Abstract
Background
To evaluate the effect of photodynamic therapy (PDT) using a modified procedure on exudative age-related macular degeneration having been conventionally difficult to treat.
Methods
The medical records of eight consecutive patients (eight eyes) with age-related macular degeneration treated with modified PDT were reviewed retrospectively. Modified PDT was used for the lesions that could not be covered by conventional use of PDT, either because the lesion was too large or too close to the optic disc. A moving PDT laser spot at constant speed, for 83 seconds, was used to cover the entire lesion, and was named “Ironing PDT.” This retrospective study was performed with informed patient consent. It was approved by the Institutional Review Board of Kansai Medical University.
Results
No exudation could be found 36 months after treatment in five eyes (62.5%). There was no significant difference between the best-corrected visual acuity before PDT (0.95 logMAR) and after PDT (1.09 logMAR). The logMAR best-corrected visual acuity was improved in one eye, maintained in five eyes, and deteriorated in two eyes.
Conclusion
Ironing PDT decreased subfoveal fluid and preserved visual acuity in some patients with age-related macular degeneration difficult to treat with conventional therapy.
Introduction
Intravitreal administration of antivascular endothelial growth factor drugs (anti-VEGF drugs) has become the standard treatment for exudative age-related macular degeneration (AMD). However, there are some patients who do not respond to the drugs at the induction phase (initial nonresponders). Some patients respond to the drugs at the induction phase, but their responses decrease after repeated administrations (tachyphylaxis). We had previously reported that 12.3% of AMD patients were identified as initial nonresponders and 4.5% were identified with tachyphylaxis.Citation1 Moreover, we had to use the anti-VEGF drugs sparingly for patients who had cerebrovascular or cardiovascular diseases. For all these patients, photodynamic therapy (PDT) is important as a second treatment option. Data from the Treatment of Age-Related Macular Degeneration with Photodynamic Therapy (TAP) study showed that verteporfin therapy of subfoveal choroidal neovascularization (CNV) of AMD patients safely reduced the risk of vision loss.Citation2–Citation5 Guidelines for the use of verteporfin in Japan showed that visual acuity was effectively maintained in all lesion types for at least 12 months.Citation6 However, it is sometimes impossible to cover a large lesion or a peripapillary lesion. We therefore introduced a modified PDT procedure when the lesion was too large or too close to the optic disc. This procedure was designated “Ironing PDT.” In the following report, the 3-year results of Ironing PDT were retrospectively determined for these AMD patients.
Materials and methods
We reviewed retrospectively eight eyes of eight patients with AMD who received Ironing PDT from April 2009 to September 2010 at the Kansai Medical University, Takii Hospital (). We chose PDT because anti-VEGF therapy was either not effective for these patients, or the patients had a history of cerebrovascular infarction or cardiovascular disease. Ironing PDT was used for the lesions that could not be covered by conventional use of PDT, either because the lesion was too large or too close to the optic disc (). Patients were followed up for more than 36 months after they received Ironing PDT. This retrospective study was performed with informed patient consent, and was conducted in accordance with the tenets of the Declaration of Helsinki. It was approved by the Institutional Review Board of Kansai Medical University.
Figure 1 Schema of PDT.
Abbreviations: AMD, age-related macular degeneration; PDT, photodynamic therapy.
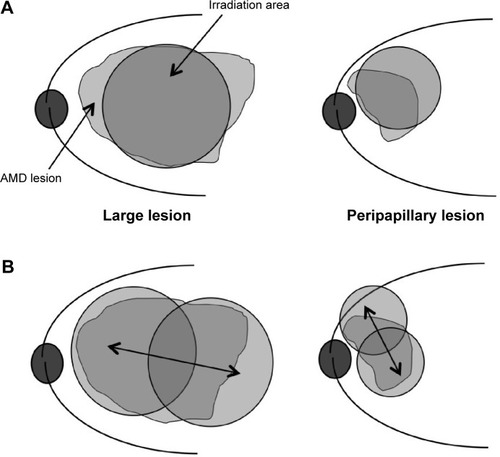
Table 1 Age-related macular degeneration patients treated with Ironing PDT
Ironing PDT was a modified PDT procedure. The dosages of verteporfin and laser power were set based on the guidelines for PDT in Japan by the Ophthalmic PDT Study Group.Citation6 The PDT laser spot moved back and forth at a constant speed, for 83 seconds, to cover the entire lesion. We moved the PDT laser spot to make one back-and-forth motion in 83 seconds as we were checking the time counter ().
There were six males and two females in this study, and the mean age of all patients was 73.9 years. There were four eyes with typical AMD and four eyes with polypoidal choroidal vasculopathy (PCV). Four eyes had a large lesion and four eyes had a lesion close to the optic disc. They were not fully treated with anti-VEGF drugs, because one eye was classified as an initial nonresponder and two eyes had tachyphylaxis. There were three eyes of three patients who had a history of cerebrovascular infarction, and one eye of one patient who had a history of cardiovascular disease. One patient did not agree to receive anti-VEGF therapy. Among eyes with typical AMD, one eye had predominantly classic CNV and three eyes had occult CNV. The greatest mean linear dimension was 7,974 (4,740–11,314) μm. We calculated the greatest linear dimension based on the analysis of both fluorescein angiography and indocyanine green angiography. We determined the spot size by using the smaller diameter of the lesion instead of the greatest linear dimension. When indocyanine green angiography-guided PDT reduced the size of the laser spot and could cover the entire lesion, patients underwent indocyanine green angiography-guided PDT instead of Ironing PDT. Ironing PDT was performed using a laser spot, after a 1,000 μm margin was added to the measurement.
Patients were seen approximately every 4 weeks. A comprehensive ophthalmic examination, including best-corrected visual acuity (BCVA) and spectral domain optical coherence tomography imaging using the RTVue-100 (Optovue Inc., Fremont, CA, USA), was performed at every visit. BCVA was measured with a Japanese standard decimal visual chart, and the logarithm of the minimum angle of resolution (logMAR) scale was used for statistical analysis. A significant change in visual acuity was defined as a difference of 0.3 or more units.
Results
shows the changes in visual acuity. The mean logMAR BCVA before treatment was 0.95±0.37 and after treatment was 1.09±0.71. There was no significant difference between them (P=0.809, paired t-test). The logMAR BCVA improved in one eye (12.5%), was maintained in five eyes (62.5%), and deteriorated in two eyes (25.0%). In five eyes (62.5%), no exudation was found and a fibrotic scar developed 36 months after the first Ironing PDT. In 36 months of follow-up, three eyes needed additional PDT. Among them, one eye received three additional Ironing PDT, one eye received two additional treatments (–), and one eye received one additional treatment. The mean number of additional PDT was 1.8 times within 36 months after the first Ironing PDT. Vitreous hemorrhage occurred in one eye with PCV (12.5%), 23 months after the Ironing PDT. The patient received vitrectomy, and a fibrotic scar developed at the lesion (). No complications due to the Ironing PDT, such as unexpected hemorrhage, were found in 36 months follow-up.
Figure 2 The change in BCVA (evaluated in logMAR).
Abbreviations: BCVA, best-corrected visual acuity; logMAR, logarithm of the minimum angle of resolution; PDT, photodynamic therapy.
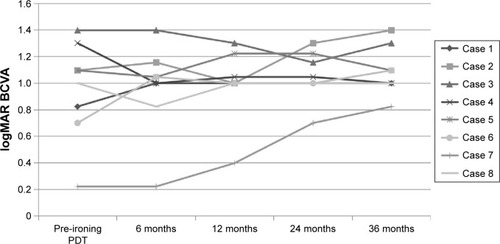
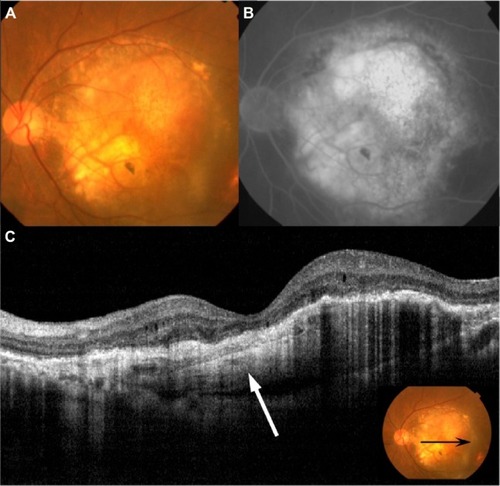
Figure 3 Fundus photography, FA, ICGA, and OCT performed in patient 7.
Abbreviations: CNV, choroidal neovascularization; FA, fluorescein angiography; ICGA, indocyanine green angiography; IVR, intravitreal injections of ranibizumab; OCT, optical coherence tomography; PED, pigment epithelial detachment; RPE, retinal pigment epithelium; SRD, serous retinal detachment.
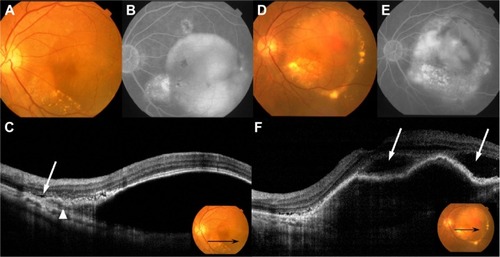
Figure 4 Ironing PDT performed in patient 7.
Abbreviations: GLD, greatest linear dimension; PDT, photodynamic therapy.
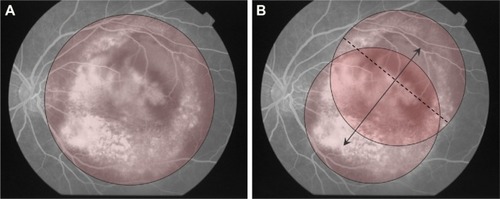
Figure 5 Fundus photography, FA and OCT 36 months after the first Ironing PDT performed in patient 7.
Abbreviations: CNV, choroidal neovascularization; FA, fluorescein angiography; OCT, optical coherence tomography; PDT, photodynamic therapy; RPE, retinal pigment epithelium.
Discussion
PDT has reduced the risk of vision loss for AMD patients with subfoveal CNV. PDT is considered to be effective, especially for PCV. Data from the EVEREST study showed that PDT combined with intravitreal ranibizumab (IVR) or alone was superior to IVR monotherapy in achieving complete regression of polyps in patients with symptomatic PCV.Citation7 However, several studies have reported that PDT damages the choriocapillaris layer, and repeated PDT led to persistent nonperfusion of choriocapillaris in most eyes.Citation8,Citation9 When compared with standard-fluence treatment, Michels et alCitation10 reported that reduced-fluence PDT led to less damage to the choriocapillaris. With Ironing PDT, moving the laser spot makes the irradiation time shorter at each spot. Yamashita et alCitation11 reported the results of a 2-year follow-up study of Japanese PCV patients treated with reduced-fluence PDT monotherapy. The logMAR BCVA was stable or improved by ≥0.3 in 95% of the eyes at the 2-year follow-up. In this same report, they reduced the light fluence to 25 J/cm2, with 83 seconds of irradiation time. Sakurai et alCitation12 reported the efficacy of IVR combined with reduced-fluence PDT for PCV. They performed PDT for 42 seconds with a fluence of 50 J/cm2. To reduce the total energy, they shorten the exposure time to half of the standard time of 83 seconds. They reported that the IVR and reduced-fluence PDT combination led to significant BCVA improvements and required fewer additional IVR treatments for at least 12 months in eyes with PCV.
Rosenblatt et alCitation13 reported the efficacy of PDT in the management of symptomatic extrafoveal peripapillary CNVCitation13 using a laser spot that was divided into three 30 seconds minimally overlapping areas that were concentric to the optic nerve. However, using this method, the lesions could be undertreated because lesions still existed that were not covered with the laser spot. Wachtlin et alCitation14 described a new “paint-brush technique” for peripapillary and large choroidal hemangiomas.Citation14 The use of this technique allowed complete treatment of choroidal hemangiomas with visual control. It involved moving the laser spot at constant speed eccentrically around the lesion’s center and over the entire tumor surface. The technique was reported to be a safe and effective modification for the treatment of symptomatic choroidal hemangiomas.
Many studies reported that aflibercept was effective for nonresponders and patients having tachyphylaxis to ranibizumab. However, we often find patients who are nonresponders or who have tachyphylaxis when given aflibercept. These cases are considered to be an indication for PDT, and Ironing PDT might be a good candidate for treating cases with large lesions or lesions close to the optic disc.
Conclusion
In conclusion, moving the laser spot makes the irradiation time shorter at each area, resulting in suppression of the exudation without excessive irradiation. Ironing PDT resolved the fluid accumulation successfully in 62.5% of the patients, but did not prevent deterioration of BCVA in some patients. There are several limitations in this study. Further research with a larger number of patients is needed to improve the technique of Ironing PDT and confirm the effect for long periods of time. Our data suggest that Ironing PDT may be a treatment option in patients with AMD difficult to treat with anti-VEGF agents or conventional PDT.
Acknowledgments
The manuscript was presented at the World Ophthalmology Congress of the International Council of Ophthalmology in Tokyo as an oral free paper presentation with interim findings on April 4, 2014.
Disclosure
The authors report no conflicts of interest in this work.
References
- OtsujiTNagaiYShoKInitial non-responders to ranibizumab in the treatment of age-related macular degeneration (AMD)Clin Ophthalmol201371487149023901256
- Treatment of Age-Related Macular Degeneration with Photodynamic Therapy (TAP) study groupPhotodynamic therapy of subfoveal choroidal neovascularization in age-related macular degeneration with verteporfin: one-year results of 2 randomized clinical trials – TAP reportArch Ophthalmol1999117101329134510532441
- Treatment of Age-Related Macular Degeneration with Photodynamic Therapy (TAP) study groupPhotodynamic therapy of subfoveal choroidal neovascularization in age-related macular degeneration with verteporfin: two-year results of 2 randomized clinical trials-tap report 2Arch Ophthalmol2001119219820711176980
- Treatment of Age-Related Macular Degeneration with Photodynamic Therapy (TAP) study groupVerteporfin therapy of subfoveal choroidal neovascularization in patients with age-related macular degeneration: additional information regarding baseline lesion composition’s impact on vision outcomes – TAP report No 3Arch Ophthalmol2002120111443145412427056
- Treatment of Age-Related Macular Degeneration with Photodynamic Therapy (TAP) study groupVerteporfin therapy for subfoveal choroidal neovascularization in age-related macular degeneration: three-year results of an open-label extension of 2 randomized clinical trials – TAP report No.5Arch Ophthalmol2002120101307131412365909
- TanoYOphthalmic PDT Study GroupGuidelines for PDT in JapanOphthalmology20081153585585.e618319105
- KohALeeWKChenLJEVEREST study: efficacy and safety of verteporfin photodynamic therapy in combination with ranibizumab or alone versus ranibizumab monotherapy in patients with symptomatic macular polypoidal choroidal vasculopathyRetina20123281453146422426346
- Schmidt-ErfurthUMichelsSBarbazettoILaquaHPhotodynamic effects on choroidal neovascularization and physiological choroidInvest Ophthalmol Vis Sci200243383084111867605
- Schmidt-ErfurthULaquaHSchlötzer-SchrehardUViestenzANaumannGOHistopathological changes following photodynamic therapy in human eyesArch Ophthalmol2002120683584412049594
- MichelsSHansmannFGeitzenauerWSchmidt-ErfurthUInfluence of treatment parameters on selectivity of verteporfin therapyInvest Ophthalmol Vis Sci200647137137616384987
- YamashitaAShiragaFShiragamiCShirakataYFujiwaraATwo-year results of reduced-fluence photodynamic therapy for polypoidal choroidal vasculopathyAm J Ophthalmol2013155196102.e122995028
- SakuraiMBabaTKitahashiMOne-year results of intravitreal ranibizumab combined with reduced-fluence photodynamic therapy for polypoidal choroidal vasculopathyClin Ophthalmol2014823524124511222
- RosenblattBJShahGKBlinderKPhotodynamic therapy with verteporfin for peripapillary choroidal neovascularizationRetina2005251333715655438
- WachtlinJSpyridakiMStrouxABechrakisNEFoersterMTherapy for peripapillary located and large choroidal haemangioma with PDT ‘paint-brush technique’Klin Monbl Augenheilkd200922611933938 German19798625
