Abstract
Objective
The aim of this study was to evaluate the effects of cyclosporine ophthalmic emulsion 0.05% on ocular surface staining and visual performance in patients with dry eye.
Methods
This was a single-center, 6-month, open-label, Phase IV study. Patients with bilateral dry eye disease and a symptom score of ≥2 on the Ocular Discomfort and 4-Symptom Questionnaire, an Ocular Surface Disease Index score of >12, at least one eye with Schirmer’s score <10 mm/5 minutes, and central corneal staining graded as ≥2 on the Ora Calibra™ Corneal and Conjunctival Staining Scale were enrolled. Cyclosporine ophthalmic emulsion 0.05% (Restasis®) was instilled twice daily in each eye. The primary efficacy endpoints were ocular surface staining and visual function at 6 months. Secondary outcome measures included Schirmer’s test, tear film breakup time, symptoms, and adverse events.
Results
A total of 40 patients with the mean age of 59.4 years (range, 40–78 years) were enrolled; 35 (87.5%) were female and 37 (92.5%) completed the study. At 6 months, inferior corneal, central corneal, total corneal, and total ocular surface fluorescein staining were significantly improved from baseline in both eyes (P<0.001). Patient responses on the Ocular Surface Disease Index showed significant improvement in blurred vision and visual function related to reading, driving at night, working with a computer or bank machine, and watching television (P≤0.041). At 6 months, 35.1% of patients achieved ≥5 mm improvement and 18.9% achieved ≥10 mm improvement in the average eye Schirmer score. Mean tear film breakup time improved by >50% in both eyes (P>0.001). Patients reported significant improvement in ocular discomfort and dry eye symptoms (P<0.001). No patients discontinued treatment because of stinging or any other ocular adverse event.
Conclusion
Dry eye patients with difficulties with day-to-day visual function demonstrated improvement in both signs and symptoms of dry eye and reported improved visual function after 6 months of treatment with cyclosporine ophthalmic emulsion 0.05%.
Introduction
Dry eye is a prevalent, chronic, multifactorial disease of the tears and ocular surface.Citation1,Citation2 Instability of the tear film accompanied by T-cell-mediated ocular surface inflammation plays a major role in disease development and progression.Citation3,Citation4 Dry eye symptoms are a key complaint of patients with dry eye. Patients with dry eye suffer from symptoms of ocular discomfort, dryness, and visual disturbances, which may be episodic and also vary during the day.Citation5–Citation7
Corneal staining with fluorescein is a diagnostic sign of dry eye and a key measure of dry eye in clinical studies. Additional diagnostic measures may include Schirmer tests and tear film evaluations such as tear film breakup time (TBUT). However, lack of a consistent association between the signs and symptoms of dry eye remains an impediment to accurate diagnosis and assessment of treatment efficacy.Citation8,Citation9
Patients with dry eye frequently report visual disturbances such as blurred or foggy vision, fluctuating vision, and problems with glare.Citation5 A quality-of-life survey study by Miljanović et alCitation10 showed an association between dry eye disease and patient difficulty with vision-related tasks including reading, using a computer, watching television, and driving. Subsequent studies using the National Eye Institute 25-Item Visual Function Questionnaire (NEI VFQ-25)Citation11–Citation13 and the disease-specific Ocular Surface Disease Index (OSDI) questionnaire have demonstrated that patients with dry eye report increased difficulty with vision-related activities compared with control subjects.Citation11 Objective assessments have also shown a reduction in functional visual acuity and reading speed, which correlate with central corneal staining, in patients with dry eye.Citation14,Citation15 Impairment of driving ability due to visual disturbance in patients with dry eye also has been confirmed objectively in a study using a driving simulator.Citation16
Topical cyclosporine ophthalmic emulsion 0.05% is an anti-inflammatory treatment that has been shown to effectively improve dry eye signs (eg, tear production and corneal staining) and symptoms (eg, blurred vision) in patients with moderate-to-severe disease.Citation17 Early treatment with cyclosporine has been associated with improvements in tear production and tear film stability, as well as reduced progression of dry eye disease.Citation18,Citation19 Although it has been over 12 years since the US Food and Drug Administration approval of topical cyclosporine emulsion for the treatment of dry eye, the impact of cyclosporine treatment on visual function in patients with dry eye in the real world is not widely recognized. This study was designed to better understand patient response to cyclosporine therapy and the therapeutic benefit of cyclosporine treatment in reducing signs and symptoms and improving visual function in patients diagnosed with dry eye.
Methods
This was a prospective, single-center, open-label, Phase IV study conducted at Andover Eye Associates (Andover, MA, USA) between April and September 2014 (ClinicalTrials.gov identifier: NCT02121847). The study adhered to the tenets of the Declaration of Helsinki and Good Clinical Practice. The Alpha Independent Review Board (San Clemente, CA, USA) approved the study protocol, and all patients provided written informed consent.
To be included in the study, patients were required to be 18 years of age or older and have a history of dry eye in both eyes. At screening, patients were required to have a history of use or desire to use eye drops for dry eye symptoms within the past 6 months, report a score of ≥2 for at least one of the dry eye symptoms assessed on the Ora Calibra™ Ocular Discomfort and 4-Symptom QuestionnaireCitation20 (Ora, Inc., Andover, MA, USA), report a composite OSDI score of >12 with a score of ≥2 on the vision-related function subscale of the OSDI, and have at least one eye with the following: a Schirmer test score (without anesthesia) of <10 mm/5 minutes, corneal fluorescein staining graded as ≥2 and <4 in at least one region and central corneal fluorescein staining graded as ≥2 on the Ora Calibra™ Corneal and Conjunctival Staining Scale,Citation20 and a conjunctival redness score of ≥1 on the Ora Calibra™ Conjunctival Redness Scale.Citation20
Key exclusion criteria at screening included ongoing ocular infection, clinically significant slit-lamp findings such as active blepharitis, meibomian gland dysfunction, lid margin inflammation, or ocular allergies requiring treatment or that in the opinion of the investigator might interfere with interpretation of the study results, use of cyclosporine ophthalmic emulsion 0.05% within the past 90 days, use of any eye drop within the past 2 hours, ocular or lid surgery within the past 6 months, keratomileusis surgery within the past 12 months or previous cataract surgery at any time in either eye, required use of any medication known to cause ocular drying or affect dry eye unless on a dosing regimen that had been stable for 30 days and would remain stable during the study, and best-corrected visual acuity of +0.6 logMAR (~20/80 Snellen equivalent) or worse in both eyes.
Consecutive patients who met the study eligibility criteria were enrolled and dispensed cyclosporine ophthalmic emulsion 0.05% (Restasis®; Allergan plc, Dublin, Ireland). Patients were instructed to instill the study medication twice daily in both eyes for 6 months. Refresh Optive® advanced artificial tears (Allergan plc) were also provided to patients for use as needed.
Study visits were at day 0 (screening/baseline), month 1, and month 6. The primary efficacy endpoints were ocular surface staining and visual function at 6 months. Secondary efficacy endpoints were the Schirmer tear test, TBUT, blink patterns, and dry eye symptoms.
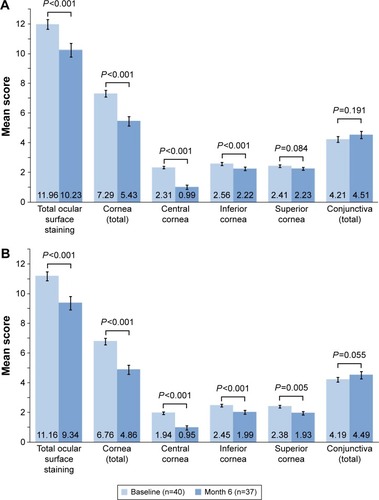
Corneal and conjunctival staining were evaluated at each visit. Fluorescein corneal staining (central, inferior, and superior regions, and their total) and fluorescein and lissamine green conjunctival staining (nasal and temporal regions) in each eye were graded on the Ora Calibra™ Corneal and Conjunctival Staining Scale (0–4 scale with 0.5 increments, where 0 is no staining and 4 is severe staining). Conjunctival fluorescein staining scores were added to the total corneal fluorescein staining score to generate an all-region combined score of ocular surface staining.
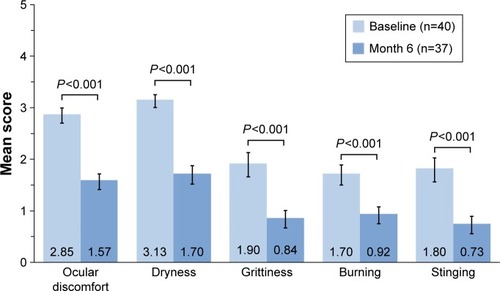
Visual function was assessed by patient responses on the OSDI questionnaire at baseline and month 6. Schirmer tear test without anesthesia and TBUT were assessed in each eye at baseline and month 6. Blink patterns were assessed in each eye over a period of 3 minutes using the automated Ocular Protection Index 2.0 System (Ora, Inc.)Citation21 at baseline, month 1, and month 6. This system measures blink frequency, which is used to calculate the interblink interval (time between blinks). Severity of dry eye symptoms was assessed with the Ora Calibra™ Ocular Discomfort and 4-Symptom Questionnaire at baseline and month 6. This questionnaire evaluates overall ocular discomfort, dryness, grittiness, burning, and stinging on a 6-point visual analog scale of 0 (none) to 5 (worst).
Safety outcome measures at each visit included adverse events, best-corrected visual acuity evaluated using an Early Treatment Diabetic Retinopathy Study (ETDRS) chart, and slit-lamp biomicroscopy. Adverse events and compliance with the dosing schedule were elicited at each visit, as well as in telephone calls to patients at week 2 and months 2, 3, 4, and 5.
Efficacy parameters were evaluated using observed values in the intent-to-treat population of all enrolled patients. Safety parameters were evaluated in the safety population of all patients who received cyclosporine treatment. The worse eye was defined as the eye with worse central corneal staining at baseline. If baseline staining was the same in each eye, the right eye was designated the worse eye.
Ocular surface staining, TBUT, and interblink interval were analyzed separately in worse treated eyes and fellow treated eyes. The analysis of Schirmer test results used the average value from both treated eyes. Statistical analysis comparing signs and symptoms after 6 months of cyclosporine treatment with those at baseline used the paired t-test or Wilcoxon signed-rank test and an alpha level of 0.05.
Results
A total of 40 patients were enrolled in the study. The mean (± standard deviation [SD]) age of the patients was 59.4±9.1 years (range, 40–78). Thirty-five (87.5%) of the patients were female, and all were Caucasian.
Thirty-seven patients (92.5%) completed the 6-month study. Two patients discontinued from the study because of non-ocular adverse events (one patient with injuries from an automobile accident, and one patient with headache), and one patient discontinued from the study for administrative reasons.
Efficacy
Total ocular surface staining scores were significantly reduced from baseline at month 6 in both worse treated eyes and fellow treated eyes (P<0.001). A significant reduction in corneal staining from baseline was observed for total cornea, central cornea, and inferior cornea in both the worse and fellow treated eyes following 6 months of treatment (P<0.001; ). The largest reductions from baseline in staining were seen in total cornea (26.9% in worse and 29.1% in fellow treated eyes) and central cornea (58.4% in worse and 52.1% in fellow treated eyes).
Figure 1 Ocular surface staining at baseline and month 6.
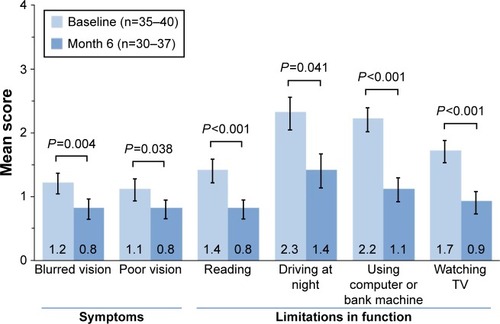
The mean (± standard error of the mean) composite OSDI score improved from 47.0±2.7 (indicating severe disease) at baseline to 26.8±3.0 (indicating moderate disease) at month 6 (P<0.001). Patient responses on the visual functioning subscale of the OSDI (four questions concerning eye problems causing difficulty in reading, driving at night, working with a computer or bank machine, and watching television within the past week) indicated that visual function related to all four tasks improved significantly after 6 months of cyclosporine treatment (). In addition, patient responses to OSDI questions regarding symptoms of poor visual function (ie, blurred vision and poor vision within the past week) indicated that the frequency of both symptoms decreased significantly after 6 months of cyclosporine treatment ().
Figure 2 Mean scores related to visual function on the OSDI.
Abbreviations: OSDI, ocular surface disease index; TV, television.
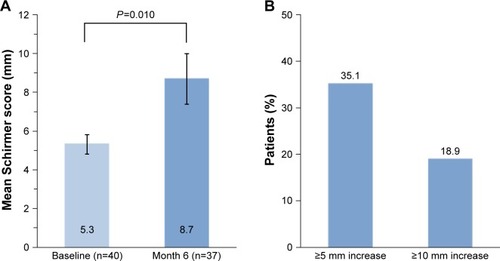
Mean average eye Schirmer test scores increased significantly from 5.3 mm at baseline to 8.7 mm at month 6 (P=0.010; ). At month 6, 35.1% of patients (13/37) achieved at least 5 mm improvement in the average eye Schirmer score, and 18.9% of patients (7/37) achieved at least 10 mm improvement in the Schirmer score.
Figure 3 Tear production.
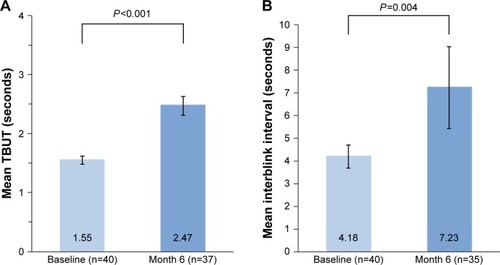
Significant improvements in TBUT compared with baseline values were observed at month 6 in both worse treated eyes (P<0.001; ) and fellow treated eyes (P<0.001). The mean increase in TBUT was 0.92 seconds (59.4%) from a baseline value of 1.55 seconds in worse treated eyes and 0.78 seconds (55.7%) from a baseline value of 1.40 seconds in fellow treated eyes. The increase in tear film stability was accompanied by a significant decrease in blink frequency (P=0.004; ). The mean increase in interblink interval was 3.04 seconds (72.7%) in worse treated eyes.
Figure 4 Mean (A) TBUT and (B) interblink interval in worse treated eyes at baseline and month 6.
Abbreviation: TBUT, tear film breakup time.
Patient responses on the Ora Calibra™ Ocular Discomfort and 4-Symptom Questionnaire at month 6 indicated a significant reduction from baseline in ocular discomfort and each symptom (P<0.001; ). The mean decrease in scores was 1.30 (45.6%) for ocular discomfort, 1.46 (46.6%) for dryness, 1.14 (60.0%) for grittiness, 0.84 (49.4%) for burning, and 1.11 (61.7%) for stinging.
Safety
All patients were treated with cyclosporine and were included in the safety population. Adverse events were reported in 17 patients (42.5%). Ocular adverse events were reported in ten patients (25%), most commonly instillation site burn (three patients, 7.5%), instillation site pain (three patients, 7.5%), and eye irritation (two patients, 5%). Only one patient (2.5%) had a non-ocular treatment-emergent adverse event (headache) that was considered to be related to treatment.
There were no safety concerns on biomicroscopy or visual acuity testing, and no anatomical changes were observed. Mean best-corrected visual acuity in worse treated eyes was 0.083 logMAR (~20/25 Snellen equivalent) at baseline and 0.085 logMAR (~20/25 Snellen equivalent) at month 6. In fellow treated eyes, mean best-corrected visual acuity was 0.062 logMAR (~20/25 Snellen equivalent) at baseline and 0.049 logMAR (20/20 Snellen equivalent) at month 6. No patient had a clinically relevant treatment-related decrease in best-corrected visual acuity.
Compliance
Patients generally reported being compliant with twice-daily dosing of cyclosporine ophthalmic emulsion 0.05% during the 6-month study period, with <3% of total doses missed across all patients.
Discussion
Dry eye is frequently associated with visual disturbances. The patients selected for this study had difficulties with daily activities related to visual function, as well as ocular surface damage seen with fluorescein staining, and both improved after twice-daily treatment with cyclosporine ophthalmic emulsion 0.05%. After 6 months of treatment, patient responses on the OSDI showed a significant reduction in the frequency of blurred vision and significant improvement in visual functioning related to reading, night driving, computer or bank machine use, and watching television. Total corneal staining and staining in the central corneal region, which is known to be critical to visual function, had decreased significantly from baseline.
Patients also demonstrated clinically significant improvements in other signs and symptoms of dry eye disease. Consistent with the approved indication for cyclosporine ophthalmic emulsion for the increase in tear production in patients whose tear production is presumed to be suppressed due to ocular inflammation associated with keratoconjunctivitis sicca, sizable increases (>60%) were observed in tear production measured with the Schirmer test. A similar magnitude of improvement was seen in tear film stability and interblink intervals. Patient-reported outcomes of ocular discomfort and other symptoms of dry eye also were significantly improved. The mean composite OSDI score improved by almost 50%, with the mean score indicating severe disease at baseline and moderate disease following 6 months of cyclosporine treatment. Patients reported excellent compliance with the dosing schedule, and treatment was well tolerated. Notably, no patient discontinued the use of cyclosporine because of stinging upon instillation.
Dry eye is underdiagnosed and potentially undertreated.Citation22 In a prospective study of the prevalence of dry eye in 136 patients who presented to nine clinical sites in the US for cataract surgery and were not being treated with topical cyclosporine, 81% had moderate-to-severe dry eye based on International Task Force criteria, yet only 22% had a previous diagnosis of dry eye.Citation23 Most of the patients (63%) had a TBUT of 5 seconds or less, and a majority of the eyes (77%) had corneal staining with central staining present in half of the eyes (50%).
The ocular discomfort and visual disturbances associated with untreated dry eye have a negative impact on the quality of life of affected individuals. In the ongoing PROOF study of the natural history of dry eye disease, the majority of patients with International Task Force Level 2 (moderate) dry eye reported moderate, severe, or very severe blurred or fluctuating vision that made them uncomfortable at baseline.Citation24 In the PROOF study, as in this study, baseline mean best-corrected visual acuity as measured on a standard Snellen eye chart was ~ 20/25. These findings reinforce the need for vision assessments beyond standard visual acuity testing. Evaluations should include the potential impact of dry eye disease on the patient’s quality of vision and day-to-day visual functioning.
The beneficial effects of cyclosporine ophthalmic emulsion treatment on the signs and symptoms of dry eye in this open-label study are consistent with the previously reported effects of cyclosporine treatment in controlled clinical trials. The percentage of patients with at least a 10 mm increase in Schirmer’s test (without anesthesia) scores at month 6 was 18.9%, similar to results in the Phase III registration trials of cyclosporine 0.05% emulsion, where ~15% of patients treated with cyclosporine versus 5% treated with vehicle had at least a 10 mm increase from baseline Schirmer’s test (with anesthesia) scores after 6 months of treatment.Citation25 The improvement in tear production (>3 mm on average) was similar to the significant increase from 3.93±1.21 mm at baseline to 7.30±1.79 mm after 3 months of cyclosporine ophthalmic emulsion 0.05% treatment that was reported in a study by Kim et al.Citation26 In a study by Demiryay et al,Citation27 the increase in mean Schirmer scores from baseline after 4 months was 3.33 mm greater in patients treated with cyclosporine ophthalmic emulsion 0.05% than in patients treated with vehicle.Citation28
The decrease in corneal fluorescein staining seen in this study, particularly in the region of the central cornea, likely contributed to the improvement in blurred vision reported by patients and is consistent with results of previous studies. In the cyclosporine ophthalmic emulsion 0.05% Phase III pivotal trials, fluorescein corneal staining and lissamine green interpalpebral conjunctival staining, measured on the Oxford scale, decreased during follow-up in both the cyclosporine and the vehicle treatment groups, but the reduction in corneal staining was significantly greater in patients treated with cyclosporine 0.05% than in patients treated with vehicle at 4 months and 6 months.Citation17 Significant decreases in corneal staining were also observed after 3 months or 4 months of cyclosporine 0.05% treatment in the studies by Kim et alCitation26 and Demiryay et al.Citation27
Each blink reforms the tear film and protects the ocular surface. Patients with dry eye blink more frequently because of tear film instability, but blinking too often may interfere with visual tasks or lead to visual fatigue. In this study, improvement in tear film stability after 6 months of cyclosporine treatment resulted in a significant increase in the interblink interval. These results are consistent with patient-reported improved performance on tasks related to visual function after 6 months.
Topical cyclosporine has been used successfully in the treatment of a number of ocular surface diseases in addition to dry eye.Citation29 The improvement in tear film stability provided by topical cyclosporine and its anti-inflammatory effects on meibomian glands have been shown to be beneficial in patients with meibomian gland dysfunction.Citation30,Citation31 Topical cyclosporine has also been shown to be an effective anti-inflammatory treatment for vernal and atopic keratoconjunctivitis.Citation29,Citation32,Citation33 Studies have demonstrated that topical cyclosporine reduces contact lens intolerance in contact lens wearers with dry eyeCitation34 and improves signs and symptoms in patients with ocular rosaceaCitation35 as well as superior limbic keratoconjunctivitis.Citation36 Most recent studies of cyclosporine treatment in ocular surface disease other than dry eye have used topical cyclosporine (0.5%–2%) or cyclosporine ophthalmic emulsion 0.05% (Restasis®) off-label. Restasis® is indicated to increase tear production in patients whose tear production is presumed to be suppressed due to ocular inflammation associated with keratoconjunctivitis sicca.Citation25
A limitation of this study was the lack of a control group. The study was open label to allow the evaluation of cyclosporine ophthalmic emulsion 0.05% effects in patients with dry eye when both eyes are treated. Data are already available from controlled clinical trials evaluating treatment versus no treatment. However, a primary objective in this study was to examine visual function, a patient-level outcome influenced by both eyes.
Day-to-day real-world visual functioning is impaired in dry eye disease and can improve with treatment. The results of this study confirm the clinical benefit of cyclosporine ophthalmic emulsion 0.05% treatment for patients with dry eye. Cyclosporine 0.05% emulsion treatment reduced the signs and symptoms of dry eye and improved the performance of patients on common vision-related tasks. Patients reported very good compliance with treatment. No patient discontinued the use of cyclosporine because of ocular intolerability, and only one systemic adverse event (headache) considered to be related to treatment was reported.
Author contributions
All authors contributed toward data interpretation, drafting and critically revising the paper, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Acknowledgments
This study was sponsored by Allergan plc, Dublin, Ireland. Writing and editorial assistance was provided to the authors by Kate Ivins, PhD, of Evidence Scientific Solutions (Philadelphia, PA, USA), and funded by Allergan plc. Neither honoraria nor payments were made for authorship.
Presented in part at the American Society of Cataract & Refractive Surgery Annual Meeting, April 17–21, 2015, San Diego, CA, USA, and at the Association for Research in Vision and Ophthalmology Annual Meeting, May 3–7, 2015, Denver, CO, USA.
Disclosure
Karl G Stonecipher is a consultant for Abbott Medical Optics, Alcon, Allergan, Bausch & Lomb, Laser ACE, Nidek, Presbia, Refocus Group, and STAAR Surgical; an employee of TLC Laser Eye Centers; and has personal financial interest in Alphaeon. Gail L Torkildsen is a consultant for Ora, Inc. George W Ousler III is an employee of Ora, Inc. Scot Morris is a consultant for Allergan, Bausch & Lomb, Essilor, Gateway Professional Network, and Marco and a speaker/educator for Alcon, Allergan, Essilor, Marco, and Vistakon. He has received grants from Alcon, Allergan, Biosciences, Essilor Laboratories of America, and Luxottica. Linda Villanueva and David A Hollander are employees of Allergan, plc. The authors report no other conflicts of interest in this work.
References
- No authors listedThe definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop (2007)Ocul Surf200752759217508116
- No authors listedThe epidemiology of dry eye disease: report of the Epidemiology Subcommittee of the International Dry Eye WorkShop (2007)Ocul Surf2007529310717508117
- PflugfelderSCCorralesRMde PaivaCST helper cytokines in dry eye diseaseExp Eye Res201311711812524012834
- StevensonWChauhanSKDanaRDry eye disease: an immune-mediated ocular surface disorderArch Ophthalmol201213019010022232476
- UchinoMSchaumbergDADry eye disease: impact on quality of life and visionCurr Ophthalmol Rep201312515723710423
- DogruMTsubotaKPharmacotherapy of dry eyeExpert Opin Pharmacother201112332533421214414
- RidderWH3rdTomlinsonAHuangJFLiJImpaired visual performance in patients with dry eyeOcul Surf201191425521338568
- SullivanBDCrewsLAMessmerEMCorrelations between commonly used objective signs and symptoms for the diagnosis of dry eye disease: clinical implicationsActa Ophthalmol201492216116623279964
- SchmidlDWitkowskaKJKayaSThe association between subjective and objective parameters for the assessment of dry-eye syndromeInvest Ophthalmol Vis Sci20155631467147225650419
- MiljanovićBDanaRSullivanDASchaumbergDAImpact of dry eye syndrome on vision-related quality of lifeAm J Ophthalmol2007143340941517317388
- LiMGongLChapinWJZhuMAssessment of vision-related quality of life in dry eye patientsInvest Ophthalmol Vis Sci20125395722572722836767
- LeQZhouXGeLWuLHongJXuJImpact of dry eye syndrome on vision-related quality of life in a non-clinic-based general populationBMC Ophthalmol2012122222799274
- PaulsenAJCruickshanksKJFischerMEDry eye in the Beaver Dam offspring study: prevalence, risk factors, and health-related quality of lifeAm J Ophthalmol2014157479980624388838
- KaidoMMatsumotoYShigenoYIshidaRDogruMTsubotaKCorneal fluorescein staining correlates with visual function in dry eye patientsInvest Ophthalmol Vis Sci201152139516952222110071
- RidderWH3rdZhangYHuangJFEvaluation of reading speed and contrast sensitivity in dry eye diseaseOptom Vis Sci2013901374423222922
- DeschampsNRicaudXRabutGLabbeABaudouinCDenoyerAThe impact of dry eye disease on visual performance while drivingAm J Ophthalmol20131561e3.184e3.18923706501
- SallKStevensonODMundorfTKReisBLCsA Phase III Study Group. Two multicenter, randomized studies of the efficacy and safety of cyclosporine ophthalmic emulsion in moderate to severe dry eye diseaseOphthalmology2000107463163910768324
- RaoSNTopical cyclosporine 0.05% for the prevention of dry eye disease progressionJ Ocul Pharmacol Ther201026215716420415623
- StevensonDTauberJReisBLEfficacy and safety of cyclosporin A ophthalmic emulsion in the treatment of moderate-to-severe dry eye disease: a dose-ranging, randomized trial. Cyclosporin A Phase II Study GroupOphthalmology2000107596797410811092
- OuslerG3rdDevriesDKKarpeckiPMCiolinoJBAn evaluation of Retaine™ ophthalmic emulsion in the management of tear film stability and ocular surface staining in patients diagnosed with dry eyeClin Ophthalmol2015923524325709384
- AbelsonRLaneKJRodriguezJValidation and verification of the OPI 2.0 SystemClin Ophthalmol2012661362222570541
- McDonnellPJDry eye: an underrecognized and undertreated diseaseJohns Hopkins Adv Stud Ophthalmol20129114
- TrattlerWBwebpage on the InternetPrevalence of dry eye in surgical populationsASCRS EyeWorld CME Supplement32013 Available from: http://www.eyeworld.org/supplements/oct-2013/Allergan_supplement_October2013.pdfAccessed September 5, 2015
- McDonnellPJPflugfelderSSchiffmanRMProgression of Ocular Findings (PROOF) study of the natural history of dry eye: study design and baseline patient characteristicsInvest Ophthalmol Vis Sci201354154338 ARVO E-Abstract 4338.
- Restasis® (cyclosporine ophthalmic emulsion) [prescribing information]Irvine, CAAllergan, Inc2014
- KimECChoiJSJooCKA comparison of vitamin a and cyclosporine a 0.05% eye drops for treatment of dry eye syndromeAm J Ophthalmol20091472e3.206e3.21318848318
- DemiryayEYaylaliVCetinENYildirimCEffects of topical cyclosporine A plus artificial tears versus artificial tears treatment on conjunctival goblet cell density in dysfunctional tear syndromeEye Contact Lens201137531231521792057
- WanKHChenLJYoungALEfficacy and safety of topical 0.05% cyclosporine eye drops in the treatment of dry eye syndrome: a systematic review and meta-analysisOcul Surf201513321322526045239
- UtineCASternMAkpekEKClinical review: topical ophthalmic use of cyclosporin AOcul Immunol Inflamm201018535236120735287
- PrabhasawatPTesavibulNMahawongWA randomized double-masked study of 0.05% cyclosporine ophthalmic emulsion in the treatment of meibomian gland dysfunctionCornea201231121386139323135530
- QiaoJYanXEmerging treatment options for meibomian gland dysfunctionClin Ophthalmol201371797180324043929
- WanKHChenLJRongSSPangCPYoungALTopical cyclosporine in the treatment of allergic conjunctivitis: a meta-analysisOphthalmology2013120112197220323743438
- ZicariAMZicariANebbiosoMHigh-mobility group box-1 (HMGB-1) and serum soluble receptor for advanced glycation end products (sRAGE) in children affected by vernal keratoconjunctivitisPediatr Allergy Immunol2014251576324236762
- HomMMUse of cyclosporine 0.05% ophthalmic emulsion for contact lens-intolerant patientsEye Contact Lens200632210911116538135
- SchechterBAKatzRSFriedmanLSEfficacy of topical cyclosporine for the treatment of ocular rosaceaAdv Ther200926665165919551353
- SahinABozkurtBIrkecMTopical cyclosporine A in the treatment of superior limbic keratoconjunctivitis: a long-term follow-upCornea200827219319518216575