Chung HY, Machado P, van der Heijde D, D’Agostino MA, Dougados M. Smokers in early axial spondyloarthritis have earlier disease onset, more disease activity, inflammation and damage, and poorer function and health-related quality of life: results from the DESIR cohort. Ann Rheum Dis. 2012;71(6):809–816. doi:10.1136/annrheumdis-2011-20018021989541
- Romanowski MW, Špiritović M, Rutkowski R, Dudek A, Samborski W, Straburzyńska-Lupa A. Comparison of deep tissue massage and therapeutic massage for lower back pain, disease activity, and functional capacity of ankylosing spondylitis patients: a randomized clinical pilot study. Evid Based Complementary Alternat Med. 2017;9894128.
- Millner JR, Barron JS, Beinke KM, et al. Exercise for ankylosing spondylitis: an evidence-based consensus statement. Semin Arth Rheum. 2016;45:411–427. doi:10.1016/j.semarthrit.2015.08.00326493464
- Chunco R. The effects of massage on pain, stiffness, and fatigue levels associated with ankylosing spondylitis: a case study. Int J Ther Massage Bodywork. 2011;4:12–17.21589691
- Baraliakos X, Kiltz U, Peters S, et al. Efficiency of treatment with non-steroidal anti-inflammatory drugs according to current recommendations in patients with radiographic and non-radiographic axial spondyloarthritis. Rheumatology (Oxford). 2017;56(1):95–102. doi:10.1093/rheumatology/kew36727997346
- Wanders A, Heijde D, Landewé R, et al. Nonsteroidal antiinflammatory drugs reduce radiographic progression in patients with ankylosing spondylitis: a randomized clinical trial. Arthritis Rheum. 2005;52(6):1756–1765. doi:10.1002/art.2105415934081
- Kroon F, Landewé R, Dougados M, van der Heijde D. Continuous NSAID use reverts the effects of inflammation on radiographic progression in patients with ankylosing spondylitis. Ann Rheum Dis. 2012;71:1623–1629. doi:10.1136/annrheumdis-2012-20137022532639
- Borer JS, Simon LS. Cardiovascular and gastrointestinal effects of COX-2 inhibitors and NSAIDs: achieving a balance. Arthritis Res Ther. 2005;7:14–22. doi:10.1186/ar1794
- Rice JB, White AG, Scarpati LM, Wan G, Nelson WW. Long-term systemic corticosteroid exposure: a systematic literature review. Clin Ther. 2017;39(11):2216–2229. doi:10.1016/j.clinthera.2017.09.01129055500
- Haibel H, Fendler C, Listing J, Callhoff J, Braun J, Sieper J. Efficacy of oral prednisolone in active ankylosing spondylitis: results of a double-blind, randomised, placebo-controlled short-term trial. Ann Rheum Dis. 2014;73(1):243–246. doi:10.1136/annrheumdis-2012-20305523625982
- Fraser AD, van Kuijk AWR, Westhovens R, et al. A randomised, double blind, placebo controlled, multicentre trial of combination therapy with methotrexate plus ciclosporin in patients with active psoriatic arthritis. Ann Rheum Dis. 2005;64:859–864. doi:10.1136/ard.2004.02446315528283
- Gossec L, Smolen JS, Ramiro S, et al. European League Against Rheumatism (EULAR) recommendations for the management of psoriatic arthritis with pharmacological therapies: 2015 update. Ann Rheum Dis. 2016;75:499–510. doi:10.1136/annrheumdis-2015-20833726644232
- van der Heijde D, Salonen D, Weissman BN, et al. Assessment of radiographic progression in the spines of patients with ankylosing spondylitis treated with adalimumab for up to 2 years. Arthritis Res Ther. 2009;11(4):R127. doi:10.1186/ar279419703304
- Baraliakos X, Listing J, Fritz C, et al. Persistent clinical efficacy and safety of infliximab in ankylosing spondylitis after 8 years–early clinical response predicts long-term outcome. Rheumatology (Oxford). 2011;50:1690–1699. doi:10.1093/rheumatology/ker19421672969
- Sepriano A, Regel A, van der Heijde D, et al. Efficacy and safety of biological and targeted-synthetic DMARDs: a systematic literature review informing the 2016 update of the ASAS/EULAR recommendations for the management of axial spondyloarthritis. RMD Open. 2017;3(1):e000396. doi:10.1136/rmdopen-2016-00039628176964
- Veale DJ, McGonagle D, McInnes IB, et al. The rationale for Janus kinase inhibitors for the treatment of spondyloarthritis. Rheumatology (Oxford). 2019;58(2):197–205. doi:10.1093/rheumatology/key07029618084
- Baraliakos X, van den Berg R, Braun J, van der Heijde D. Update of the literature review on treatment with biologics as a basis for the first update of the ASAS/EULAR management recommendations of ankylosing spondylitis. Rheumatology. 2012;51(8):1378–1387. doi:10.1093/rheumatology/kes02622427410
- Saougou I, Markatseli TE, Voulgari PV, Drosos AA. Maintained clinical response of infliximab treatment in ankylosing spondylitis: a 6-year long-term study. Joint Bone Spine. 2010;77(4):325–329. doi:10.1016/j.jbspin.2010.02.01420452801
- Wu D, Guo -Y-Y, Xu -N-N, et al. Efficacy of anti–tumor necrosis factor therapy for extra-articular manifestations in patients with ankylosing spondylitis: a meta–analysis. BMC Musculoskelet Disord. 2015;16(1):19. doi:10.1186/s12891-015-0489-225888248
- Calvo-Río V, Blanco R, Santos-Gómez M, et al. Golimumab in refractory uveitis related to spondyloarthritis. Multicenter study of 15 patients. Semin Arthritis Rheum. 2016;46:95–101. doi:10.1016/j.semarthrit.2016.03.00227060872
- Braun J, Baraliakos X, Listing J, et al. Differences in the incidence of flares or new onset of inflammatory bowel diseases in patients with ankylosing spondylitis exposed to therapy with anti-tumor necrosis factor alpha agents. Arthritis Rheum. 2007;57:639–647. doi:10.1002/art.2266917471540
- Jeon C, Sekhon S, Yan D, Afifi L, Nakamura M, Bhutani T. Monoclonal antibodies inhibiting IL-12, −23, and −17 for the treatment of psoriasis. Hum Vaccin Immunother. 2017;13(10):2247–2259. doi:10.1080/21645515.2017.135649828825875
- Langley RG, Elewski BE, Lebwohl M, et al. Secukinumab in plaque psoriasis–results of two Phase 3 trials. N Engl J Med. 2014;371:326–338. doi:10.1056/NEJMoa131425825007392
- Thibodaux RJ, Triche MW, Espinoza LR. Ustekinumab for the treatment of psoriasis and psoriatic arthritis: a drug evaluation and literature review. Expert Opin Biol Ther. 2018;18(7):821–827. doi:10.1080/14712598.2018.149254529949399
- Kehl AS, Corr M, Weisman WH. Enthesitis. New insights into pathogenesis, diagnostic modalities, and treatment. Arthritis Rheumatol. 2016;68:312–322. doi:10.1002/art.3945826473401
- van der Heijde D, Braun J, Deodhar A, et al. Comparison of three enthesitis indices in a multicentre, randomized, placebo-controlled trial of golimumab in ankylosing (GO-RAISE). Rheumatology. 2013;52:321–325. doi:10.1093/rheumatology/kes25123024015
- Rudwaleit M, Claudepierre P, Kron M, Kary S, Wong R, Kupper H. Effectiveness of adalimumab in treating patients with ankylosing spondylitis associated with enthesitis and peripheral arthritis. Arthritis Res Ther. 2010;12(2):R43. doi:10.1186/ar295320230622
- Feuchtenberger M, Schäfer A, Nigg AP, Kraus MR. Hepatitis B serology in patients with rheumatic diseases. Open Rheumatol J. 2016;10(1):39–48. doi:10.2174/187431290161001003927708728
- Xie X, Li F, Chen JW, Wang J. Risk of tuberculosis infection in anti-TNF-a biological therapy: from bench to bedside. J Microbiol Immunol Infect. 2014;47:268–274. doi:10.1016/j.jmii.2013.03.00523727394
- Mori S, Fujiyama S. Hepatitis B virus reactivation associated with antirheumatic therapy: risk and prophylaxis recommendations. World J Gastroenterol. 2016;21:10274–10289. doi:10.3748/wjg.v21.i36.10274
- World Health Organization. Guidelines on the management of latent tuberculosis infection. Available from: http://www.who.int/tb/publications/ltbi_document_page/en/. Accessed 291 2019.
- World Health Organization. Chest radiography in tuberculosis detection. Available from: https://www.who.int/tb/publications/chest-radiography/en/. Accessed 291 2019.
- Ledingham J, Gullick N, Irving K, et al. BSR and BHPR guideline for the prescription and monitoring of non-biologic disease-modifying anti-rheumatic drugs. Rheumatology. 2017;56(6):865–868. doi:10.1093/rheumatology/kew47928339817
- Ossum AM, Palm Ø, Lunder AK, et al. ankylosing spondylitis and axial spondyloarthritis in patients with long-term inflammatory bowel disease: results from 20 years of follow-up in the IBSEN study. J Crohns Colitis. 2018;12(1):96–104. doi:10.1093/ecco-jcc/jjx12628961700
- Mitulescu TC, Popescu C, Naie A, et al. Acute anterior uveitis and other extra-articular manifestations of spondyloarthritis. J Med Life. 2015;8(3):319–325.
- Ogdie A, Schwartzman S, Husni ME. Recognizing and managing comorbidities in psoriatic arthritis. Curr Opin Rheumatol. 2015;27(2):118–126. doi:10.1097/BOR.000000000000015225603040
- Lee S, Mendelsohn A, Sarnes E. The burden of psoriatic arthritis: a literature review from a global health systems perspective. P T. 2010;35:680–689.21197266
- Winthrop KL, Strand V, van der Heijde DM, et al. The unmet need in rheumatology: reports from the targeted therapies meeting 2016. Clin Exp Rheumatol. 2016;4(Suppl 98):69–76.
- Gelfand J. Metabolic syndrome in patients with psoriatic disease. J Rheumatol Suppl. 2012;89:24–28. doi:10.3899/jrheum.12023722751586
- Mehta NN, Yu Y, Saboury B, et al. Systemic and vascular inflammation in patients with moderate to severe psoriasis as measured by [18F]-fluorodeoxyglucose positron emission tomography-computed tomography (FDG-PET/CT): a pilot study. Arch Dermatol. 2011;147:1031–1039. doi:10.1001/archdermatol.2011.11921576552
- Gelfand JM, Dommasch ED, Shin DB, et al. The risk of stroke in patients with psoriasis. J Invest Dermatol. 2009;129(10):2411–2418. doi:10.1038/jid.2009.11219458634
- Ludwig RJ, Herzog C, Rostock A, et al. Psoriasis: a possible risk factor for development of coronary artery calcification. Br J Dermatol. 2007;156(2):271–276. doi:10.1111/j.1365-2133.2006.07562.x17223866
- Gladman DD, Ang M, Su L, Tom BD, Schentag CT, Farewell VT. Cardiovascular morbidity in psoriatic arthritis. Ann Rheum Dis. 2009;68(7):1131–1135. doi:10.1136/ard.2008.09483918697777
- Raychaudhuri SK, Chatterjee S, Nguyen C, Kaur M, Jialal I, Raychaudhuri SP. Increased prevalence of the metabolic syndrome in patients with psoriatic arthritis. Metab Syndr Relat Disord. 2010;8(4):331–334. doi:10.1089/met.2009.012420367239