Abstract
Rheumatoid arthritis (RA) is a chronic inflammatory disease that is associated with joint damage and progressive disability, an increased risk of morbidity related to comorbid conditions and substantial socioeconomic costs. Tumor necrosis factor-alpha (TNF-α) is a proinflammatory cytokine known to have a central role in the initial host response to infection and in the pathogenesis of various immune-mediated diseases, such as RA, ankylosing spondylitis, psoriasis and/or psoriatic arthritis, Crohn’s disease, and systemic lupus erythematosus. Five TNF-α inhibitors are available for the clinical use: infliximab; adalimumab; etanercept; golimumab; and certolizumab pegol. Infliximab is a chimeric human/murine IgG1 monoclonal antibody (mAb); adalimumab, and golimumab are human mAbs; certolizumab pegol is composed of the fragment antigen-binding anti-binding domain of a humanized anti-TNF-α mAb, combined with polyethylene glycol to increase its half-life in the body; etanercept is a fusion protein that acts as a “decoy receptor” for TNF-α. In this paper, we will briefly review the current data on efficacy and safety of adalimumab in patients with RA, its potential beneficial effects upon comorbid conditions, such as endothelial dysfunction and accelerated atherosclerosis in RA, and the immunogenicity.
Introduction
Rheumatoid arthritis (RA) is a chronic inflammatory immune-mediated disease that is burdened by progressive joint damage and disability, increased risk of comorbidity and socioeconomic costs.Citation1–Citation3 The ongoing progresses in the knowledge of the pathogenic mechanisms of various immune-mediated diseases, such as RA, ankylosing spondylitis (AS), psoriasis (Ps) and/or psoriatic arthritis (PsA), Crohn’s disease (CD), systemic lupus erythematosus (SLE), and the availability of innovative biotechnological approaches, have led to the development of new drugs that add to conventional treatments. In particular, efforts have been made to design biologic drugs that are able to counteract the activity of different molecules (ie, tumor necrosis factor-α [TNF-α], interleukin 1 (IL-1), CD20, CD22, and CD11a). TNF-α is a proinflammatory cytokine known to have a central role in the initial host response to infection and in the pathogenesis of the above-mentioned diseases.Citation4 TNF-α inhibitors have demonstrated efficacy in large, randomized controlled clinical trials either as monotherapy or in combination with other anti-inflammatory or disease modifying antirheumatic drugs (DMARDs).Citation5–Citation10 Five TNF-α inhibitors are available for the clinical use: infliximab, adalimumab, etanercept, golimumab, and certolizumab pegol. All these agents block the biologic effects of TNF-α, although there are some differences in their structure, pharmacokinetics, and mechanisms of action. The efficacy and safety profile of the TNF-α inhibitors can be considered, in general, as a class effect. Nevertheless, some differences may exist among the five agents. Infliximab is a chimeric human/murine IgG1 monoclonal antibody (mAb), adalimumab, and golimumab are human mAbs, certolizumab pegol is composed of the fragment antigen-binding (Fab) domain of a humanized anti-TNF-α mAb combined with polyethylene glycol to increase its half-life in the body, etanercept is a fusion protein that acts as a “decoy receptor” for TNF-α.Citation5–Citation12 In particular, adalimumab is a fully recombinant human IgG1 anti-TNF-α-specific mAb which is approved for the treatment of Ps, PsA, RA, AS, and CD.Citation5–Citation12 However, the efficacy and safety of adalimumab administered as monotherapy or in combination with methotrexate (MTX) for the treatment of RA has been well-established in clinical trials.Citation13–Citation19 In this paper, we will briefly review the current data upon efficacy and safety of adalimumab in patients with RA, its potential beneficial effects upon comorbid conditions, such as endothelial dysfunction and accelerated atherosclerosis in RA, and the immunogenicity.
Efficacy and safety of adalimumab: how to optimize the treatment of RA
Adalimumab is a fully recombinant human immunoglobulin G1 (IgG1) anti-TNF-α-specific mAb that is capable of complement fixation and fragment crystallizable-(Fc) receptor binding. The plasma half-lives of antibodies appear to be largely related to the binding of their Fc regions to the neonatal Fc receptors (FcRn) on endothelial cells. The long plasma half-life of adalimumab suggests that it binds to FcRn like natural IgG1 molecules. Adalimumab is usually administered subcutaneously (40 mg every other week).Citation5–Citation12 Adalimumab was approved by the US Food and Drug Administration (FDA) in 2002 and was granted approval from the European Medicines Agency (EMA) in September 2003 for the treatment of RA.
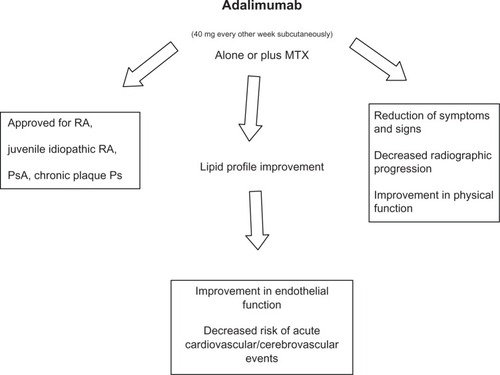
Adalimumab was subsequently approved by the FDA for the following indications: PsA (in 2005), AS (in 2006), and CD (in 2007), as well as for juvenile-idiopathic RA and chronic plaque Ps, (both in 2008). Adalimumab is also approved for the treatment of these diseases by the EMA.Citation5–Citation12 Patients with RA must meet the following criteria before TNF-α inhibitors can be administered:
failure to respond to an adequate trial of at least two DMARDs, including MTX at an optimal dose (at least 15 mg/week and maximum dose 25 mg/week) for a minimum of 3 months, or intolerance for MTX;
clinical evidence of active disease (multiple actively inflamed joints and/or Disease Activity Score uses 28 joint counts [DAS28] >3.2);
persistently elevated inflammatory markers, such as erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP).Citation8,Citation20
Notably, DAS28 has been widely used to monitor the disease activity of patients with RA. The DAS28 cut-off points of 2.6, 3.2, and 5.1 have been proposed to be indicative of remission, low-disease activity and high-disease activity, respectively.Citation8,Citation21 In particular, treatment is judged effective in case of improvement of the DAS28 ≥ 1.2 or after reaching DAS28 < 3.2 after 12 weeks of therapy. The PREMIER Study,Citation13 a multicenter randomized double-blind clinical trial of combination therapy with adalimumab plus MTX, enrolled 799 patients with early aggressive RA.Citation12 Patients were randomized to one of three treatment groups: adalimumab plus MTX (20 mg/week), adalimumab plus placebo, and MTX plus placebo. After 2 years of treatment, the combination therapy adalimumab plus MTX resulted significantly superior to adalimumab alone or MTX alone in improving symptoms and signs of the disease, decreasing radiographic progression and, finally, favoring disease remission.
The percentages of patients reporting both serious adverse events (AEs) and infectious AEs were similar in the three groups of treatment. Nevertheless, the rate of serious infections in the adalimumab monotherapy group was significantly lower than that in the combination treatment group, but not different compared with the MTX monotherapy group. In particular, in the combination treatment arm, one female patient developed pleural tuberculosis (TB). This patient had no purified protein derivative test performed, had a negative chest X-ray at baseline, and did not receive primary prophylactic isoniazid before starting treatment. However, as we have previously reported, although TNF-α inhibitors are generally well-tolerated, physicians should be aware of the potential adverse events of these drugs.Citation5–Citation12 Indeed, TNF-α favors the recruitment and the activation of lymphocytes, neutrophils, and platelets, the expression of adhesion molecules (intercellular adhesion molecule-1 [ICAM-I], vascular cell adhesion molecule-1 [VCAM-1], selectins) on endothelial cells and induces the neo-angiogenesis in the sites of phlogosis. TNF has a central role in the initial host response to infection.Citation6 In TB, it results in macrophage activation, cell recruitment, granuloma formation, and maintenance of granuloma integrity.Citation4,Citation8,Citation22 The overall risk of reactivation of latent TB and opportunistic infections should be considered before the beginning of therapy. Appropriate screening with the Mantoux test and chest X-ray should always be performed before starting treatment. Skin induration of 5 mm or greater should be interpreted as a positive result for latent TB (LTB) in any patient considered for anti-TNF-α treatment. However, the negative Mantoux test should be interpreted with caution in any patient who is under treatment with immunosuppressive drugs as they are more likely to have false–negative Mantoux test results.Citation8
Accordingly, a number of TB cases have occurred in subjects who had a negative Mantoux test before starting TNF-α inhibitor therapy.Citation8,Citation23 By contrast, previous Bacillus Calmette–Guérin vaccination may cause false–positive Mantoux test results. However, the PREMIER Study confirmed that the patients at high risk for reactivation or primary TB infection must take isoniazid before starting treatment with adalimumab, as well as with the other TNF-α inhibitors.Citation8,Citation13 In the PREMIER Study, one case of lymphoma was reported in the MTX monotherapy arm, confirming the evidence that in RA patients, the risk for lymphoma is irrespective of treatment and does not exert a major influence over decisions to start or continue TNF-α inhibitor therapy in patients with rapidly progressing joint destruction.Citation8,Citation23 Furthermore, one case of lupus like reaction occurred in the combination treatment group. Increased frequencies of autoantibodies, such as antinuclear antibodies and anti-double-stranded DNA antibodies, have been reported in subjects under TNF-α inhibitor treatment. However, the risk for development of systemic autoimmune diseases is low, and at present there is no recommendation for the monitoring of autoantibody titers during anti-TNF-α therapy.Citation8,Citation20,Citation24 Van der Heijde et alCitation14 reported the data from 799 patients who participated in an ongoing open-label extension study of the PREMIER Study. Of these, 497 patients completed a further 3 years of open-label adalimumab therapy after the initial 2 years of double-blind treatment, allowing an analysis of a total of 5 years of treatment. The results obtained confirmed that the initial combination treatment with adalimumab plus MTX led to better long-term clinical, functional, and radiographic outcomes than either initial adalimumab alone or MTX alone. Only two cases of TB were reported, but no serious opportunistic infection AEs occurred. Only one case of lymphoma and one case of nonmelanoma skin cancer were reported.
Eleven cases of malignancies other than lymphoma and nonmelanoma skin cancer occurred. There were no serious AEs of lupus like syndrome or demyelinating disease reported. Therefore, the long-term efficacy and safety data suggest a favorable benefit–risk profile for the use of adalimumab for more than 5 years in patients with early RA. Pavelka et alCitation25 presented and discussed the results of Phase 3 clinical trials using mAbs that inhibit T-lymphocyte activation, deplete B-lymphocytes or target cytokines such as TNF-α involved in the pathogenesis of RA in patients who did not respond to DMARDs. In particular, a pivotal 52-week, double-blind, placebo-controlled trial, 619 patients were randomized to receive placebo, adalimumab 20 mg/week or adalimumab 40 mg every other week plus their usual MTX dose.Citation17 The study confirmed that the adding of adalimumab to the MTX regimen in patients partially responsive to MTX provided additional benefit with the inhibition of joint damage, reduction of symptoms and signs, and improvement in physical function. In the OPTIMA study,Citation19 1032 patients with active RA were enrolled and assigned 1:1 to adalimumab plus MTX or placebo plus MTX for 26 weeks.
However, 44% of patients treated with adalimumab plus MTX and 24% of patients treated with placebo plus MTX achieved stable low disease activity, confirming that combination therapy was superior to MTX alone in obtaining higher American College of Rheumatology (ACR) criteria (ACR 20, 50, and 70) responses, more clinical remissions, greater mean decrease in DAS28, no radiographic progression, and normal functional status at week 26. Furthermore, the frequencies of total AEs and serious AEs were similar between the two groups of therapy. In particular, one case of Pneumocystis jiroveci (carinii) pneumonia, two malignancies (malignant melanoma in situ and squamous cell carcinoma), and one case of lupus like syndrome occurred in the adalimumab plus MTX group.
Krieckaert et alCitation26 enrolled 184 RA patients who were treated with adalimumab for at least 1 year. Bone mineral density (BMD) measurements of the total hip and lumbar spine were performed using dual-energy X-ray absorptiometry, and metacarpal cortex BMD was measured using digital X-ray radiogrammetry. This study demonstrated that the loss of BMD in the spine was arrested over 4 years of adalimumab therapy, whereas BMD of the hands and hip continued to decrease after 1 and 4 years, respectively. However, the changes in BMD were related to the disease activity and the current use of prednisone. Therefore, these results confirm and underline the need to monitor the degree of disease activity, not only for the long-term impact of the inflammation on local bone and the formation/progression of erosions, but also on the generalized bone loss, osteoporosis, and the additional risk of fractures. Finally, a few studies reported an increase in BMI during 2 years of therapy with TNF-α inhibitors.Citation27,Citation28 The increase in BMI may be favored by the release of adipocytokines, even if their actual role remains still not entirely clear.Citation29 Takeuchi et alCitation30 enrolled 334 Japanese patients with early RA (HOPEFUL 1 study) and randomized 171 patients to receive adalimumab plus MTX (6 mg–8 mg/week) and 163 to receive MTX plus placebo. The study confirmed that the combination adalimumab plus MTX regimen inhibited radiographic progression and favored the achievement of ACR20, ACR50, and ACR70. The study also confirmed the safety of adalimumab therapy. Indeed, there were no significant differences in the percentage of patients with AEs in the adalimumab plus MTX arm and in the MTX-alone arm, and the incidence of serious AEs were rare. As is widely known, the synovium in RA is characterized by a dense infiltrate, consisting of T- and B-lymphocytes, plasma cells, macrophages, dendritic cells (DCs) and other cells. Chemerin is a recently discovered chemokine that specifically modulates chemotaxis and activation of macrophages and DCs (in particular plasmocytoid DCs and monocyte-derived DCs).Citation31 Herenius et alCitation32 measured the chemerin serum levels in 49 patients with active RA before and after 16 weeks of treatment with adalimumab. Adalimumab treatment decreased the chemerin serum levels which was also correlated with the decrease in DAS28, in serum levels of IL-6 and, finally, in the macrophage migration inhibitory factor. These findings confirm that adalimumab treatment promotes the improvement of the clinical parameters of disease activity. Notably, all studies confirmed the low incidence of cutaneous injection site reactions (ie, local erythema and swelling) as we have described.Citation5–Citation11 Finally, lymphotoxin (LT)-α seems to play a role in the development of flogosis of immune-mediated disease such as RA. Indeed, in human RA, in addition to TNF-α, also lymphotoxin (LT)-α expression in the synovium is elevated.Citation33 T-helper(Th)-1 and Th-17 lymphocytes have been associated with autoimmune diseases, such as RA, and expressed LT-α.Citation34,Citation35 Depletion of LT-α–expressing Th-1 and Th-17 lymphocytes with LT-α–specific mAb may be beneficial in the treatment of autoimmune disease such as RA.Citation35 However, is it possible that adalimumab could bind LT-α as etanercept as demonstrated in PsA patients?Citation36 Further studies are required to better define the role of LT-α and LT-α blockade both in PsA and RA patients.
Potential beneficial effects on endothelial dysfunction and accelerated atherosclerosis in RA
Autoimmune rheumatic diseases have been associated with accelerated atherosclerosis and various types of vasculopathies.Citation34 The terms “endothelial activation” and “dysfunction” are used to describe the changes in endothelial homeostatic control mechanisms. Indeed, in response to a variety of noxious stimuli, endothelium undergoes a phenotypic modulation from the normal state to a nonadaptive state known as endothelial dysfunction. This pathophysiological condition is associated with increased expression of adhesion molecules such as Intercellullar adhesion molecule (ICAM)-1, vascular cellular adhesion molecule (VCAM)-1 and selectins,Citation37–Citation41 and pro-inflammatory cytokines (ie, TNF-α, IL-1, IL-6, and interferon-γ) and prothrombotic factors as well as with oxidative stress upregulation and abnormal vascular tone modulation. Systemic chronic inflammation may add to excessive oxidative stress leading to the formation and accumulation of advanced glycation end products. It is known that several systems generating reactive oxygen species (ROS) may catalyze a variety of modifications to nucleic acids, lipids, and proteins favoring the appearance of neo-cryptic epitopes which may behave as autoantigens.Citation34–Citation37
Furthermore, vascular endothelial growth factor (VEGF) is a critical mediator of inflammation both in chronic immune-mediated and allergic diseases.Citation37,Citation41–Citation43 It is known that VEGF is a pro-angiogenic factor which alters the microvascular network and, thus, correlates and may contribute to the development and progression of atherosclerosis. Indeed, it is now accepted that atherosclerosis is an inflammatory condition which starts as a “response to injury” that adds to traditional cardiovascular and genetic risk factors favoring endothelial dysfunction which arises before the appearance of the first morphological signs of atherosclerosis. The increase of common carotid intima-media thickness (ccIMT) and the impairment of brachial artery flow-mediated (FMD) and nitroglycerine-mediated (NMD) vasodilatation represent, with the above reported methodological limitations, good early indicators of accelerated atherosclerosis in RA and SLE patients with negative history for vascular disease.Citation37,Citation44–Citation47 However, Kerekes et alCitation44 confirmed that TNF-α inhibitors (etanercept, infliximab, adalimumab) may have a beneficial effect on arterial stiffness in patients with RA slowing the progression of accelerated atherosclerosis and consequently reducing the cardiovascular risk. Szekanecz et alCitation48 suggested that etanercept and adalimumab may exert beneficial effects on the lipid profile improving the endothelial dysfunction. Furthermore, TNF-α inhibitors are able to reduce the expression and production of VEGF, nitric oxide (NO), and inducible NO synthase.Citation38 Gonzalez-Juanatey et alCitation49 treated 34 RA patients with adalimumab and analyzed FMD values at baseline and after 12 months of therapy. Adalimumab treatment favored persistent improvement of endothelial function as proven by the increase in FMD value after 12 months of therapy.
In summary, the administration of TNF-α inhibitors reduces the systemic inflammation in patients with chronic immune-mediated diseases, improves both the clinical course of the disease itself and the endothelial function, and thus may decrease the risk of acute cardiovascular and/or cerebrovascular events.
Therapeutic indications and beneficial effects of adalimumab are depicted in .
Immunogenicity
Although adalimumab sequence is fully humanized and it is supposed to be less immunogenic than murine or chimeric monoclonal antibodies, the production of antibodies to adalimumab has been reported, and it still remains unclear which part of its molecule induces the antibody response.Citation8,Citation50 However, the production of antibodies to adalimumab may reduce the efficacy of the drug and induce the development of adverse drug-reactions and exanthema.Citation8,Citation50
Conclusion
TNF-α inhibitors represent a new class of drugs which have revolutionized the clinical management of chronic inflammatory diseases such as RA. Moreover, adalimumab as the other TNF-α inhibitors may favor the improvement of the endothelial dysfunction, and, thus, reduce the risk of cardiovascular and cerebrovascular diseases. However, physicians need to be aware of the potential efficacy and risks of treatment with these agents.
Disclosure
The authors declare no conflicts of interest in this work.
References
- FilipovicIWalkerDForsterFCurryASQuantifying the economic burden of productivity loss in rheumatoid arthritisRheumatology (Oxford)20115061083109021245074
- ScottDLWolfeFHuizingaTWRheumatoid arthritisLancet201037697461094110820870100
- TakeuchiTRevolutionary change in rheumatoid arthritis management with biological therapyKeio J Med2011603758121979826
- PeschonJJTorranceDSStockingKLTNF receptor-deficient mice reveal divergent roles for p55 and p75 in several models of inflammationJ Immunol199816029439529551933
- MurdacaGColomboBMCagnatiPGulliRSpanòFPuppoFUpdate upon efficacy and safety of TNF-α inhibitorsExpert Opin Drug Saf20121111522010813
- MurdacaGColomboBMPuppoFEmerging biological drugs: a new therapeutic approach for systemic lupus erythematosus. An update upon efficacy and adverse eventsAutoimmun Rev2011111566021835271
- MurdacaGColomboBMPuppoFAdalimumab for the treatment of immune-mediated diseases: an update on old and recent indicationsDrugs Today (Barc)201147427728821573251
- MurdacaGColomboBMPuppoFAnti-TNF-alpha inhibitors: a new therapeutic approach for inflammatory immune-mediated diseases: an update upon efficacy and adverse eventsInt J Immunopathol Pharmacol200922355756519822072
- MurdacaGColomboBMBarabinoGCaitiMCagnatiPPuppoFAnti-tumor necrosis factor-α treatment with infliximab for disseminated granuloma annulareAm J Clin Dermatol201011643743920515080
- PuppoFMurdacaGGhioMIndiveriFEmerging biologic drugs for the treatment of rheumatoid arthritisAutoimmun Rev20054853754116214092
- MurdacaGSpanòFPuppoFSelective TNF-α inhibitor-induced injection site reactionsExpert Opin Drug Saf201312218719323330811
- MurdacaGSpanòFMiglinoMPuppoFEffects of TNF-α inhibitors upon the mechanisms of action of VEGFImmunotherapy20135211311523413901
- BreedveldFCWeismanMHKavanaughAFThe PREMIER study: a multicenter, randomized, double-blind clinical trial of combination therapy with adalimumab plus methotrexate versus methotrexate alone or adalimumab alone in patients with early, aggressive rheumatoid arthritis who had not had previous methotrexate treatmentArthritis Rheum2006541263716385520
- van der HeijdeDBreedveldFCKavanaughADisease activity, physical function, and radiographic progression after long-term therapy with adalimumab plus methotrexate: 5-year results of PREMIERJ Rheumatol201037112237224620889601
- WeinblattMEKeystoneECFurstDEAdalimumab, a fully human anti-tumor necrosis factor alpha monoclonal antibody, for the treatment of rheumatoid arthritis in patients taking concomitant methotrexate: the ARMADA trialArthritis Rheum2003481354512528101
- van de PutteLBAtkinsCMalaiseMEfficacy and safety of adalimumab as monotherapy in patients with rheumatoid arthritis for whom previous disease modifying antirheumatic drug treatment has failedAnn Rheum Dis200463550851615082480
- KeystoneECKavanaughAFSharpJTRadiographic, clinical, and functional outcomes of treatment with adalimumab (a human anti-tumor necrosis factor monoclonal antibody) in patients with active rheumatoid arthritis receiving concomitant methotrexate therapy: a randomized, placebo-controlled, 52-week trialArthritis Rheum20045051400141115146409
- FurstDESchiffMHFleischmannRMAdalimumab, a fully human anti-tumor necrosis factor-alpha monoclonal antibody, and concomitant standard antirheumatic therapy for the treatment of rheumatoid arthritis: results of STAR (Safety Trial of Adalimumab in Rheumatoid Arthritis)J Rheumatol200330122563257114719195
- KavanaughAFleischmannRMEmeryPClinical, functional, and radiographic consequences of achieving stable low disease activity and remission with adalimumab plus methotrexate or methotrexate alone in early rheumatoid arthritis: 26-week results from the randomized, controlled OPTIMA studyAnn Rheum Dis2013721647122562973
- ChangJGirgisLClinical use of anti-TNF-alpha biological agents – a guide for GPsAust Fam Physician200736121035103818075630
- InoueEYamanakaHHaraMTomatsuTKamataniNComparison of Disease Activity Score (DAS)28-erythrocyte sedimentation rate and DAS28-C-reactive protein threshold valuesAnn Rheum Dis200766340740916926186
- RoachDRBeanAGDemangelCFranceMPBriscoeHBrittonWJTNF regulates chemokine induction essential for cell recruitment, granuloma formation, and clearance of mycobacterial infectionJ Immunol200216894620462711971010
- WinthropKLRisk and prevention of tuberculosis and other serious opportunistic infections associated with the inhibition of tumor necrosis factorNat Clin Pract Rheumatol200621160261017075599
- Gonnet-GraciaCBarnetcheTRichezCBlancoPDehaisJSchaeverbekeTAntinuclear antibodies, anti-DNA, and C4 complement evolution in rheumatoid arthritis and ankylosing spondylitis treated with TNF-alpha blockersClin Exp Rheumatol200826340140718578960
- PavelkaKKavanaughAFRubbert-RothAFerraccioliGOptimizing outcomes in rheumatoid arthritis patients with inadequate responses to disease-modifying antirheumatic drugsRheumatology (Oxford)201251Suppl 5v12v2122718922
- KrieckaertCLNurmohamedMTWolbinkGLemsWFChanges in bone mineral density during long-term treatment with adalimumab in patients with rheumatoid arthritis: a cohort studyRheumatology (Oxford)201352354755323221326
- EngvallILTengstrandBBrismarKHafströmIInfliximab therapy increases body fat mass in early rheumatoid arthritis independently of changes in disease activity and levels of leptin and adiponectin: a randomized study over 21 monthsArthritis Res Ther2010125R19720964833
- BriotKGossecLKoltaSDougadosMRouxCProspective assessment of body weight, body composition, and bone density changes in patients with spondyloarthropathy receiving antitumor necrosis factor-alpha treatmentJ Rheumatol200835585586118381782
- BakerJFGeorgeMBakerDGToedterGVon FeldtJMLeonardMBAssociations between body mass, radiographic joint damage, adipokines and risk factors for bone loss in rheumatoid arthritisRheumatology (Oxford)201150112100210721890621
- TakeuchiTYamanakaHIshiguroNAdalimumab, a human anti-TNF monoclonal antibody, outcome study for the prevention of joint damage in Japanese patients with early rheumatoid arthritis: the HOPEFUL 1 studyAnn Rheum Dis Epub1112013
- WittamerVGrégoireFRobberechtPVassartGCommuniDParmentierMThe C-terminal nonapeptide of mature chemerin activates the chemerin receptor with low nanomolar potencyJ Biol Chem2004279119956996214701797
- HereniusMMOliveiraASWijbrandtsCAGerlagDMTakPPLebreMCAnti-TNF therapy reduces serum levels of chemerin in rheumatoid arthritis: a new mechanism by which anti-TNF might reduce inflammationPLoS One201382e5780223460910
- TakemuraSBraunACrowsonCLymphoid neogenesis in rheumatoid synovitisJ Immunol200116721072108011441118
- MurdacaGColomboBMPuppoFThe role of Th17 lymphocytes in the autoimmune and chronic inflammatory diseasesIntern Emerg Med20116648749521258875
- ChiangEYKolumamGAYuXTargeted depletion of lymphotoxin-alpha-expressing TH1 and TH17 cells inhibits autoimmune diseaseNat Med200915776677319561618
- MurdacaGColomboBMContiniPPuppoFDetermination of lymphotoxin-alpha levels in patients with psoriatic arthritis undergoing etanercept treatmentJ Interferon Cytokine Res201232627727922480318
- MurdacaGColomboBMCagnatiPGulliRSpanòFPuppoFEndothelial dysfunction in rheumatic autoimmune diseasesAtherosclerosis2012224230931722673743
- PudduPPudduGMCraveroEDe PascalisSMuscariAThe emerging role of cardiovascular risk factor-induced mitochondrial dysfunction in atherogenesisJ Biomed Sci20091611220003216
- ButtariBProfumoEMatteiVOxidized beta2-glycoprotein I induces human dendritic cell maturation and promotes a T helper type 1 responseBlood2005106123880388716099886
- ArvieuxJRegnaultVHachullaEDarnigeLBerthouFYouinouPOxidation of beta2-glycoprotein I (beta2GPI) by the hydroxil radical alters phospholipid binding and modulates recognition by anti-beta2GPI autoantibodiesThromb Haemost20018641070107611686326
- MurdacaGSpanòFCagnatiPPuppoFFree radicals and endothelial dysfunction: potential positive effects of TNF-α inhibitorsRedox reportin press
- CiprandiGMurdacaGColomboBMDe AmiciMMarsegliaGLSerum vascular endothelial growth factor in allergic rhinitis and systemic lupus erythematosusHum Immunol200869851051218577409
- CiprandiGColomboBMMurdacaGDe AmiciMSerum vascular endothelial growth factor and sublingual immunotherapyAllergy200863794594618588566
- KerekesGSzekaneczZDérHEndothelial dysfunction and atherosclerosis in rheumatoid arthritis: a multiparametric analysis using imaging techniques and laboratory markers of inflammation and autoimmunityJ Rheumatol200835339840618203326
- ColomboBMCacciapagliaFPuntoniMTraditional and nontraditional risk factors in accelerated atherosclerosis in systemic lupus erythematosus: role of vascular endothelial growth factor (VEGATS Study)Autoimmun Rev20098430931518976721
- ColomboBMMurdacaGCaitiMIntima-media thickness: a marker of accelerated atherosclerosis in women with systemic lupus erythematosusAnn N Y Acad Sci2007110812112617893978
- DulaiRPerryMTwycross-LewisRMorrisseyDAtzeniFGreenwaldSThe effect of tumor necrosis factor-α antagonists on arterial stiffness in rheumatoid arthritis: a literature reviewSemin Arthritis Rheum20124211822475245
- SzekaneczZKerekesGSoltészPVascular effects of biologic agents in RA and spondyloarthropathiesNat Rev Rheumatol200951267768419901918
- Gonzalez-JuanateyCVazquez-RodriguezTRMiranda-FilloyJAAnti-TNF-alpha-adalimumab therapy is associated with persistent improvement of endothelial function without progression of carotid intima-media wall thickness in patients with rheumatoid arthritis refractory to conventional therapyMediators Inflamm2012201267426522899879
- BenderNKHeiligCEDröllBWohlgemuthJArmbrusterFPHeiligBImmunogenicity, efficacy, and adverse events of adalimumab in RA patientsRheumatol Int200727326927417006705