Abstract
Purpose
The purpose of this study was to evaluate the risk factors for tube exposure after glaucoma drainage implant surgery.
Patients and methods
This was a retrospective case-controlled observational study of 64 eyes from 64 patients. Thirty-two eyes of 32 patients with tube erosion requiring surgical revision were compared with 32 matched control eyes of 32 patients. Univariate and multivariate risk factor analyses were performed.
Results
Mean age was significantly younger in the tube exposure group compared with the control group (48.2±28.1 years versus 67.3±18.0 years, respectively; P=0.003). The proportion of diabetic patients (12.5%) in the tube exposure group was significantly less (P=0.041) compared with the control group (37.5%). Comparisons of the type and position of the drainage implant were not significantly different between the two groups. The average time to tube exposure was 17.2±18.0 months after implantation of the drainage device. In both univariate and multivariate analyses, younger age (P=0.005 and P=0.027) and inflammation prior to tube exposure (P≤0.001 and P=0.004) were significant risk factors. Diabetes was a significant risk factor only in the univariate analysis (P=0.027).
Conclusion
Younger age and inflammation were significant risk factors for tube exposure after drainage implant surgery.
Introduction
Patch graft thinning and conjunctival erosion with exposure of the silicone tube is a complication that may occur in the late postoperative period in 2%–7% of eyes after glaucoma drainage device implantation.Citation1–Citation9 Tube exposure can lead to ocular inflammation, hypotony, poor vision, and phthisis and represents a major risk factor for the development of late endophthalmitis, with the exposed tube providing a pathway for organisms to enter the eye from the ocular surface.Citation10,Citation11 Various methods of treatment of exposed tubes have been described, including debridement and placement of patch graft material, with or without repositioning the tube.Citation12–Citation15 Sclera, dura, and pericardium patch graft materials were compared and found to have similar patch graft survival.Citation16 Prevention of tube exposure has been attempted by varying techniques, including a long scleral tunnel, different patch graft materials,Citation17–Citation19 and doubling of processed pericardium allograft.Citation20
Several mechanisms have been proposed for tube exposure, including mechanical rubbing of the conjunctiva over the tube, excessive tension of the conjunctiva over the tube, or abnormal positioning of the tube. Patch graft melting has also been observed without conjunctival erosion, suggesting a role for patch graft absorption in the development of tube exposure.Citation21 Previous studies have not clearly identified the risk factors for tube exposure. The purpose of this study was to identify the risk factors for tube exposure after glaucoma drainage implant surgery.
Patients and methods
This was a retrospective, comparative study of 64 eyes, including 32 eyes with tube erosion and 32 control eyes from 64 patients. Tube erosion patients were identified from consecutive medical records of patients who had surgical revision of glaucoma drainage implants for tube exposure from two glaucoma surgeons (DJR, n=16; PAN, n=16) over a 4-year period. Patients of all ages were included in the study, with no exclusion criteria for the study. Tube erosion patients were matched with the control group, identified as the subsequent consecutive primary glaucoma drainage device implanted by the same surgeon. The study was approved by the Institutional Review Board of the University of Tennessee Health Science Center and Wills Eye Hospital and conformed to the requirements of the United States Health Insurance Portability and Privacy Act. Written informed patient consent was deemed not necessary due to the retrospective nature of this study.
All patients had glaucoma drainage device implantation prior to tube exposure, including 35 Ahmed Glaucoma Valves (New World Medical, Inc., Rancho Cucamonga, CA, USA) and 29 Baerveldt implants (Advanced Medical Optics, Inc., Santa Ana, CA, USA). Tube exposures and controls treated with the Ahmed Glaucoma Valve included eyes with Model S-2 (n=16 and n=13, respectively), Model FP-7 (n=2 and n=2, respectively), and Model B-1 (n=1 and n=1, respectively). All patients with the Baerveldt implant were treated with the Baerveldt 350 model. Nearly all patients (61 of 64 patients, 95%) had implants placed in the superotemporal quadrant, while the device was located in the inferonasal quadrant in one eye with tube erosion and in the inferotemporal quadrant in one eye in both groups. Irradiated donor pericardium (Tutoplast; IOP Ophthalmics, Cosa Mesa, CA, USA and New World Medical, Inc.) was the material used for the patch graft at the time of the original glaucoma drainage implant surgery in all patients.
Baseline clinical characteristics and demographic data from both the tube exposure and control groups were collected from the records. Preoperative data were from the visit prior to surgical revision of the exposed tube, and postoperative data were from the final follow-up visit after surgical revision. Steroid use and inflammation were compared using data at diagnosis of tube erosion for cases and the nearest time point after drainage device implantation in controls. Sample size of at least 30 in each group was determined from the ability to detect a 20% difference between groups with a SD of 25% (power =0.86) and the ability to detect a relative risk of 1.75 with proportion unexposed of 0.5 (power =0.90). Mann–Whitney U-tests were used to compare continuous parameters between the two groups. Chi-square and Fisher’s exact tests were used for categorical variables. Cox proportional hazards regression model analysis was performed to assess the relationship between tube erosion and multiple variables. P<0.05 was considered as statistically significant.
Results
Eyes with exposed tubes had thinning of the allograft pericardium and erosion of the conjunctiva over the silicone tube of the glaucoma drainage implant (), requiring surgical revision. Mean follow-up from the original glaucoma drainage implant surgery was 2.3 years in the tube exposure and control groups. The characteristics of patients in both the tube exposure and control groups are shown in . The mean age was significantly younger (P=0.003) in the patients in the tube exposure group (48.2±28.1 years) compared with the control group (67.3±17.9 years). There were no significant differences of sex, ethnicity, or lens status between the tube exposure and control groups. There was no significant difference in the number of previous surgical procedures between the tube exposure (1.5±1.05) and control (1.75±0.93) groups (P=0.424). Significantly fewer (P=0.041) diabetic patients (12.5%) were found in the tube exposure group compared to the control group (37.5%). History of hypertension or immune system abnormalities was not significantly different between the two groups. No significant differences were found in comparison of the type of glaucoma drainage implant used in the two groups (P=0.451). The glaucoma diagnosis differed in the two groups, with a larger number of pediatric and uveitic glaucomas in the tube erosion group compared with the control group.
Figure 1 Clinical photograph of one of the eyes in this study with an exposed tube (arrow), with thinning of the allograft pericardium and erosion of the conjunctiva over the silicone tube of the glaucoma drainage device.
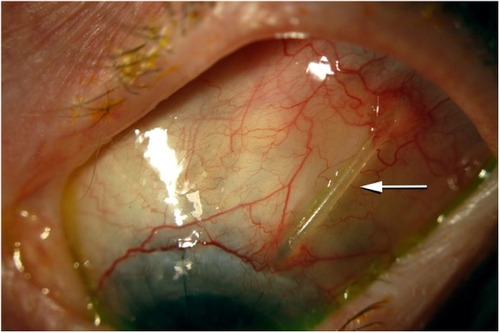
Table 1 Characteristics of patients with tube exposure and controls
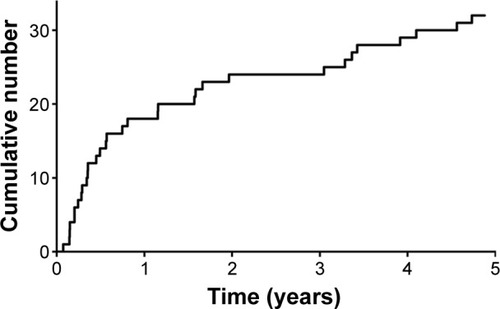
shows the cumulative number of eyes with tube exposure over time after glaucoma drainage device implantation. Following drainage implant surgery, the average ± SD time to tube exposure was 17.2±18.0 months (median 7.9 months). All patients with tube exposure underwent surgical revision to cover the tube. Average follow-up from surgical revision was 11.1 months. One of the 32 patients required multiple surgeries to cover the eroded tube. Two of the 32 patients with an eroded tube presented with an infection at the time of diagnosis of tube exposure, which required an anterior chamber injection of antibiotics.
Figure 2 Cumulative number of eyes with tube exposure over time after glaucoma drainage device implantation. After drainage implant surgery, the mean ± SD time to tube exposure was 17.2±18.0 months (median 7.9 months).
At the time of surgical revision of tube exposure, the visual acuity in the tube exposure group ranged from 20/30 to light perception, with 17 eyes ranging from 20/300 to light perception (logMAR >1.0), ten eyes ranging from 20/50 to 20/200 (0.3< logMAR ≤1.0), and five eyes with 20/40 or better (log-MAR ≤0.3). Mean intraocular pressure was 14.2±6.6 mmHg (range 5–30 mmHg). At the last follow-up visit after surgical revision in the tube exposure group, final visual acuity ranged from 20/25 to no light perception in the tube exposure group. The no light perception vision was secondary to cyclitic membrane formation and hypotony. Postoperatively, 27 eyes had the same visual acuity or showed a change within only two lines from preoperative vision on the Snellen chart, and five eyes lost more than two lines of visual acuity on the Snellen chart. Postoperatively, the mean final intraocular pressure was 15.4±8.0 mmHg (range =7–40 mmHg) in the tube exposure group. This was not significantly different (P=0.123) from the mean intraocular pressure prior to surgical revision. The tube exposure group was using an average of 1.0±1.4 glaucoma medications at the final follow-up visit.
Comparisons of outcomes at the last follow-up visit are shown in . The mean intraocular pressure in the tube exposure group (15.0±8.2 mmHg, range =7–40 mmHg) was significantly lower (P=0.039) compared with the control group (18.6±8.9 mmHg, range =2–40 mmHg). The average number of glaucoma medications at final follow-up was not significantly different between the two groups (P=0.076), with 1.0±1.4 (range =0–4) and 1.5±1.3 (range =0–4) medications in the tube exposure and control groups, respectively.
Table 2 Outcomes at last follow-up examination
As shown in , the proportion of patients using steroids at diagnosis of tube exposure (78.1%) was significantly higher (P<0.0001) compared with the control group (12.5%) at the nearest time point after drainage device implantation. The proportion of patients with inflammation prior to tube exposure (65.6%) was significantly higher (P<0.0001) compared with the control eyes at the nearest time point after glaucoma drainage implant surgery (9.4%). Cox proportional hazards regression model analysis for risk of tube exposure is shown in . In both the univariate and multivariate analyses, younger age (P=0.005 and P=0.027) and the presence of inflammation prior to tube exposure (P≤0.001 and P=0.004) were significant risk factors for tube erosion. A history of diabetes was significant only in the univariate analysis (P=0.027) and not in the multivariate analysis (P=0.598). Other variables, such as sex, African-American race, hypertension, and follow-up time, were evaluated and were not statistically significant.
Table 3 Comparison of steroid use and inflammation
Table 4 Outcomes of Cox proportional hazards regression model for tube exposure
Discussion
Exposure of glaucoma drainage device tube is associated with potentially vision-threatening complications and requires surgical revision or removal of the device. In this study, we compared eyes with tube exposure to control eyes. Our results indicated that patients with tube exposure developed this complication, on average, at 1.43±1.50 years after glaucoma drainage device implantation. Stewart et alCitation22 found an overall incidence of exposure of 2.0%±2.6% in their meta-analysis of 38 studies including 3,255 eyes. In this study, 64 eyes developed tube exposure at a rate of 0.09%±0.14% per month.Citation22 We found no difference in the time to erosion between eyes receiving Baerveldt versus Ahmed shunts. As both implant models utilize tubes made from medical grade silicone, this is not a surprising finding. We also found that younger age and inflammation were significant risk factors for developing exposure of the glaucoma drainage device tube.
Our average time to tube exposure from the drainage device implantation was comparable to the time to exposure in eleven eyes reported by Byun et alCitation1 (1.18±1.37 years). Unlike our study, however, their most common diagnosis was neovascular glaucoma, which was found in 45.4% of their tube exposure cases.Citation1 Lama and Fechtner reported two cases of tube exposure in patients with primary open-angle glaucoma after glaucoma drainage implant surgery occurring 7 months and 8 months postoperatively.Citation23 Lankaranian et alCitation20 reported an average time to tube exposure of 9 months in five eyes (range 4–14 months), with open-angle glaucoma in three of the five eyes (60%). In contrast, more recent studies reported a longer duration to tube exposure. Low et alCitation24 reported an average time to exposure for the tube in 19 eyes to be 5.3±3.8 years. Huddleston et alCitation25 reported an average duration of 21.5±28.4 months to tube exposure. Geffen et alCitation26 retrospectively reviewed 158 eyes with 14 exposures (twelve of which were tube exposures) with time to exposure to be 33.2±24.5 months.
In this study, younger age and the presence of inflammation prior to tube exposure were risk factors in both the univariate and multivariate analyses. In four patients with tube exposure evaluated by Joos et al,Citation13 three patients had recurrent chronic inflammation. Similarly, Smith et alCitation8 reported that one of the two patients who experienced tube erosion was associated with a low-grade active uveitis within 6 months of implantation of glaucoma drainage implant. Furthermore, Rachmiel et al reported tube erosion in two of 15 (13.3%) uveitic glaucoma patients compared with one of 53 (1.8%) open-angle glaucoma patients who had been treated with the Ahmed Glaucoma Valve (P=0.120). Only the uveitic glaucoma patients required tube removal (P=0.018).Citation4 In contrast, Geffen et alCitation26 did not find ocular inflammatory disease to be a significant risk factor (P=0.33), although they included patients with ocular surface disease in the same category.
We found no significant difference of implant type in eyes with tube exposure compared with controls. Similarly, Lankaranian et alCitation20 found no significant difference in the type of implant (Ahmed Glaucoma Valve or Baerveldt implant) in patients who developed tube exposure. Stewart et alCitation22 also found no significant difference between the type of implant used and exposure rate in their meta-analysis. Several studies have shown, however, that the location of the implant may be significant. Pakravan et al found more significant complications including exposure with inferior-placed Ahmed Glaucoma Valve implants (25%) compared to those placed superiorly (5.2%) in their study of 106 eyes followed for an average of 10.58±6.75 months.Citation27 Levinson et alCitation28 also found inferior implants exposed more frequently at 15.6% compared to superior placed implants at 6.3% (P=0.2761). Trubnik et alCitation29 retrospectively evaluated 28 eyes (8.3%) with tube erosion in 339 eyes treated with glaucoma drainage implants but found no significant relationship between location and erosion. However, they state that fewer tubes were placed in inferior-located positions in their study.Citation29
The type of patch graft used to cover the tube has been studied and the exposure rates after implantation have shown to be quite variable. Smith et alCitation8 reported a 4.3% exposure rate in 23 eyes covered with donor sclera (ethanol preserved), a 5.6% exposure rate in 18 eyes covered with dura (Tutoplast), and a 0% exposure rate in 23 eyes covered with pericardium; however, these differences were not statistically significant. Lankaranian et alCitation20 found 16% exposure rate with single thickness processed pericardium in 31 eyes significantly compared to 0% of tubes in 59 eyes covered with double thickness processed pericardium in their study (P=0.0002). Lawrence and NetlandCitation30 found no erosions of gamma-irradiated cornea (VisionGraft; Tissue Banks International, Baltimore, MD, USA) in a small pilot study with limited follow-up. However, in a larger group of patients with longer follow-up, Ekici et alCitation31 reported a 1.8% exposure rate of tubes in 169 eyes covered with VisionGraft gamma-irradiated cornea. In contrast, Levinson et alCitation28 found that tubes covered with donor cornea (9.2%) and pericardium (7.9%) exposed more often than scleral patch grafts (0.5%, P=0.72). Authors of this study suggested that the higher rate of exposure in the corneal patch graft group was related to the more frequent use of this type of patch graft with inferiorly placed tubes, which have been shown to expose more often.Citation28 Trubnik et al,Citation29 however, found no significant difference of tube erosion with different patch graft materials.
Byun et alCitation1 found previous ocular surgery as a risk factor for tube exposure; however, Geffen et alCitation26 found no significant risk of tube erosion with previous glaucoma procedures. In these studies, age and diabetes were not risk factors for tube exposure.Citation1,Citation26 In our study, fewer patients with exposed tubes had diabetes compared with controls, suggesting that diabetes was not a risk factor for exposure of drainage implant tubes. Other ocular risk factors, including previous ocular trauma and postoperative use of corticosteroids for exposure, were evaluated by Geffen et al,Citation26 but none were found to be significant. Only mean preoperative number of glaucoma hypotensive medications was found to be a significant risk factor in the tube exposure group (P=0.01) in their study.Citation26 Huddleston et alCitation25 also found a correlation between higher number of preoperative medications and exposure. In contrast, Trubnik et alCitation29 found no significant risk of tube exposure associated with preoperative medications, but that concomitant surgical procedures were a significant risk factor for exposure. We found no significant difference in the number of previous surgical procedures in patients with exposed tubes compared with controls, although we did not evaluate subgroups of cataract surgery or other procedures because of the small number of patients in the study.
A limitation of many of the previous studies on tube exposure is the small number of subjects. Limitations of this study also include relatively small sample size as well as variation in surgeon technique and bias. Nonetheless, we identified younger age and inflammation as risk factors for tube erosion. The association of inflammation with tube exposure here and in other studies suggests possible involvement of immune mechanisms in the etiology of tube exposure and possible prevention strategies with improved modulation of the immune response.
Disclosure
Dr Netland and the University of Virginia receive research support for unrelated studies from PTC Therapeutics (South Plainfield, NJ, USA) and New World Medical (Rancho Cucamonga, CA, USA). The authors report no other conflicts of interest in this work. The authors have no proprietary interest in this material.
References
- ByunYSLeeNYParkCKRisk factors of implant exposure outside the conjunctiva after Ahmed Glaucoma Valve implantationJpn J Ophthalmol20095311411919333694
- HuangMCNetlandPAColemanALSiegnerSWMosterMRHillRAIntermediate-term clinical experience with the Ahmed Glaucoma Valve implantAm J Ophthalmol199912727339932995
- WishartPKChoudharyAWongDAhmed Glaucoma Valves in refractory glaucoma: a 7-year auditBr J Ophthalmol2010941174117919965829
- RachmielRTropeGEBuysYMFlanaganJGChipmanMLAhmed Glaucoma Valve implantation in uveitic glaucoma versus open-angle glaucoma patientsCan J Ophthalmol20084346246718711462
- MillsRPReynoldsAEmondMJBarlowWELeenMMLong-term Survival of Molteno glaucoma drainage devicesOphthalmology19961032993058594518
- Gil-CarrascoFSalinas-VanOrmanERecillas-GispertCPaczkaJAGilbertMEArellanes-GarcíaLAhmed Valve implant for uncontrolled uveitic glaucomaOcul Immunol Inflamm1998627379798191
- VuoriMLMolteno aqueous shunt as a primary surgical intervention for uveitic glaucoma: long-term resultsActa Ophthalmol201088333619900205
- SmithMFDoyleJWTierneyJWA comparison of glaucoma drainage implant tube coverageJ Glaucoma20021114314711912362
- SiegnerSWNetlandPAUrbanRCClinical experience with the Baerveldt glaucoma drainage implantOphthalmology1995102129813079097766
- Al-TorbakAAAl-ShahwanSAl-JadaanIAl-HommadiAEdwardDPEndophthalmitis associated with the Ahmed Glaucoma Valve implantBr J Ophthalmol20058945445815774923
- GeddeSJScottIUTabandehHLate endophthalmitis associated with glaucoma drainage implantsOphthalmology20011081323132711425695
- AinsworthGRotchfordADuaHSKingAJA novel use of amniotic membrane in the management of tube exposure following glaucoma tube shunt surgeryBr J Ophthalmol20069041741916547316
- JoosKMLaviñaAMTawansyKAAgarwalAPosterior repositioning of glaucoma implants for anterior segment complicationsOphthalmology200110827928411158799
- RootmanDBTropeGERootmanDSGlaucoma aqueous drainage device erosion repair with buccalmucous membrane graftsJ Glaucoma20091861862219826391
- MerrillKDSuhrAWLimMCLong-term success in the correction of exposed glaucoma drainage tubes with a tube extenderAm J Ophthalmol200714413613717601438
- RavivTGreenfieldDSLiebmannJMSidotiPAIshikawaHRitchRPericardial patch grafts in glaucoma implant surgeryJ Glaucoma1998727329493112
- OllilaMFalckAAiraksinenPJPlacing the Molteno implant in a long scleral tunnel to prevent postoperative tube exposureActa Ophthalmol Scand20058330230515948781
- Gutiérrez-DíazEMontero-RodríguezMMencía-GutiérrezECabelloAMonescilloJLong-term persistence of fascia lata patch graft in glaucoma drainage device surgeryEur J Ophthalmol20051541241415945014
- TanjiTMLundyDCMincklerDSHeuerDKVarmaRFascia lata patch graft in glaucoma tube surgeryOphthalmology1996103130913128764803
- LankaranianDReisRHendererJDChoeSMosterMRComparison of single thickness and double thickness processed pericardium patch graft in glaucoma drainage device surgeryJ Glaucoma200817485118303385
- HeuerDKBudenzDColemanAAqueous shunt tube erosionJ Glaucoma20011049349611740221
- StewartWCKristoffersenCJDemosCMFsadniMGStewartJAIncidence of conjunctival exposure following drainage device implantation in patients with glaucomaEur J Ophthalmol20102012413019927268
- LamaPJFechtnerRDTube erosion following insertion of a glaucoma drainage device with a pericardial patch graftArch Ophthalmol19991171243124410496400
- LowSARootmanDBRootmanDSTropeGERepair of eroded glaucoma drainage devices: mid-term outcomesJ Glaucoma20122161962222828001
- HuddlestonSMFeldmanRMBudenzDLAqueous shunt exposure: a retrospective review of repair outcomeJ Glaucoma20132243343821673598
- GeffenNBuysYMSmithMConjunctival complications related to Ahmed Glaucoma Valve insertionJ Glaucoma20142310911423059483
- PakravanMYazdaniSShahabiCYaseriMSuperior versus inferior Ahmed Glaucoma Valve implantationOphthalmology200911620821319062098
- LevinsonJDGiangiacomoALBeckALGlaucoma drainage devices: risk of exposure and infectionAm J Ophthlamol2015160516521
- TrubnikVMosterMRChiaTEvaluation of risk factors for glaucoma drainage device-related erosions: a retrospective case-control studyJ Glaucoma20152449850224326968
- LawrenceSDNetlandPAGamma-irradiated cornea allograft for glaucoma surgeryJ Glaucoma20132235535723429612
- EkiciFMosterMRCvintalVHuWDWaisbourdMTube shunt coverage with gamma-irradiated cornea allograft (VisionGraft)Clin Ophthalmol2015975175525995612
