Abstract
Latanoprostene bunod (LBN) is a novel nitric oxide-donating prostaglandin F2α receptor agonist in clinical development for intraocular pressure lowering in open-angle glaucoma and ocular hypertension. Currently in Phase III clinical trials in the USA, European Union, and Japan, LBN has demonstrated promising efficacy while maintaining safety and tolerability. We review preclinical and clinical developmental efforts and evaluate the potential role of LBN monotherapy in the management of open-angle glaucoma and ocular hypertension. The current LBN clinical development program comprises eight trials, four of which have resulted in publication of complete methodology and outcomes. We additionally pool adverse events data to determine incidences across three pivotal studies. Evidence thus far indicates that LBN may be a safe and effective ocular hypotensive agent, although the potential neuroprotective effects and the impact on visual field loss remain to be evaluated.
Introduction
Glaucoma, the second leading cause of blindness worldwide, is characterized by permanent visual field loss from apoptosis and atrophy of retinal ganglion cells. Intra-ocular pressure (IOP) plays a pivotal role in the pathogenesis of glaucoma, although recent evidence has also implicated pressure-independent etiologies.Citation1,Citation2 However, IOP is currently the only modifiable risk factor, and its lowering remains the objective of most current medical and surgical management. Visual disability from glaucoma is preventable with timely and effective treatment. In the management of open-angle glaucoma (OAG) and ocular hypertension (OHTN), topical ocular antihypertensive medications are the most commonly used initial therapy. Of these, prostaglandin analogs (PGAs) such as latanoprost (Xalatan®; Pfizer, Inc., New York, NY, USA) are the preferred first-line medication.
PGAs have demonstrated enhanced efficacy, safety, and tolerability compared to other classes of topical ocular antihypertensives and can significantly forestall disease progression.Citation3 In addition, the once-daily regimen is associated with improved compliance compared to more frequently dosed eye drops.Citation4–Citation7 Nonetheless, patient compliance with these medications is still a challenge, with adherence and persistence rates generally below 50%.Citation4,Citation8 As such, the development of more potent medications – without sacrificing safety, tolerability, and convenience of dosing – may be beneficial in optimizing patient outcomes.
Latanoprostene bunod (LBN: NicOx S.A., Sophia-Antipolis, France, and Bausch + Lomb, Rochester, New York, NY, USA; initially developed by Pfizer, Inc.) is a novel nitric oxide (NO)-donating PGA currently being investigated for use in the reduction of IOP in individuals with OAG and OHTN. LBN (also known as PF-03187207, BOL-303259-X, NCX-116, and Vesneo™ [Bausch + Lomb]) has demonstrated profound IOP lowering and favorable safety profile in clinical trials in humans.
We review the preclinical and clinical development programs for LBN as of June 2016 and discuss the potential role of LBN in the management of OAG and OHTN. Publications considered for inclusion in this analysis were selected in PubMed using the search terms “latanoprostene bunod”, “PF-03187207”, “BOL-303259-X”, “NCX-116”, “Vesneo”, “nitric oxide”, “comparative study [publication type]”, and “glaucoma/drug therapy*[MeSH].” Clinical studies were referenced with their respective listings on ClinicalTrials.gov. Identification of unindexed clinical trials was achieved using the search terms “latanoprostene bunod,” “PF-03187207”, “BOL-303259-X”, and “NCX-116” in the ClinicalTrials.gov database. Trials were characterized as pivotal based on the strength of methodology and the availability of outcome data. To expand our search results and to review reports not currently indexed, we performed ad hoc searches using these search parameters for articles and abstracts from meetings of the following societies: Association for Research in Vision and Ophthalmology, American Academy of Ophthalmology, International Society for Eye Research, American Glaucoma Society, European Glaucoma Society, and World Glaucoma Congress.
Pharmacology
Mechanism of action
The molecular structure of LBN is nearly identical to that of latanoprost (). LBN is distinguished, however, by the integration of an NO-donating moiety (a terminal butyl nitrate ester functional group) in lieu of an isopropyl ester. The putative ocular hypotensive mechanisms of LBN are outlined in . Upon topical administration at the ocular surface, LBN is hydrolyzed by endogenous corneal esterases into latanoprost acid – the active component of latanoprost – and butanediol mononitrate, which is further metabolized to NO and the inactive 1,4-butanediol.Citation9
Figure 1 Molecular structures of (A) PGF2α, (B) latanoprost, and (C) LBN.
Abbreviations: PGF2α, prostglandin F2α; LBN, latanoprostene bunod; NO, nitric oxide.
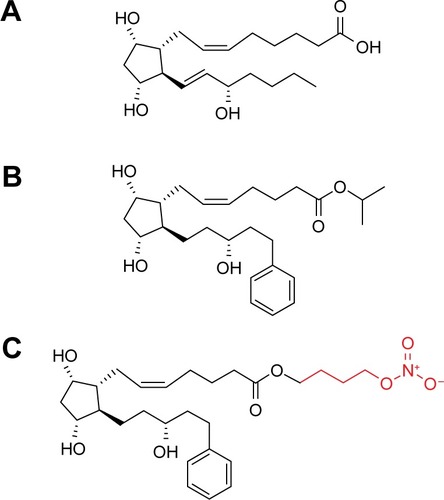
Figure 2 Putative mechanism of action of LBN.
Abbreviations: GTP, guanosine triphosphate; sGC, soluble guanylate cyclase; cGMP, cyclic guanosine monophosphate; PKG, protein kinase G; P, phosphate; Pi, inorganic phosphate; LBN, latanoprostene bunod; BDMN, butanediol mononitrate; ECM, extracellular matrix; MMPs, matrix metalloproteinases; SC, Schlemm’s canal; TM, trabecular meshwork; NO, nitric oxide; MLC-2, myosin light chain-2; BKCa, large-conductance calcium-activated potassium channel.
Primary open-angle glaucoma (POAG) and OHTN are associated with greater resistance to aqueous outflow through trabecular meshwork (TM) and Schlemm’s canal (SC, conventional pathway), although the pathophysiology associated with this outflow dysfunction is not fully understood.Citation10 Latanoprost is structurally similar to prostaglandin F2α (PGF2α; ) and acts as a selective PGF2α receptor (FP receptor) agonist. FP receptors have been identified in the ciliary muscle, ciliary epithelium, and sclera, and an in vitro analysis has revealed significant PGA agonist activity at cloned human ciliary body FP receptors.Citation11,Citation12 Latanoprost acid increases aqueous humor drainage primarily by reducing outflow resistance through the uveoscleral pathway. This is thought to occur by an increased expression of matrix metalloproteinases (MMPs) relative to their inhibitors (tissue inhibitors of metalloproteinases). Most notably, enhanced expressionCitation13 of MMP-1, -3, and -9 promotes degradation of collagen types I, III, and IV in the longitudinal bundles of the ciliary muscleCitation14–Citation16 and surrounding sclera.Citation17–Citation19 The resulting extracellular matrix remodeling yields increased permeability and reduced outflow resistance. Latanoprost-enhanced ciliary muscle permeability may be further augmented by morphologic changes in ciliary muscle cells caused by actin and vinculin reorganization.Citation20
In humans, latanoprost acid has also been demonstrated to increase conventional outflow through the TM and SC, although the impact is minor compared to the enhancement of uveoscleral outflow.Citation21–Citation22 The mechanism of this effect has not been fully elucidated, but is likely similar to latanoprost’s action on the ciliary muscle and sclera, involving MMP-mediated reorganization of the TM extracellular matrix milieu.Citation19 This is supported by the presence of FP receptors on human TM cells, at which PGAs exert agonistic activity.Citation12,Citation21 Furthermore, latanoprost has also been demonstrated to induce cyclooxygenase-2-dependent expression of MMP-1 by nonpigmented ciliary epithelium cells.Citation23 Subsequent diffusion of MMP-1 to the ciliary muscle and TM may augment outflow through both pathways.
The PGA metabolite of the LBN prodrug recapitulates the effects of latanoprost on both the uveoscleral and conventional outflow pathways. The unique NO component, however, exerts additional pharmacologic action on the conventional outflow pathway that is not characteristic of latanoprost and other PGAs.
Although NO-donating compounds have been used in medicine for over a century, LBN is among the first to be evaluated for topical ophthalmic use. The ocular hypotensive effects of NO-releasing molecules have been well characterized in animal and in vitro studies. NO-donating compounds have been demonstrated to lower IOP in animal models of glaucoma and OHTN,Citation24–Citation28 as well as in humans with OAG and angle-closure glaucoma upon oral administration.Citation29
NO is a gas and can freely diffuse across plasma membranes. The relaxing effect of NO and its second messenger, cyclic guanosine monophosphate (cGMP), on vascular smooth muscle cells has been well described;Citation30 in fact, these discoveries by Louis Ignarro, Robert Furchgott, and Ferid Murad earned them the Nobel Prize in Physiology and Medicine in 1998. TM cells, like vascular smooth muscle cells, are highly contractile and may therefore respond to NO in a similar fashion. In cultured human TM cells, NO donors induce a reduction in cell volume and contractility.Citation31 These effects are dependent upon soluble guanylate cyclase (sGC), protein kinase G (PKG), and large-conductance calcium-activated potassium (BKCa) channels and are mimicked by radiolabeled cGMP.Citation31,Citation32 Dismuke et alCitation31 therefore proposed that NO induces these TM cell morphologic and biomechanical changes by activation of the sGC/cGMP/PKG pathway, leading to phosphorylation of BKCa channels and subsequent efflux of potassium ions. These changes also correspond to a decrease in myosin light chain (MLC) phosphorylationCitation33 – which may further promote TM cell relaxationCitation34 – and are associated with increased outflow facility in perfused porcine anterior segments.Citation31 NO-mediated increase in conventional outflow, with corresponding cGMP increase, has also been demonstrated in anterior segments of human donor eyes.Citation24
NO may also induce relaxation of the inner wall of SCCitation35 and reduction in SC endothelial cell volume via the sGC/cGMP/PKG cascadeCitation36 and subsequent disruption of intercellular adherens junctions.Citation37 These biomechanical changes likely further enhance the egress of aqueous via the conventional outflow tract. NO might also act downstream of SC by promoting relaxation of smooth muscle cells surrounding collector channels to decrease distal outflow resistance.Citation38 In addition, although NO may modulate episcleral venous pressure (EVP), this process has not been fully elucidated. Investigations of the effects of topical NO donors on EVP have yielded discrepant results, with some authors reporting a decrease in EVPCitation39 and others describing an increase.Citation40,Citation41 In the episcleral circulation, NO likely primarily causes vasodilation of arterioles and arteriovenous anastomoses rather than acting upon veins, thereby increasing blood flow to veins and subsequently elevating the EVP.Citation39 In contrast, the effect of latanoprost acid on EVP is likely minimal.Citation21
Animal model and in vitro studies of LBN, in particular, lend credence to the putative effects of NO donors on TM and also provide further mechanistic insights (). Data from Saeki et alCitation42 suggest that the NO-associated effects of LBN are independent from the PGA-mediated effects. In PGF2α receptor (FP receptor) knockout mice, LBN yielded significant IOP reduction, whereas latanoprost did not. The IOP-lowering effects of LBN persist even when FP signaling is abolished indicate that the effects of NO donation alone may be sufficient to reduce IOP. Krauss et alCitation9,Citation43 report that topical administration of LBN resulted in significant IOP reduction in three animal models of elevated IOP. These IOP-lowering effects are associated with increases in anterior segment cGMP and latanoprost acid concentrations.
Table 1 Preclinical studies of latanoprostene bunodTable Footnotea
Studying endothelin-1-contracted human TM cells, Cavet et alCitation44,Citation45 similarly found increases in sGC-dependent cGMP concentrations after treatment with LBN. This was associated with diminutions in MLC-2 phosphorylation, F-actin-mediated contractility, and vinculin-associated adhesion of TM cells to underlying substrate. These biochemical and structural changes were significantly greater with LBN compared to latanoprost. These data suggest that the NO/cGMP signaling cascade triggered by other NO-releasing compounds in TM cells is also likely induced by LBN, promoting rearrangement of cytoskeletal architecture, increased cell relaxation, and reduced cell adhesion.
The potent actions of LBN on both the uveoscleral and conventional outflow pathways have been hypothesized to enhance its ocular hypotensive effects in comparison to other PGAs.
Pharmacokinetics
The ocular and systemic pharmacokinetics of LBN in humans has not been well described. However, ocular pharmacokinetic parameters have been characterized in mammalian model studies. Krauss et alCitation9,Citation43 measured concentrations of latanoprost acid in anterior segment structures of rabbits and monkeys after administration of topical LBN 0.01% versus latanoprost 0.012%. Peak concentration of latanoprost acid in these anterior segment structures was similar between the two compounds, suggesting comparable penetration rates. Following instillation of LBN versus latanoprost in rabbits, time to maximum concentration (Tmax) of latanoprost acid was identical for the two drugs as measured in cornea (0.5 hours), aqueous (1 hour), and iris/ciliary body (1 hour). In monkeys, Tmax of latanoprost acid was similar for the two drugs as measured in cornea (0.5 hours) and aqueous (0.5 hours), but longer for LBN (1 hour) compared to latanoprost (0.5 hours) as measured in iris/ciliary body.
Half-life (T1/2) of latanoprost acid in anterior segment compartments was also similar after administration of the two drugs. In rabbits, T1/2 after application of LBN was 1.8 hours in cornea, 2.1 hours in aqueous, and 4.6 hours in iris/ciliary body. These were not statistically significantly different from T1/2 after instillation of latanoprost (1.7 hours in cornea, 3.0 hours in aqueous, and 2.6 hours in iris/ciliary body).
Importantly, however, these pharmacokinetic data for the monkey model were obtained from a limited sample size (N=2) that precluded statistical comparison of these parameters between the two compounds.
The metabolism of the latanoprost acid component of LBN may be similar to that of latanoprost acid derived from latanoprost, although this has not been demonstrably verified. In adult humans, latanoprost acid derived from latanoprost reaches a maximum concentration of 53 pg/mL in the systemic circulation 5 minutes after administration. It is metabolized by the liver and excreted primarily in the urine.Citation46
Efficacy and comparative studies
Preclinical studies
All preclinical basic science studies of LBN were sponsored by or performed in collaboration with Pfizer, Inc. or Bausch + Lomb. These studies have highlighted the superior ocular hypotensive effects of topical LBN compared to latanoprost in animal and in vitro models (). In beagles with glaucoma, LBN produced a 34% maximal decrease in IOP (approximately 9.2 mmHg, adjusting for decrease associated with LBN vehicle) at 2 hours after dosing.Citation9 Equimolar latanoprost (0.030%), in contrast, was associated with an 18% maximal IOP reduction (approximately 4.5 mmHg, adjusting for decrease associated with latanoprost vehicle) at 6 hours postadministration.
The comparatively greater efficacy of LBN to latano-prost has also been demonstrated in cynomolgus monkeys with OHTN secondary to laser-induced trabecular photocoagulation,Citation9 a model that is sensitive to the pharmacologic effects of high-dose latanoprost.Citation47,Citation48 Interestingly, IOP reduction in this model after instillation of LBN 0.030% was more profound than that of both equimolar and high-dose (0.10%) latanoprost, suggesting that the NO-mediated activity of LBN is robust in the context of a significantly restricted conventional outflow. LBN was associated with a 28% maximal reduction of IOP (approximately 13.6 mmHg) within 6 hours of instillation, whereas equimolar latanoprost did not produce a statistically significant reduction in IOP. High-dose latanoprost yielded a maximal 14.3% diminution in IOP (approximately 6.1 mmHg), which was still inferior to the ocular hypotensive effect of LBN.
As both LBN and latanoprost were associated with similar ocular penetration rates in animal models (see “Pharmacokinetics” section), the greater IOP-lowering effects observed with LBN were likely a consequence of the NO moiety.Citation9 This is corroborated by the observation that LBN produced significant reduction in IOP in pigmented rabbits, which are not sensitive to PGF2α agonists.Citation49,Citation50 In this rabbit model with transient saline-induced OHTN, LBN 0.036% was associated with a 30% mean decrease in IOP (−13.5±2.0 mmHg), with maximal effect at 0.5–1.5 hours after instillation.Citation9 Conversely, equimolar latanoprost did not produce a statistically significant diminution in IOP in this model.
Cavet et alCitation44,Citation45 demonstrated greater reductions in in vitro human TM cell contractility and adhesion with LBN compared to latanoprost, lending insight into the mechanistic basis underlying the greater ocular hypotensive activity of LBN. Only one preclinical study found no significant difference in the efficacy of the two drugs, with LBN 0.006% and latanoprost 0.005% producing similar IOP-lowering effects in a wild-type mouse model for up to 6 hours after administration.Citation42 Superiority of LBN to latanoprost was observed, however, in FP-receptor knockout mice, highlighting the role of NO in lowering IOP.
Clinical experiments
To date, eight US Food and Drug Administration clinical trials of LBN involving human subjects have been performed since 2007 (). All trials were sponsored by Pfizer, Inc. or Bausch + Lomb. Complete methodology and results have been published under peer review for four of these trials. In all efficacy and comparative studies described herein, one drop of LBN or a comparative agent was administered topically to subjects in the dosing regimen specified. Efficacy outcomes of six major trials are summarized in , depicting the IOP-lowering effects of LBN compared to those of latanoprost and timolol.
Figure 3 Mean reduction in IOP from baseline associated with LBN 0.024% qPM, latanoprost 0.005% qPM, and timolol 0.5% BID from six major clinical trials.
Abbreviations: IOP, intraocular pressure; LBN, latanoprostene bunod; qPM, every evening; BID, twice daily.
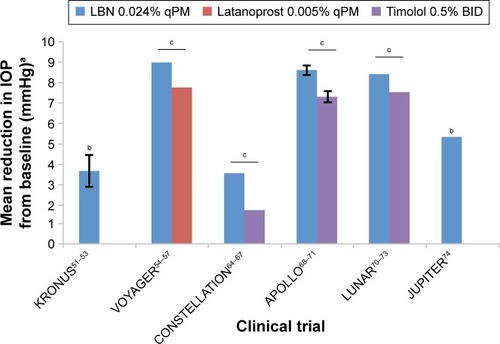
Table 2 Latanoprostene bunodTable Footnotea clinical developmental programs as of June 2016
Phase I trials
KRONUS: proof of principle (ClinicalTrials.gov Study ID: NCT01895985)
KRONUSCitation51–Citation53 is the only Phase I trial of LBN performed to date. This trial was performed after dose-finding studies (see “Phase II Trials”; “VOYAGER” sections),Citation54–Citation57 and as such evaluated LBN 0.024%, which previously demonstrated optimal efficacy and safety profile.
This single-arm, controlled, open-label study performed at a single center in Japan assessed the effects on IOP of topical LBN 0.024% dosed once daily at 8 PM for 14 days in healthy adult Japanese males with no ocular pathology (mean age =26.8±6.3 years; mean baseline IOP =13.6±1.3 mmHg). IOP measurements were recorded at nine time points over a 24-hour monitoring period on day 14; the primary efficacy end point was change in IOP from baseline at all of these time points. A reduction in IOP was observed at all time points (P<0.001), with a 27% mean reduction (3.6±0.8 mmHg) across 24 hours. Maximal IOP-lowering (30%, 4.2±1.8 mmHg) occurred 12 hours after dosing, while trough IOP decrease (20%, 2.8±2.2 mmHg) occurred 24 hours postadministration (at 8 AM and 8 PM, respectively).
The results of this pivotal trial serve as proof of principle of the profound IOP reduction associated with LBN 0.024% monotherapy. The demonstration of potent IOP lowering over 24 hours in Japanese subjects with normal IOP may also justify the clinical evaluation of LBN in normal tension glaucoma, which is common in Japanese populationsCitation58 and may be associated with diurnal fluctuations in IOP.Citation59,Citation60
Phase II trials
ClinicalTrials.gov Study ID: NCT00441883
NCT00441883 was the first trial of LBN in human subjects. This was a controlled, randomized, parallel-group, double-masked, dose-finding study comparing the safety and efficacy of LBN (PF-03187207) to latanoprost at multiple centers in the USA. Adult subjects with POAG, OHTN, pigmentary glaucoma, and pseudoexfoliative glaucoma were recruited, resulting in a total of 242 enrolled. Subjects were randomized to receive either one of five doses of LBN ranging from 0.003% to 0.040%, or latanoprost 0.005%, dosed once daily in the study eye. Outcomes included change in IOP from baseline at 7, 14, 21, and 28 days after study commencement and proportion of individuals reaching target IOPs. Complete methodology – including exact timing and frequency of dosing, timing of IOP measurement, as well as target IOPs – and results remain unpublished to date. Partial dose-finding results, however, were presented in the form of a meta-analysis estimating a median effective dose of 0.0054%/d and maximal IOP lowering of 6.90 mmHg.Citation61
ClinicalTrials.gov Study ID: NCT00595101
Similar to NCT00441883 in the USA, this controlled, randomized, double-masked, dose-finding study at multiple sites in Japan assessed the safety and efficacy of LBN (PF-03187207) compared to latanoprost in adult patients with POAG or OHTN. Subjects were randomized to receive one of five concentrations of LBN ranging from 0.003% to 0.040%, or latanoprost 0.005% dosed once daily either in the morning (qAM) or evening (qPM). IOP was measured at baseline and at four time points after dosing (days 7, 14, 21, and 28), at 8 AM, 10 AM, 1 PM and 4 PM on each of these days. The primary efficacy end point was change in mean diurnal IOP in the study eye on day 28, and secondary efficacy measures included IOP at day 7, 14, and 21 as well as a proportion of subjects reaching target IOPs on these days.
Only partial methodology and results of this trial have been published.Citation62,Citation63 Reduction in IOP with LBN 0.040% qPM was numerically greatest compared to IOP lowering with other LBN formulations as well as latanoprost qAM and qPM, although these differences were not statistically significant at the primary end point. Mean difference between LBN 0.040% qPM and latanoprost qPM, however, was statistically significant on day 21 (1.86 mmHg; P=0.017). (The reporting of these results involves discrepancies in the number of enrolled patients, with 128 documented by ClinicalTrials.gov, 176 reported by Bosworth et al,Citation62 and 133 noted by Raber et alCitation63).
VOYAGER: pivotal comparison to latanoprost (ClinicalTrials.gov Study ID: NCT01223378)
As a controlled, randomized, investigator-masked, dose-finding study at multiple centers in the USA and EU, this pivotal study compared the safety and efficacy of LBN (BOL-303259-X) and latanoprost in adult subjects with OHTN or OAG, including pseudoexfoliative and pigmentary glaucoma.Citation54–Citation57 VOYAGER also sought to determine the optimal dose of LBN in lowering IOP. This was the first comparative study with latanoprost detailing all clinical methodology and results, including all adverse events. Subjects (mean age =61.0±11.44 years; range of mean baseline IOPs in treatment groups =26.01±1.67–26.25±1.79 mmHg) were randomized to receive one of four LBN concentrations or latanoprost 0.005% in the study eye once daily at 8 PM for 28 days.
Mean diurnal IOP (average of readings at 8 AM, 12 PM, and 4 PM) was assessed at baseline and on days 7, 14, 28, and 29. The primary efficacy measure was change in mean diurnal IOP from baseline at day 28. Secondary efficacy outcomes were as follows: 1) change in mean diurnal IOP from baseline on days 7, 14, and 29; 2) change from baseline IOP at each of the three measurement time points on measurement days; 3) percentage of individuals with IOP of 18 mmHg or less at measurement time points.
Of 413 subjects randomized, 396 completed the study (LBN 0.006%, N=76; LBN 0.012%, N=81; LBN 0.024%, N=80; LBN 0.040%, N=80; latanoprost, N=79). Primary and secondary efficacy end points demonstrated greater IOP-lowering activity of LBN 0.024% and 0.040% compared to latanoprost. The IOP-reducing effect of LBN was dose-dependent, plateauing at 0.024%. The greatest diurnal IOP reductions with LBN on day 28 were 9.00 mmHg (0.024%) and 8.93 mmHg (0.040%); standard deviations not reported. These were the only statistically significantly greater changes (P=0.005 and 0.009, respectively) compared to IOP reduction with latanoprost (7.77 mmHg).
LBN 0.024% also showed significantly greater mean diurnal IOP reduction compared to latanoprost on day 7 (P=0.033) and day 14 (P=0.015), but not 36–44 hours after last instillation of each (day 29; P=0.051). Among these measurement points, LBN 0.040% was superior to latano-prost only on day 7 (P=0.009). LBN 0.024% demonstrated significantly greater reduction in IOP than latanoprost at multiple specified time points, with the exception of the 8 AM measurements on day 7 and day 21, as well as the 8 AM and 12 PM measurements on day 29 (P>0.05 for each). Of note, LBN 0.024% still showed a greater effect than latanoprost at 4 PM on day 29, 36–44 hours after cessation of therapy (P=0.045). LBN 0.040% similarly produced a greater IOP reduction compared to latanoprost at several specified time points, with the exception of 8 AM on day 7, 8 AM and 12 PM on day 14, and all time points on day 29 (P>0.05 for each).
The LBN 0.024% group also had a significantly greater percentage of individuals with mean diurnal IOP ≤18 mmHg compared to that in the latanoprost group on all measurement visits after treatment commenced (days 7, 14, 28, 29; P≤0.046 for each). The LBN 0.040% group had a greater percentage versus the latanoprost group on days 7 (P=0.007) and 28 (P=0.039).
CONSTELLATION: initial comparison to timolol (ClinicalTrials.gov Study ID: NCT01707381)
CONSTELLATION was the first trial to compare the efficacy and safety of LBN (BOL-303259-X) and timolol.Citation64–Citation67 In this controlled, open-label, two-period crossover study at a single center in the USA, 20 adult subjects with OAG or OHTN were randomized 1:1 and first given either LBN 0.024% daily at 8 PM or timolol maleate 0.5% twice daily (BID; 8 AM and 8 PM) in the study eye (Period 1). After 4 weeks of treatment, subjects were crossed over to the comparator arm for four additional weeks (Period 2). To date, this has been the only study of LBN with crossover data as the primary efficacy end point. Mean diurnal IOP was measured every 2 hours during a 24-hour period at baseline, 4 weeks (end of Period 1), and 8 weeks (end of Period 2). Primary efficacy end point was change in mean 24-hour IOP from baseline from both periods.
Mean change in 24-hour IOP after treatment was significantly greater for LBN (3.5±0.24 mmHg) than for timolol (1.7±0.25 mmHg; P<0.05). This change was also numerically greater for LBN during the diurnal period (3.9±0.28 versus 2.4±0.29 mmHg) and nocturnal period (2.75±0.45 versus 0.2±0.46 mmHg); the statistical significance of these differences, however, was not specified.
Phase III trials
APOLLO: pivotal, large-scale comparison to timolol (ClinicalTrials.gov Study ID: NCT01749904)
This pivotal randomized, controlled, double-masked trial, evaluated the efficacy and safety of LBN (BOL-303259-X) compared to timolol on a large scale and with a noncrossover design for primary end points.Citation68–Citation71 Four hundred and twenty adult subjects with OHTN or OAG, including pseudoexfoliative and pigmentary glaucoma, at multiple centers throughout the USA and EU were randomized 2:1 to receive either LBN 0.024% once daily at 8 PM or timolol maleate 0.5% BID (8 AM and 8 PM) in the study eye for 3 months.
The primary efficacy outcome was IOP recorded at three time points – 8 AM, 12 PM, and 4 PM – at postbaseline visits 2 weeks, 6 weeks, and 3 months after treatment commenced. Secondary end points included the following: 1) percentage of subjects with IOP ≤18 mmHg at all nine measurement time points; 2) percentage of subjects with 25% or greater decrease in IOP from baseline at all nine measurement time points; 3) change in IOP from baseline at each time point; 4) change in diurnal IOP (average of 8 AM, 12 PM, and 4 PM readings) from baseline at each postbaseline visit.
Three hundred and eighty seven patients completed the study (LBN, N=263; timolol, N=124). The results demonstrated that IOP was significantly lower in the LBN group (range =17.8–18.7 mmHg) than in the timolol group (range =19.1–19.8) at all nine time points (P≤0.002 for each). Of note, the greater efficacy of LBN at these time points did not differ based on subject age (<65 versus 65 years or older) or prior treatment status.
In the LBN group, a significantly higher proportion of subjects (22.9%) had IOP measurements ≤18 mmHg at all nine time points than in the timolol group (11.3%; P=0.005). Similarly, the LBN group had a greater percentage (34.9%) with an IOP decrease of ≥25% from baseline at all nine time points than in the timolol group (19.5%; P=0.001). In addition, IOP decrease from baseline was superior at all nine time points in the LBN group (range =7.7–9.1 mmHg) versus in the timolol group (range =6.6–8.0 mmHg; standard deviations not reported), with P≤0.002 for each time point. Decrease in diurnal IOP was significantly greater at all three postbaseline visits for LBN (range =8.4–8.6 mmHg) compared to timolol (range =7.1–7.3 mmHg), with P<0.001 for each visit (standard deviations not reported).
After completion of the 3-month treatment phase, all subjects were administered LBN 0.024% qPM for an additional 9 months as part of an open-label safety extension phase; IOP measurements were recorded during this time period at 6, 9, and 12 months postrandomization. These results were pooled with data from the LUNAR study (see “LUNAR” section), with a total of 737 subjects completing these extension phases.Citation70 LBN demonstrated sustained IOP lowering compared to baseline over 12 months, with a reduction from baseline in mean diurnal IOP among all subjects ranging from 32% to 34% (P<0.001 at each time point). Importantly, subjects crossed over to LBN from timolol during this phase experienced an additional 6.3%–8.3% diminution in mean diurnal IOP.
LUNAR (ClinicalTrials.gov Study ID: NCT01749930)
LUNAR was a randomized, double-masked study comparing the efficacy and safety of LBN (BOL-303259-X) to timolol at multiple sites in the USA and EU.Citation70–Citation73 The design and efficacy end points of this study were comparable to those of APOLLO; however, the open-label safety extension phase lasted 3 months after treatment, as opposed to 9 months as in APOLLO. Four hundred and twenty adults with OAG or OHTN were randomized 2:1 to receive a 3-month regimen of either LBN 0.024% qPM or timolol 0.05% BID in the study eye. Three hundred and eighty seven subjects completed the trial (LBN, N=259; timolol, N=128).
At almost all nine time points measured over 3 months (with the exception of the week 2 measurement at 8 AM), mean IOP was significantly lower with LBN than with timolol (P≤0.025). IOP at these time points ranged from 17.7 to 19.2 mmHg with LBN and 18.8 to 19.6 mmHg with timolol. Reduction in mean diurnal IOP was also significantly greater at all visits (P≤0.034) with LBN compared to timolol (8.0 versus 7.3, 8.4 versus 7.5, and 8.4 versus 7.3 mmHg at weeks 2, 6, and 12, respectively; standard deviation values not specified).Citation73 Around 17.7% of subjects demonstrated IOP lowering to a target of ≤18 mmHg at all nine time points, compared to 11.1% with timolol, although unlike in APOLLO this was not statistically significant (P=0.084). Conversely, 31.0% of subjects in the LBN group had a reduction from baseline in IOP of ≥25%, versus only 18.5% in the timolol group; this difference was statistically significant (P=0.007).
After completion of the 3-month treatment phase, all subjects were administered LBN 0.024% qPM for an additional 3 months as part of an open-label safety extension phase; IOP measurements were recorded during this time period at 6 months postrandomization. These results were pooled with data from the APOLLO study; all subjects demonstrated IOP lowering from baseline over this period (see “APOLLO” section).Citation68–Citation71
JUPITER (ClinicalTrials.gov Study ID: NCT01895972)
JUPITER was a single-arm, open-label trial of LBN at multiple centers in Japan. Partial methodology and results of this trial have been published.Citation74 Individuals ≥20 years old with either OAG or OHTN (mean baseline IOP =19.6±2.9 mmHg) were administered LBN 0.024% qPM in the study eye for a duration of 1 year. IOP was measured every 4 weeks at 10 AM, for a total of 13 time points over the treatment period. Efficacy outcomes included IOP value and change from baseline IOP at each time point. Hundred and thirty subjects were randomized, with 121 completing the study. The results demonstrated sustained IOP lowering throughout the year-long treatment period. Mean IOP was significantly decreased from baseline at all 13 points (P<0.001 for each), with reductions ranging from 4.3 to 5.3 mmHg (standard deviations not reported). IOP at week 4 had decreased 22.0% from baseline to 15.3±3.0 mmHg and was even lower at week 52 (26.3% reduction from baseline to 14.4±2.7 mmHg.)
Safety and tolerability
The chronic, long-term use of topical medications for management of glaucoma necessitates the development of eye drops with favorable safety and tolerability profiles. Ocular irritation and hyperemia secondary to preservatives such as benzalkonium chloride in antiglaucomatous eye drops have been well demonstrated.Citation75,Citation76 Various treatment-emergent adverse events (TEAEs) associated with LBN have been reported in clinical trials ().
Table 3 TEAEsTable Footnotea reported by three pivotal trials of LBN
Pooled data from three pivotal trials demonstrate that 22.0% of subjects experienced at least one ocular TEAE during treatment with LBN. Most ocular adverse events noted with LBN were mild and transient. The risk of serious adverse events is exceedingly low, with none documented in any study to date. The incidence of adverse events was comparable between different concentrations of LBN.Citation54–Citation57 The proportion of subjects experiencing ocular adverse events experienced with LBN was numerically higher compared to latanoprost (19.7% versus 12.2%), similar in comparison to timolol (13.4% versus 11.9%) in the APOLLO study, and higher compared to timolol (23.8% versus 13.3%) in the LUNAR study.
LBN likely has no appreciable effect on visual acuity, with only 1/637 pooled subjects (0.2%) experiencing decreased visual acuity associated with treatment. Overall, LBN was associated with high tolerability and compliance, with only 1.4% of patients discontinuing therapy secondary to adverse events. However, the majority of adverse event data for LBN is derived from trials lasting 3 months or shorter. The incidence of ocular adverse events was markedly higher in the year-long JUPITER trial.Citation74 This may indicate that the risk of developing ocular side effects increases with prolonged use.
Hyperemia
Hyperemia is among the most common side effects of glaucoma medications and similarly was among the most frequently observed adverse events of LBN in clinical trials. Across three pivotal studies, 4.9% of individuals experienced conjunctival hyperemia and 1.9% of subjects developed ocular hyperemia (as the VOYAGER study distinguished between “conjunctival hyperemia” and “ocular hyperemia”, these were maintained as independent categories in the present review for statistical analysis). The incidence and severity of hyperemia did not differ among different concentrations of LBN. Compared to the incidence of hyperemia with latanoprost, the potential for an increased risk with LBN is uncertain. In head-to-head comparison with latanoprost, ocular hyperemia was more frequent with latanoprost (8.5% versus 3.6%), whereas conjunctival hyperemia was higher for LBN (3.3% versus 0%) after 3 months.Citation54–Citation57 In a large-scale trial involving latanoprost (N=411), the incidence of hyperemia was 47.1% after 3 months.Citation77 Of note, the incidence of conjunctival hyperemia was higher (17.7%) in the year-long JUPITER trial than in other trials of LBN.
The majority of hyperemia was mild in severity. However, the incidence of more profound and prolonged hyperemia with LBN may be greater compared to that associated with timolol. In APOLLO, the LBN group had a higher percentage of individuals with moderate-to-severe hyperemia compared to the group receiving timolol at all postbaseline time points (9.6% versus 0.7% at week 2; 11.8% versus 3.8% at week 6; 8.5% versus 2.4% at month 3).
Ocular irritation and pain
Pain and irritation associated with LBN were characterized as either instillation site pain, eye irritation, or eye pain, with respective incidences of 8.3%, 1.7%, and 1.1% across three pivotal studies. These adverse effects therefore have an approximate combined incidence of 11.1%. However, this group of TEAEs are mostly mild and do not appear to affect tolerability. In APOLLO, only one subject in the LBN group discontinued therapy secondary to mild conjunctival irritation and edema, compared to one subject in the timolol group withdrawing secondary to moderate eye irritation. The year-long JUPITER trial noted greater incidences of discomfort-related effects (eye irritation, 11.5%; eye pain, 10.0%).
Instillation site pain occurred more frequently with LBN (15.2%) versus latanoprost (6.1%), but did not result in reduced adherence. Incidences of irritation and pain were similar between LBN and timolol.
Dry eye and punctate keratitis
Preserved eye drop formulations are known to impair ocular surface health, especially with prolonged use.Citation78 A total of 3.0% of LBN-treated individuals from pooled study data demonstrated evidence of punctate keratitis on biomicroscopy. In VOYAGER, incidence with LBN (1.8%) was similar to that with latanoprost (1.2%). LBN was associated with a 0.9% incidence of dry eye in pooled data, which was higher than the incidence with latanoprost but comparable to that with timolol.
Iris and eyelid pigmentation
Increased pigmentation of the iris and eyelid is the distinctive side effect of PGAs, although generally do not arise until after several months of treatment. As expected, therefore, these side effects were not observed in any shorter-term trials of LBN (≤3 months), but were noted in the year-long JUPITER study. In this trial, 3.8% and 3.1% of individuals demonstrated iris hyperpigmentation and blepharal pigmentation, respectively. Other features of prostaglandin-associated periorbitopathyCitation79 – such as blepharoptosis, deepening of the upper lid sulcus, and involution of dermatochalasis – have not been described with LBN.
Hypertrichosis
PGAs may induce eyelash growth. Only one study, JUPITER, has reported hypertrichosis as a TEAE of LBN, with 16.2% of individuals (21/103) experiencing eyelash growth. Although this may be a desirable cosmetic effect in some patients, it can present an aesthetic imbalance when occurring unilaterally, and in certain cases may interfere with eye drop administration. Further evaluation of the incidence and magnitude of this potential side effect of LBN – and its likelihood compared to that with other PGAs – may be warranted.
Nonocular adverse events
Nonocular and systemic side effects are rare with LBN, experienced by fewer than 1% of treated individuals (). The most common of these adverse events is headache, which is nonetheless exceedingly rare (3/637, 0.5%). There were no treatment-related changes in vital signs or any significant safety concerns associated with LBN in any study.
Patient-focused perspectives
Patients with vision loss from glaucoma, on average, report impaired quality of life.Citation80,Citation81 Profound disease and faster rate of visual field loss are also associated with poorer patient psychological outcomes.Citation82 Accordingly, the need for effective treatment regimens that halt disease progression without further impairing the quality of life and overall well-being is paramount.
Assessments of patient satisfaction and acceptability with LBN, however, have not been reported in any clinical trial to date. However, PGAs may enhance certain aspects of patients’ quality of life. For example, OAG and OHTN patients switching to latanoprost from other monotherapies report improved quality of life.Citation83 This may be a function of the more favorable side-effect profiles and once-daily dosing regimen of PGAs compared to other ocular hypotensive medications.
Many barriers impede the delivery of effective medical treatment to individuals with OHTN or glaucoma, including eye drop intolerability,Citation84–Citation86 ineffectiveness of treatment,Citation83 regimen complexity or inconvenience,Citation84,Citation86 and medication cost.Citation84,Citation87 Medication compliance – a manifestation of adherence and persistence – depends upon tolerability, convenience, and affordability.
The tolerability of LBN, as demonstrated by clinical trials, may promote patient compliance. Although risk and severity of some adverse reactions from LBN may be marginally increased compared to those of latanoprost, most of these reactions are nonetheless infrequent and mild and do not appear to reduce adherence. Furthermore, side effects of LBN are somewhat comparable to those of timolol, which, combined with LBN’s markedly greater efficacy, may favor LBN as a first-line therapy. The potential risk for more severe hyperemia with LBN, however, is not insignificant. Hyperemia is a significant risk factor for patient noncompliance.Citation88 In cases of drug discontinuation related to side effects, hyperemia represented the most commonly cited reason, accounting for 65%.Citation89
The potent effects of LBN as a monotherapy may promote tolerability and compliance. The use of monotherapy not only enhances convenience,Citation90 but also likely reduces exposure to preservatives and risk of adverse effects compared with combination therapy.Citation91 Complexity of eye drop regimen may also account for suboptimal compliance. Use of an effective monotherapy could minimize this barrier and discourage discontinuation.
Timing of dosing may be important in promoting compliance. Although many individuals find nightly dosing of PGAs practical and convenient, morning dosing schedules may be associated with improved adherence, particularly among males.Citation92,Citation93 LBN 0.024% dosed once nightly has demonstrated robust ocular hypotensive effects. The only published direct comparison of LBN 0.024% qAM versus qPM found no significant difference between the two at the primary end point.Citation62,Citation63 Timing of dose may be of importance, although complete head-to-head results comparing LBN 0.024% qAM versus qPM have not been published. These results may have significant implications on efficacy and patient compliance. A direct, rigorous comparison of the two may therefore be of clinical relevance.
Conclusion
Multiple preclinical and clinical studies underscore the efficacy, safety, and tolerability of LBN as an ocular hypotensive agent. The profound IOP-lowering effects of LBN monotherapy have been consistent and reproducible across trials, surpassing those of latanoprost or timolol monotherapy. Targeting of both the uveoscleral and conventional aqueous outflow pathways may account for LBN’s robust therapeutic activity.
Clinical trials demonstrate that LBN can offer, on average, approximately 1–3 mmHg additional improvement over latanoprost and timolol. This is not insignificant, as data from the Ocular Hypertension Treatment Study and Early Manifest Glaucoma Trial indicate that each mmHg reduction in IOP from baseline reduces the risk of onset or progression of glaucoma in eyes with elevated IOP.Citation94,Citation95
LBN 0.024% once daily has been demonstrated as an optimal dosing regimen, with superior ocular hypotensive effect and comparable risk of adverse events to other concentrations. This dose has demonstrated profound IOP reductions both in mean diurnal, nocturnal, and 24-hour IOP as well as across multiple time points throughout the day.
LBN may have marginally increased risk of ocular adverse reactions compared to latanoprost, although the overwhelming majority are transient and mild. Longer-term treatment with LBN may result in increased risk of development of ocular side effects such as eye-related irritation and pain. Despite this, LBN has been associated with high tolerability and compliance.
LBN is an effective pharmacologic agent emerging from the developmental pipeline for controlling IOP in OAG and OHTN. The promising results of LBN to date warrant further clinical evaluation, particularly in individuals with normal-tension glaucoma. LBN may be an effective therapeutic tool in the ophthalmologist’s arsenal to combat visual disability and improve patients’ quality of life.
Acknowledgments
The author KYL would like to thank Professor John P. Cooke at Stanford University for introducing him to the fascinating world of vascular biology and nitric oxide, which partly inspired the present article. The Department of Ophthalmology at University of California, Irvine, is a recipient of an institutional Research to Prevent Blindness unrestricted grant.
Disclosure
The authors report no conflicts of interest in this work.
References
- CharlsonMEde MoraesCGLinkANocturnal systemic hypotension increases the risk of glaucoma progressionOphthalmology20141212004201224869467
- KamalDHitchingsRNormal tension glaucoma – a practical approachBr J Ophthalmol19988278358409924383
- LeeAJMcCluskeyPClinical utility and differential effects of prostaglandin analogs in the management of raised intraocular pressure and ocular hypertensionClin Ophthalmol2010474176420689791
- SchwartzGFQuigleyHAAdherence and persistence with glaucoma therapySurv Ophthalmol200853Suppl 1S57S6819038625
- NordstromBLFriedmanDSMozaffariEQuigleyHAWalkerAMPersistence and adherence with topical glaucoma therapyAm J Ophthalmol2005140459860616226511
- SpoonerJJBullanoMFIkedaLIRates of discontinuation and change of glaucoma therapy in a managed care settingAm J Manag Care20028S262S27012188169
- PatelSCSpaethGLCompliance in patients prescribed eyedrops for glaucomaOphthalmic Surg1995262332367651690
- CampbellJHSchwartzGFLaBountyBKowalskiJWPatelVDPatient adherence and persistence with topical ocular hypotensive therapy in real-world practice: a comparison of bimatoprost 0.01% and travoprost Z 0.004% ophthalmic solutionsClin Ophthalmol2014892793524868144
- KraussAHImpagnatielloFTorisCBOcular hypotensive activity of BOL-303259-X, a nitric oxide donating prostaglandin F2α agonist, in preclinical modelsExp Eye Res201193325025521396362
- StamerWDAcottTSCurrent understanding of conventional outflow dysfunction in glaucomaCurr Opin Ophthalmol201223213514322262082
- SharifNAKellyCRCriderJYAgonist activity of bimatoprost, travo-prost, latanoprost, unoprostone isopropyl ester and other prostaglandin analogs at the cloned human ciliary body FP prostaglandin receptorJ Ocul Pharmacol Ther200218431332412222762
- Schlotzer-SchrehardtUZenkelMNüsingRMExpression and localization of FP and EP prostanoid receptor subtypes in human ocular tissuesInvest Ophthalmol Vis Sci20024351475148711980863
- WeinrebRNLindseyJDMetalloproteinase gene transcription in human ciliary muscle cells with latanoprostInvest Ophthalmol Vis Sci200243371672211867589
- SagaraTGatonDDLindseyJDGabeltBTKaufmanPLWeinrebRNTopical prostaglandin F2α treatment reduces collagen types I, III, and IV in the monkey uveoscleral outflow pathwayArch Ophthalmol199911779480110369592
- OcklindAEffect of latanoprost on the extracellular matrix of the ciliary muscle. A study on cultured cells and tissue sectionsExp Eye Res19986721791919733584
- WeinrebRNKashiwagiKKashiwagiFTsukaharaSLindseyJDProstaglandins increase matrix metalloproteinase release from human ciliary smooth muscle cellsInvest Ophthalmol Vis Sci19973813277227809418730
- WeinrebRNLindseyJDMarchenkoGMarchenkoNAngertMStronginAProstaglandin FP agonists alter metalloproteinase gene expression in scleraInvest Ophthalmol Vis Sci200445124368437715557445
- AnthonyTLLindseyJDWeinrebRNLatanoprost’s effects on TIMP-1 and TIMP-2 expression in human ciliary muscle cellsInvest Ophthalmol Vis Sci200243123705371112454040
- KimJWLindseyJDWangNWeinrebRNIncreased human scleral permeability with prostaglandin exposureInvest Ophthalmol Vis Sci20014271514152111381055
- StjernschantzJSelénGOcklindAResulBEffects of latanoprost and related prostaglandin analoguesAlmAWeinrebRNUveoscleral Outflow: Biology and Clinical AspectsLondon, UKMosby International Limited19985772
- TorisCBGabeltBTKaufmanPLUpdate on the mechanism of action of topical prostaglandins for intraocular pressure reductionSurv Ophthalmol200853Suppl 1S107S12019038618
- LimKSNauCBO’ByrneMMMechanism of action of bimato-prost, latanoprost, and travoprost in healthy subjects. A crossover studyOphthalmology20081155790795.e418452763
- HinzBRöschSRamerRTammERBruneKLatanoprost induces matrix metalloproteinase-1 expression in human nonpigmented ciliary epithelial cells through a cyclooxygenase-2-dependent mechanismFASEB J200519131929193116076963
- StamerWDLeiYBoussommier-CallejaAOverbyDREthierCReNOS, a pressure-dependent regulator of intraocular pressureInvest Ophthalmol Vis Sci201152139438944422039240
- KotikoskiHAlajuumaPMoilanenEComparison of nitric oxide donors in lowering intraocular pressure in rabbits: role of cyclic GMPJ Ocul Pharmacol Ther2002181112311858611
- ChumanHChumanTNao-iNSawadaAThe effect of L-arginine on intraocular pressure in the human eyeCurr Eye Res200020651151610980664
- SchumanJSEricksonKNathansonJANitrovasodilator effects on intraocular pressure and outflow facility in monkeysExp Eye Res1994581991058157106
- NathansonJANitrovasodilators as a new class of ocular hypotensive agentsJ Pharmacol Exp Ther199226039569651532035
- WizemannAJWizemannVOrganic nitrate therapy in glaucomaAm J Ophthalmol19809011061096772033
- ArnalJFDinh-XuanATPueyoMDarbladeBRamiJEndothelium-derived nitric oxide and vascular physiology and pathologyCell Mol Life Sci1999551078108710442089
- DismukeWMMbadughaCCEllisDZNO-induced regulation of human trabecular meshwork cell volume and aqueous humor outflow facility involve the BKCa ion channelAm J Physiol Cell Physiol2008294C1378C138618385281
- EllisDZDismukeWMChokshiBMCharacterization of soluble guanylate cyclase in NO-induced increases in aqueous humor outflow facility and in the trabecular meshworkInvest Ophthalmol Vis Sci20095041808181319074804
- DismukeWMLiangJOverbyDRStamerWDConcentration-related effects of nitric oxide and endothelin-1 on human trabecular meshwork cell contractilityExp Eye Res2014120283524374036
- RaoPVDengPSasakiYEpsteinDLRegulation of myosin light chain phosphorylation in the trabecular meshwork: role in aqueous humour outflow facilityExp Eye Res200580219720615670798
- SchneemannADijkstraBGvan den BergTJKamphuisWHoyngPFNitric oxide/guanylate cyclase pathways and flow in anterior segment perfusionGraefes Arch Clin Exp Ophthalmol20022401193694112486517
- EllisDZSharifNADismukeWMEndogenous regulation of human Schlemm’s canal cell volume by nitric oxide signalingInvest Ophthalmol Vis Sci201051115817582420484594
- HeimarkRLKaocharSStamerWDHuman Schlemm’s canal cells express the endothelial adherens proteins, VE-cadherin and PECAM-1Curr Eye Res200225529930812658549
- de KaterAWSpurr-MichaudSJGipsonIKLocalization of smooth muscle myosin-containing cells in the aqueous outflow pathwayInvest Ophthalmol Vis Sci19903123473532406217
- KrupinTWeissABeckerBHolmbergNFritzCIncreased intraocular pressure following topical azide or niroprussideInvest Ophthalmol Vis Sci1977161110021007199556
- ZamoraDOKielJWEpiscleral venous pressure responses to topical nitroprusside and N-Nitro-L-arginine methyl esterInvest Ophthalmol Vis Sci20105131614162019875657
- FunkRHGehrJRohenJWShort-term hemodynamic changes in episcleral arteriovenous anastomoses correlate with venous pressure and IOP changes in the albino rabbitCurr Eye Res199615187938631208
- SaekiTTsurugaHAiharaMAraieMRittenhouseKDose-response profile of PF-03187207 (PF-207) and peak IOP lowering response following single topical administration to FP receptor knockout mice vs wild type miceInvest Ophthalmol Vis Sci20095013 ARVO E-Abstract 4064
- KraussAHTorisCAKallbergMEOcular hypotensive activity of PF-03187207, a nitric oxide donating prostaglandin analog, in preclinical modelsInvest Ophthalmol Vis Sci200950 ARVO E-Abstract 1471
- CavetMEVollmerTRHarringtonKVanDerMeidKRichardsonMNO-induced regulation of primary human trabecular meshwork cell contractility by latanoprostene bunodInvest Ophthalmol Vis Sci20145513 ARVO E-Abstract 546
- CavetMEVollmerTRHarringtonKLVanDerMeidKRichardsonMERegulation of endothelin-1-induced trabecular meshwork cell contractility by latanoprostene bunodInvest Ophthalmol Vis Sci20155664108411626114488
- SjöquistBStjernschantzJOcular and systemic pharmacokinetics of latanoprost in humansSurv Ophthalmol200247Suppl 1S6S1212204697
- ResulBStjernschantzJSelénGBitoLStructure-activity relationships and receptor profiles of some ocular hypotensive prostanoidsSurv Ophthalmol199741Suppl 2S47S529154276
- StjernschantzKSelénGSjöquistBResulBPreclinical pharmacology of latanoprost, a phenyl-substituted PGF2 alpha analogueAdv Prostaglandin Thromboxane Leukot Res1995235135187732899
- OrihashiMShimaYTsunekiHKimuraIPotent reduction of intraocular pressure by nipradilol plus latanoprost in ocular hypertensive rabbitsBiol Pharm Bull2005281656815635165
- WoodwardDFBurkeJAWilliamsLSProstaglandin F2 alpha effects on intraocular pressure negatively correlate with FP-receptor stimulationInvest Ophthalmol Vis Sci1989308183818422759797
- AraieMSforzoliniBSVittitowJWeinrebRNEvaluation of the effect of latanoprostene bunod ophthalmic solution, 0.024% in lowering intraocular pressure over 24 h in healthy Japanese subjectsAdv Ther201532111128113926563323
- AraieMScassellati-SforzoliniBNgumahQVittitowJWeinrebREfficacy of Vesneo™ (Latanoprostene bunod ophthalmic solution, 0.024%) in Lowering Intraocular Pressure Over 24 hours in Healthy Japanese SubjectsPoster presented at: 6th World Glaucoma CongressJune 6–9, 2015Hong Kong, China Abstract P-S-056
- AraieMOngTScassellati-SforzoliniBNgumahQVittitowJLWeinrebRNEfficacy of latanoprostene bunod ophthalmic solution, 0.024%, in lowering intraocular pressure over 24-hours in normal Japanese subjects (KRONUS)Invest Ophthalmol Vis Sci20145513 ARVO E-Abstract 548
- WeinrebRNOngTScassellati SforzoliniBA randomised, controlled comparison of latanoprostene bunod and latanoprost 0.005% in the treatment of ocular hypertension and open angle glaucoma: the VOYAGER studyBr J Ophthalmol201599673874525488946
- WeinrebRNOngTScassellatiBA prospective randomized, multicenter, single-masked, parallel, dose ranging (VOYAGER) study to compare the safety and efficacy of BOL-303259-X to latanoprost in subjects with open-angle glaucoma or ocular hypertensionPaper presented at: 23rd Annual Meeting of the American Glaucoma Society2013Vancouver, Canada
- WeinrebRNA prospective randomized, multicenter, single-masked, parallel, dose ranging (VOYAGER) study to compare the safety and efficacy of BOL-303259-X to latanoprost in subjects with open-angle glaucoma or ocular hypertension2013World Glaucoma Congress Paper presentation
- KatzLJKaufmanPOngTScassellati-SforzoliniBVittitowJLatanoprostene bunod 0.024% significantly reduces and maintains mean diurnal intra-ocular pressure (IOP) compared to latanoprost 0.005% in subjects with open angle glaucoma or ocular hypertensionInvest Ophthalmol Vis Sci20135413 ARVO E-Abstract 460
- IwaseASuzukiYAraieMThe prevalence of primary open-angle glaucoma in Japanese: the Tajimi StudyOphthalmology200411191641164815350316
- QuarantaLKatsanosARussoARivaI24-hour intraocular pressure and ocular perfusion pressure in glaucomaSurv Ophthalmol2013581264123217586
- DeokuleSWeinrebRNRelationships among systemic blood pressure, intraocular pressure, and open-angle glaucomaCan J Ophthalmol200843330230718493272
- NickensDMandemaJCourtneyRRaberSBosworthCA model-based dose-response meta-analysis of single agent intraocular pressure (IOP) therapies used to evaluate efficacy of a potential new therapy (PF-03187207) in glaucoma patientsInvest Ophthalmol Vis Sci20095013 ARVO E-Abstract 2479
- BosworthCFZhangMCourtneyRRaberSEvelethDBeekmanMEfficacy and safety of PF-03187207, a novel nitric oxide donating prostaglandin F2-alpha analogue, vs latanoprost in hypertensive eyesInvest Ophthalmol Vis Sci20095013 ARVO E-Abstract 2481
- RaberSZhangMCourtneyREvelethDComparison of once-daily morning or evening dosing of PF-03187207, a novel nitric oxide donating prostaglandin f2-alpha analogue, vs latanoprost in hypertensive eyesFlorence, ItalyEuropean Glaucoma Society2010 Abstract P3.1
- LiuJHKVittitowJLNgumahQWeinrebRNEfficacy of Latanoprostene bunod ophthalmic solution 0.024% compared with timolol maleate ophthalmic solution 0.5% in lowering IOP over 24 hours in Subjects with open angle glaucoma or ocular hypertension (CONSTELLATION)Invest Ophthalmol Vis Sci20145513 ARVO E-Abstract
- LiuJVittitowJSforzoliniBWeinrebREffects of latanoprostene bunod compared with timolol maleate on ocular perfusion pressure in subjects with open-angle glaucoma or ocular hypertension2015American Glaucoma Society Abstract PO015
- Scassellati SforzoliniBVittitowJWeinrebREfficacy of Vesneo™ (latanoprostene bunod ophthalmic solution, 0.024%) compared with timolol maleate ophthalmic solution 0.5% in subjects with open-angle glaucoma or ocular hypertension2015World Glaucoma Congress Abstract RF-T-03-07
- LiuJVittitowJScassellati SforzoliniBWeinrebROcular perfusion pressure effects of Vesneo™ (latanoprostene bunod ophthalmic solution, 0.024%) timolol maleate ophthalmic solution 0.5% in subjects with open-angle glaucoma or ocular hypertension2015World Glaucoma Congress Abstract P-S-074
- WeinrebRNScassellati SforzoliniBVittitowJLiebmannJLatanoprostene bunod 0.024% versus timolol maleate 0.5% in subjects with open-angle glaucoma or ocular hypertension: the APOLLO StudyOphthalmology2016123596597326875002
- VittitowJLiebmannJMWeinrebRThe effect of latanoprostene bunod 0.024% vs timolol maleate 0.5% on lowering intraocular pressure in patients with open-angle glaucoma or ocular hypertension: the APOLLO Study2016American Glaucoma Society Abstract PO086
- VittitowJLLiebmannJMKaufmanPLMedeirosFAMartinKRWeinrebRNLong-term efficacy and safety of latanoprostene bunod 0.024% for intraocular pressure lowering in patients with open-angle glaucoma or ocular hypertension: APOLLO and LUNAR studiesInvest Ophthalmol Vis Sci201657 ARVO E-Abstract 3030
- KaufmanPLLiebmannJMVittitowJLWeinrebRNIntegrated efficacy of latanoprostene bunod 0.024% vs timolol maleate 0.5% for intraocular pressure lowering in patients with open-angle glaucoma or ocular hypertension: APOLLO and LUNAR studiesInvest Ophthalmol Vis Sci201657 ARVO E-Abstract 3036
- MedeirosFAMartinKRPeaceJScassellati SforzoliniBVittitowJLWeinrebRNComparison of Latanoprostene Bunod 0.024% and Timolol Maleate 0.5% in Open-Angle Glaucoma or Ocular Hypertension: the LUNAR StudyAm J Ophthalmol Epub2016519
- PeaceJHMedeirosFAMartinKRVittitowJLWeinrebRNEfficacy of latanoprostene bunod 0.024% vs timolol maleate 0.5% for intraocular pressure lowering in patients with open angle glaucoma or ocular hypertension: the LUNAR studyInvest Ophthalmol Vis Sci201657 ARVO E-Abstract 3035
- KawaseKVittitowJLYamamotoTAraieMLong-term safety and intraocular pressure lowering efficacy of latanoprostene bunod 0.024% in Japanese subjects with open angle glaucoma or ocular hypertension: the JUPITER studyInvest Ophthalmol Vis Sci201657 ARVO E-Abstract 3037
- HenryJCPeaceJHStewartJAStewartWCEfficacy, safety, and improved tolerability of travoprost BAK-free ophthalmic solution compared with prior prostaglandin therapyClin Ophthalmol2008261362119668762
- GuenounJMBaudouinCRatPPaulyAWarnetJMBrignole-BaudouinFIn vitro study of inflammatory potential and toxicity profile of latanoprost, travoprost, and bimatoprost in conjunctiva-derived epithelial cellsInvest Ophthalmol Vis Sci2005462444245015980234
- ParrishRKPalmbergPSheuWPXLT Study GroupA comparison of latanoprost, bimatoprost, and travoprost in patients with elevated intraocular pressure: a 12-week, randomized, masked-evaluator multicenter studyAm J Ophthalmol2003135568870312719078
- BaudouinCDetrimental effect of preservatives in eyedrops: implications for the treatment of glaucomaActa Ophthalmol200886771672618537937
- BerkeSJPAP: New concerns for prostaglandin useRev Ophthalmol2012191070
- NelsonPAspinallPPapasouliotisOWortonBO’BrienCQuality of life in glaucoma and its relationship with visual functionJ Glaucoma200312213915012671469
- SkalickySGoldbergIDepression and quality of life in patients with glaucoma: a cross-sectional analysis using the Geriatric Depression Scale-15, assessment of function related to vision, and the Glaucoma Quality of Life-15J Glaucoma200817754655118854731
- Diniz-FilhoAAbeRYChoHJBaigSGracitelliCPMedeirosFAFast visual field progression Is associated with depressive symptoms in patients with glaucomaOphthalmology2016123475475926920097
- ZimmermanTJStewartWCLatanoprost Axis Study GroupIntraocular pressure, safety, and quality of life in glaucoma patients switching to latanoprost from monotherapy treatmentsJ Ocul Pharmacol Ther200319540541514583133
- StrykerJEBeckADPrimoSAAn exploratory study of factors influencing glaucoma treatment adherenceJ Glaucoma2010191667220075676
- TsaiJCMcClureCARamosSESchlundtDGPichertJWCompliance barriers in glaucoma: a systematic classificationJ Glaucoma200312539339814520147
- TaylorSAGalbraithSMMillsRPCauses of non-compliance with drug regimens in glaucoma patients: a qualitative studyJ Ocul Pharmacol Ther200218540140912419091
- LaceyJCateHBroadwayDCBarriers to adherence with glaucoma medications: a qualitative research studyEye (Lond)200923492493218437182
- FriedmanDSHahnSRGelbLDoctor-patient communication, health-related beliefs, and adherence in glaucoma results from the glaucoma adherence and persistency Study (GAPS)Ophthalmology200811581320132718321582
- FeldmanRMConjunctival hyperemia and the use of topical prostaglandins in glaucoma and ocular hypertensionJ Ocul Pharmacol Ther2003191233512648301
- AbelsonMBNetlandPAChapinMJSwitching patients with glaucoma or ocular hypertension from dual therapy to monotherapy: evaluation of brimonidine as a modelAdv Ther200118628229711841198
- PisellaPJPouliquenPBaudouinCPrevalence of ocular symptoms and signs with preserved and preservative free glaucoma medicationBr J Ophthalmol20028641842311914211
- KahookMYNoeckerRJEvaluation of adherence to morning versus evening glaucoma medication dosing regimensClin Ophthalmol200711798319668470
- FordBAGooiMCarlssonACrichtonACMorning dosing of once-daily glaucoma medication is more convenient and may lead to greater adherence than evening dosingJ Glaucoma20132211421946541
- KassMAHeuerDKHigginbothamEJThe Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucomaArch Ophthalmol20021206701713 discussion 829–83012049574
- LeskeMCHeijlAHusseinMFactors for glaucoma progression and the effect of treatment: the early manifest glaucoma trialArch Ophthalmol2003121485612523884
