Abstract
Background
Fatigue is a prevalent symptom affecting primary Sjögren’s syndrome (pSS) patients. The purpose of this study is to determine the prevalence of fatigue in Saudi pSS patients and its correlation with disease features and outcome measures using a validated tool.
Methods
This is a cross-sectional study evaluating fatigue in pSS using the Arabic version of the fatigue severity scale (FSS). The EULAR Sjögren’s syndrome disease activity index (ESSDAI) and EULAR Sjögren’s syndrome patient reported index (ESSPRI) were calculated.
Results
Forty-one patients met the sample criteria and were involved in the final report. There were predominantly females (78%) with a mean (±SD) age and disease duration of 58.76±12.7 and 4.6±2.28 years, respectively. Based on the FSS, 18 (43.9%) patients had a positive test with a mean score of 5.43±0.76. The mean ESSDAI was 9.95±7.73, while the mean EESPRI was 5.17±2.4 with individual component scores were dryness (5.23±2.62), fatigue (5.4±2.88), and pain (4.88±3.31). The FSS had a significant correlation with PGA (r=0.559; p<0.001), PhGA (r=0.671; p<0.001), ESSDAI (r=0.402; p=0.01), ESSPRI fatigue component (r=0.0.621; p<0.001), ESSPRI pain component (r=0.558; p<0.001), and missed significance for the ESSPRI dryness component (r=0.289; p=0.071). There was no correlation between the total ESSPRI score and presence of fatigue (r=−0.261; p=0.104) nor the FSS score (r=−0.136; p=0.409).
Conclusion
Fatigue is prevalent in Saudi pSS patients. FSS correlated with ESSDAI and ESSPRI components but not its total score signaling other unmeasured factors contributing to fatigue development.
Keywords:
Background
Primary Sjögren’s syndrome (pSS) is a chronic autoimmune inflammatory disease which is characterized by infiltration of exocrine glands by autoreactive lymphocytes leading to destruction and glandular dysfunction manifested by sicca symptoms.Citation1–Citation3 It primarily affects people with a male/female ratio of 1:9, and the disease peak prevalence is about 50 years of age. The etiology of pSS is not yet completely known.Citation4 The pSS is clinically defined by fatigue, dryness and disabling pain and is immunologically consistent with La/SSB, Ro/SSA, and antinuclear antibodies and the development of ectopic lymphoid structures formed in the target organs of the condition, primarily lacrimal and salivary glands, in up to one-third of the patients.Citation5 The systemic disease occurs in up to 81.8% of patients with different organ involvement.Citation6 The estimated incidence rate of pSS is 61 in 10,000 patients a year, and the overall incidence rate of pSS is between 0.1% and 4.8%. However, since there are other non-specific symptoms, the incidence could be undervalued.Citation4 Fatigue is one of the most common general symptoms, which arises in patients with pSS up to 70% to 80% and is associated with decreased quality of life.Citation7–Citation9 The nature of fatigue suggested different and unclear complex mechanisms including genetics and inflammatory factors and its one of the most important predictor outcome measures in many chronic autoimmune diseases including pSS. Using the multidimensional inventory of fatigue (MFI), the fatigue in pSS has been well documented, in which the ratings of pSS patients were twice the worst in all aspects relative to controls. Besides, pSS also displays chronic pain owing to underlying polyarthralgia and/or fibromyalgia.Citation10 The Sjogren’s syndrome prognosis is favourable. The average life span of patients with pSS is equivalent to that of the general population. Nevertheless, the quality of life of the patients is affected by the multiple forms of the disease. The significant causes of mortality are lymphoma, solid tumours, infections, and cardiovascular disease.Citation11 A number of variable clinical studies have been used different existing methods and questionnaires to measure fatigue in systemic rheumatic diseases including pSS patients; however, not all of these tools are specific for pSS and it is not clear that the fatigue measurement methods will be able to understand the nature and improvement of fatigue inpatient with pSS in response to medical intervention.Citation12 Fatigue has been included as one of the components of the European League Against Rheumatism (EULAR) Sjogren’s syndrome patient reported index (ESSPRI) evaluated by a visual analogue scale (VAS) from 0 to 10.Citation13 Different types of questionnaires including the multidimensional fatigue inventory, fatigue impact scale, fatigue severity scale (FSS), and visual analogue fatigue scale to be analyzed for the assessment of symptoms.Citation12,Citation14 For the measure of fatigue severity in patients with systemic lupus erythematosus (SLE), and multiple sclerosis (MS), the FSS was initially developed by Krupp and his co-workers in the years of 1989, the FSS is a simple and easy to use self-reported questionnaire. It consists of nine statements that assess the seriousness of the patient’s symptoms of exhaustion in terms of how these symptoms influence activities of daily living, physical function, exercise, and motivation around the day over the past week.Citation15 The FSS is commonly used in research and clinical practice and translated into a variety of languages. The validated Arabic variant of FSS-Arabic (FSS-Ar) on patients with systemic lupus erythematosus and multiple sclerosis but not on pSS patientsCitation16 with proven good psychometric properties in patients with stroke could provide excellent benefits to researchers and clinicians to examine the effect and incidence of fatigue associated with pSS patients. Therefore, the current study aims to evaluate the prevalence of fatigue in Saudi pSS utilizing the Arabic version of FSS (FSS-Ar), analyze its individual components, and evaluate its correlation with clinical characteristics and pSS composite outcome measures.
Materials and Methods
Study Design and Selection of Patients
The current cross-sectional study was conducted in a single center involving patients with pSS following in both the rheumatology and pulmonary clinics at King Saud University Medical City in Saudi Arabia. This study was conducted between October 2018 and May 2019 with 46 patients with pSS. Saudi patients aged ≥18 years with a diagnosis of pSS based on the classification criteria of the American College of Rheumatology (ACR)/EULAR (14) were invited to participate in the study. The exclusion criteria of the study included a confirmed diagnosis of malignancy, major psychiatric disorder, and the presence of end-organ failure. The clinical part of the research involves genetic study, cytokine profile, and clinical analysis of pSS. Patients’ clinical characteristics, medications, laboratory investigations, autoimmune profile were collected from patients’ charts. During clinic visits, the fatigue severity scale (FSS), patient global assessment (PGA), physician global assessment (PhGA), and clinical components of ESSPRI were completed. The ESSPRI components were calculated individually and as a single factor composed of the mean of the three components (pain, fatigue, and dryness). Based on the 12 domains’ score, the EULAR Sjögren’s syndrome disease activity index (ESSDAI) was measured based on.Citation17–Citation19 The patients were divided into seronegative defined as having pSS with no positivity in ANA, SSA, SSB nor rheumatoid factor (RF), while seropositive if any antibody is positive at least once.
Evaluation of Fatigue
The Arabic version of FSS (FSS-Ar) was used for the evaluation of fatigue in patients with pSS. The FSS consists of 9 questions that are used for the evaluation of the effect of fatigue over the last week with each component scoring from 1 to 7 with 7 signifying a worse score. An FSS was considered if the patient scored ≥4 of the mean score.
Statistical Analysis
The statistical analysis was carried out using version 20.0 of the SPSS software (IBM Corporation, Armonk, NY, US) and Microsoft Excel. Characteristics of the disease and demographic data were presented by descriptive analysis. Recorded results have been described as mean ± standard deviation for clinical research. The correlation coefficient of Spearman’s rank (or Spearman’s rho) was used to analyze the relationship between two variables of alpha 0.05.
Ethical Consideration
This study was approved by the institutional research board (IRB) at the College of Medicine at King Saud University (E-18-3206) and IRB at Princess Nourah bint Abdulrahman University IRB (19–0100). All procedures were performed according to the declaration of Helsinki. Informed consents were obtained from all participating patients in this study.
Results
Patient Characteristics
Among the screened 46 patients in this study, 41 patients were included in the final investigation of the study. Compared to males, the females were the most predominant in this study (78%) with a mean (±SD) age and disease duration of 58.76±12.7 and 4.6±2.28 years, respectively, as shown in which present Patient demographics, Laboratory variable and outcome measures.
Table 1 Present Patient Demographics, Laboratory Variable and Outcome Measures are Presented as Means (±SD) and Percentages
Global Assessment of Physician and Patients
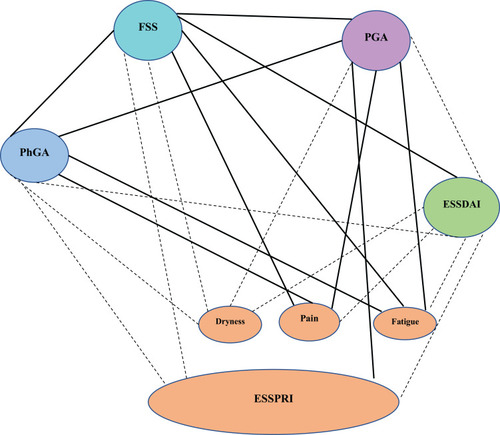
The mean value of ESSDAI and ESSPRI was 9.95±7.73 and 5.17±2.4, respectively, with a lack of correlation (r=0.066; p =0.68). The mean PGA and PhGA were 5.2±2.37 and 4.46±2.06, respectively, with both correlating to each other significantly (r=0.659; p <0.001). PGA and PhGA had a good correlation with the ESSPRI pain component (r=0.773; p<0.001) and (r=0.659; p<0.001) respectively, and the ESSPRI fatigue component (r=0. 0.696; p<0.001) and (r=0.650; p<0.001), respectively. While there was a signal with the ESSPRI dryness component, but it did not reach statistical significance (r=0.303; p=0.057) and (r=0. 0.286; p=0.073). On the other hand, there was no significant correlation between ESSDAI score and PGA (r=0.045; p=0.783) nor with the PhGA (r=0.174; p=0.276).
Fatigue and Disease Outcome Measures
The mean FSS value of the whole cohort was 3.75±1.73. Based on the FSS, 18 (43.9%) patients had a positive test with a mean score of 5.43±0.76. The result of FSS questions was measured by the positive and negative impact of fatigue in patients with pSS. The evaluation of the impact of fatigue by FSS showed question 1(My motivation is lower when I am fatigued) highest mean score followed by question 5 (Fatigue causes frequent problems for me) (). The mean ESSDAI and EESPRI were 9.95±7.73 and 5.17±2.4, respectively. The mean ESSPRI individual component scores were dryness (5.23±2.62), fatigue (5.4±2.88), and pain (4.88±3.31). The FSS had a significant correlation with PGA (r=0.559; p<0.001), PhGA (r=0.671; p<0.001), ESSDAI (r=0.402; p=0.01), ESSPRI fatigue component (r=0.0.621; p<0.001), ESSPRI pain component (r=0.558; p<0.001) and missed significance for the ESSPRI dryness component (r=0.289; p=0.071). There was no significant correlation between the total ESSPRI score and presence of fatigue (r=−0.261; p=0.104) nor the FSS score (r=−0.136; p=0.409) ()
Figure 1 Graphical representation of mean values of the fatigue severity score sub-scores with a radar chart in patients who scored positive (solid lines) and negative (dashed lines).Q1 My motivation is lower when I am fatigued, Q2 Exercise brings on my fatigue, Q3 I am easily fatigued, Q4 Fatigue interferes with my physical functioning, Q5 Fatigue causes frequent problems for me, Q6 My fatigue prevents sustained physical functioning, Q7 Fatigue interferes with carrying out certain duties and responsibilities, Q8 Fatigue is among my most disabling symptoms, Q9 Fatigue interferes with my work, family, or social life.
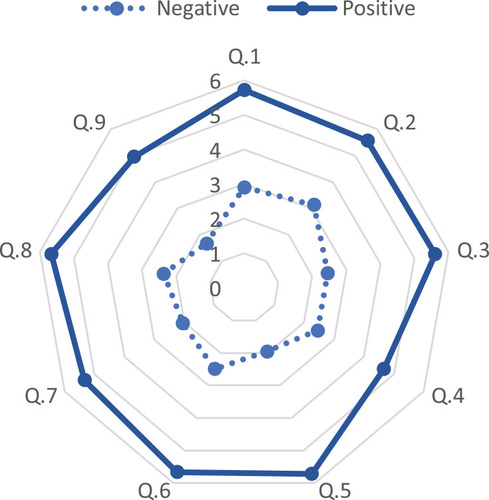
Figure 2 The statistical correlation between the fatigue severity score and disease-related measures using the Spearman correlation with solid line representing a statistically significant correlation (p<0.05) and the dashed line representing a non-significant correlation.
Fatigue and Antibody Seropositivity
The rate of antibody positivity was as follows: ANA, RF, SSA, and SSB were in 32 (79%), 10 (24.4%), 19 (46.3%), and 12 (29.3%) patients, respectively, while 8 (19.5%) patients were tested negative for all 4 autoantibodies. Six (75%) seronegative patients had a positive FSS compared to 12 (36.4%) seropositive. There was no correlation between the FSS and the focus score on salivary gland biopsy (n=24)
Discussion
The quality of life and well-being of patients are greatly affected by fatigue and it is the most incapacitating symptom.Citation20,Citation21 Fatigue is the most frequent general symptom that arises up to 70% to 80% of patients with pSS. It is the most debilitating concern and the most challenging to treat in many patients with pSS.Citation18 In this study, we evaluated the fatigue in pSS patients using FSS-Ar, a validated Arabic version, and the fatigue component of the ESSPRI. Previously several researchers evaluated the fatigue using FSS which is similar to the present study.Citation22–Citation25 Our study is the first in the Arabian Gulf region. It has shown that fatigue is not only prevalent but also correlated with disease activity outcome measures and patient/physician reported outcome measures emphasizing its importance and interaction with disease components. The study showed 43.9% of the positive fatigue score of patients with pSS measured by the FSS. Similarly, Segal and co-workersCitation14 evaluated 94 patients with pSS and reported a positive fatigue score in 67% of patients using the FSS. Depression, helplessness, and pain were the most important predictors of fatigue.
In this study, the antibodies ANA, RF, SSA, and SSB were positively associated with patients with pSS. There was no correlation between the FSS and the focus score on salivary gland biopsy (n=24). In contrast, the study by Haldorsen et alCitation22 reported poor correlations with the high serum anti-Sjögren syndrome A antigen (anti-SSA) and strong baseline fatigue and lower baseline unstimulated whole salivary volume in patients with elevated fatigue. A prospective study by Haldorsen et alCitation22 reported the FSS score of ≥4 was found in 70.7% of among 122 patients with pSS. At year 5, 35% reported a clinically significant increase in FSS of ≥6 points but neither in the FACIT-F nor the fatigue VAS. In our study, we have found an excellent correlation between the FSS and fatigue component of the ESSPRI indicating that a simple VAS for fatigue might be adequate to screen for fatigue in pSS patients. Limitations of our study include sample size, selection bias, the cross-sectional nature of the study, and the lack of fibromyalgia assessment in the studied population which could be the target of a future study. Despite that, this report highlights the high prevalence of fatigue in Saudi pSS patients and its interaction with disease activity and patient-reported outcome measures. There is a need to perform larger prospective studies to better understand the natural course of fatigue in Saudi patients with pSS.
Conclusion
In conclusion, the ability to assess the frequency and extent of fatigue includes the usage of accurate and appropriate measurement instruments to successfully treat individuals with fatigue as a primary symptom, such as those with pSS disorders. In this study, using Arabic version of FSS (FSS-Ar) which could measure the fatigue presence and severity in a sample of Saudi pSS patients, encouraging their use in clinical practice and research.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
All authors disclose no relevant conflicts of interest related to this work.
Additional information
Funding
References
- Retamozo S, Acar-Denizli N, Rasmussen A, et al. Systemic manifestations of primary Sjögren’s syndrome out of the ESSDAI classification: prevalence and clinical relevance in a large international, multi-ethnic cohort of patients. Clin Exp Rheumatol. 2019.
- Sandhya P, Kurien B, Danda D, Scofield R. Update on pathogenesis of Sjogren’s syndrome. Curr Rheumatol Rev. 2016. doi:10.2174/1573397112666160714164149
- Bayetto K, Logan RM. Sjögren’s syndrome: a review of aetiology, pathogenesis, diagnosis and management. Aust Dent J. 2010;55:39–47. doi:10.1111/j.1834-7819.2010.01197.x20553243
- Qin B, Wang J, Yang Z, et al. Epidemiology of primary Sjögren’s syndrome: a systematic review and meta-analysis. Ann Rheum Dis. 2015;74(11):1983–1989. doi:10.1136/annrheumdis-2014-20537524938285
- Brito-Zerón P, Acar-Denizli N, Ng WF, et al. How immunological profile drives clinical phenotype of primary Sjögren’s syndrome at diagnosis: analysis of 10,500 patients (Sjögren big data project). Clin Exp Rheumatol. 2018.
- Brito-Zerón P, Acar-Denizli N, Ng WF, et al. Epidemiological profile and north-south gradient driving baseline systemic involvement of primary Sjögren’s syndrome. Rheumatology (Oxford). 2020;59(9):2350–2359. doi:10.1093/rheumatology/kez57831873754
- Barendregt PJ, Visser MRM, Smets EMA, et al. Fatigue in primary Sjogren’s syndrome. Ann Rheum Dis. 1998;57(5):291–295. doi:10.1136/ard.57.5.2919741313
- Westhoff G, Dörner T, Zink A. Fatigue and depression predict physician visits and work disability in women with primary Sjögren’s syndrome: results from a cohort study. Rheumatology. 2012;51(2):262–269. doi:10.1093/rheumatology/ker20821705778
- Lackner A, Ficjan A, Stradner MH, et al. It’s more than dryness and fatigue: the patient perspective on health-related quality of life in primary SjoÈgren’s syndrome - a qualitative study. PLoS One. 2017;12(2):e0172056. doi:10.1371/journal.pone.017205628182787
- Jousse-Joulin S, Morvan J, Devauchelle-Pensec V, Saraux A. Ultrasound assessment of the entheses in primary Sjögren syndrome. Ultrasound Med Biol. 2013;39(12):2485–2487. doi:10.1016/j.ultrasmedbio.2013.05.01324035411
- Singh AG, Singh S, Matteson EL. Rate, risk factors and causes of mortality in patients with Sjögren’s syndrome: a systematic review and meta-analysis of cohort studies. Rheumatol. 2016. doi:10.1093/rheumatology/kev354
- Miyamoto ST, Lendrem DW, Ng WF, Hackett KL, Valim V. Managing fatigue in patients with primary Sjögren’s syndrome: challenges and solutions. Open Access Rheumatol. 2019. doi:10.2147/OARRR.S167990
- Shiboski CH, Shiboski SC, Seror R, et al. 2016 American college of rheumatology/European league against rheumatism classification criteria for primary Sjögren’s syndrome: a consensus and data-driven methodology involving three international patient cohorts. Arthritis Rheum. 2017;69(1):35–45. doi:10.1002/art.39859
- Segal B, Thomas W, Rogers T, et al. Prevalence, severity, and predictors of fatigue in subjects with primary Sjögren’s syndrome. Arthritis Care Res. 2008;59(12):1780–1787. doi:10.1002/art.24311
- Krupp LB. The fatigue severity scale. Arch Neurol. 1989;46(10):1121. doi:10.1001/archneur.1989.005204601150222803071
- Al-Sobayel HI, Al-Hugail HA, Alsaif RM, et al. Validation of an Arabic version of fatigue severity scale. Saudi Med J. 2016;37(1):73–78. doi:10.15537/smj.2016.1.1305526739978
- Seror R, Bootsma H, Saraux A, et al. Defining disease activity states and clinically meaningful improvement in primary Sjögren’s syndrome with EULAR primary Sjögren’s syndrome disease activity (ESSDAI) and patient-reported indexes (ESSPRI). Ann Rheum Dis. 2016;75(2):382–389. doi:10.1136/annrheumdis-2014-20600825480887
- Brito-Zerón P, Theander E, Baldini C, et al. Early diagnosis of primary Sjögrens syndrome: EULAR-SS task force clinical recommendations. Expert Rev Clin Immunol. 2016;12(2):137–156. doi:10.1586/1744666X.2016.110944926691952
- Seror R, Theander E, Brun JG, et al. Validation of EULAR primary Sjögren’s syndrome disease activity (ESSDAI) and patient indexes (ESSPRI). Ann Rheum Dis. 2015;74(5):859–866. doi:10.1136/annrheumdis-2013-20461524442883
- Meijer JM, Meiners PM, Slater JJRH, et al. Health-related quality of life, employment and disability in patients with Sjögren’s syndrome. Rheumatology. 2009;48(9):1077–1082. doi:10.1093/rheumatology/kep14119553376
- Cornec D, Devauchelle-Pensec V, Mariette X, et al. Severe health-related quality of life impairment in active primary Sjögren’s syndrome and patient-reported outcomes: data from a large therapeutic trial. Arthritis Care Res. 2017;69(4):528–535. doi:10.1002/acr.22974
- Haldorsen K, Bjelland I, Bolstad AI, Jonsson R, Brun JG. A five-year prospective study of fatigue in primary Sjögren’s syndrome. Arthritis Res Ther. 2011;13(5):R167. doi:10.1186/ar348721996338
- Norheim KB, Harboe E, Gøransson LG, Omdal R. Interleukin-1 inhibition and fatigue in primary Sjögren’s syndrome - a double blind, randomised clinical trial. PLoS One. 2012;7(1):e30123. doi:10.1371/journal.pone.003012322253903
- Yacoub YI, Rostom S, Laatiris A, Hajjaj-Hassouni N. Primary Sjögren’s syndrome in Moroccan patients: characteristics, fatigue and quality of life. Rheumatol Int. 2012. doi:10.1007/s00296-011-2009-5
- Milin M, Cornec D, Chastaing M, et al. Sicca symptoms are associated with similar fatigue, anxiety, depression, and quality-of-life impairments in patients with and without primary Sjögren’s syndrome. Joint Bone Spine. 2016;83(6):681–685. doi:10.1016/j.jbspin.2015.10.00526774177
