Abstract
Implantation of Ahmed glaucoma valve is an effective surgical technique to reduce intraocular pressure in patients affected with glaucoma. While in the past, the use of this device was reserved to glaucoma refractory to multiple filtration surgical procedures, up-to-date mounting experience has encouraged its use also as a primary surgery for selected cases. Implantation of Ahmed glaucoma valve can be challenging for the surgeon, especially in patients who already underwent previous multiple surgeries. Several tips have to be acquired by the surgeon, and a long learning curve is always needed. Although the valve mechanism embedded in the Ahmed glaucoma valve decreases the risk of postoperative hypotony-related complications, it does not avoid the need of a careful follow-up. Complications related to this type of surgery include early and late postoperative hypotony, excessive capsule fibrosis around the plate, erosion of the tube or plate edge, and very rarely infection. The aim of this review is to describe surgical technique for Ahmed glaucoma valve implantation and to report related complications.
Introduction
Glaucoma, the second leading cause of blindness in the world, is defined as a chronic optic neuropathy, linked to progressive visual field defects. In 2013, the total number of people (ged 40–80 years) with glaucoma worldwide was estimated to be 64.3 million.Citation1 Asia alone accounted for ~60% of the world’s total glaucoma cases (39 million), and Africa had the second highest number of cases with 8.3 million (13%). Europe and North America had 6.77 and 3.36 million cases, respectively.Citation1
Although many risk factors have been described for glaucoma development and progression (intraocular pressure [IOP],Citation2–Citation5 age,Citation3–Citation5 genetic predisposition,Citation6 and vascular parametersCitation7–Citation9), lowering IOP is the only scientifically demonstrated method to slow the progression of the disease. The Early Manifest Glaucoma Trial has demonstrated a 10% reduction of the risk of glaucoma progression with each mmHg of IOP decrease from baseline (hazard ratio [HR]=0.90 per mmHg decrease; 95% confidence interval [CI], 0.86–0.94).Citation2 Similarly, in the UK Glaucoma Treatment Study, a 19% decrease of the risk of visual field progression has been found with each mmHg reduction from baseline.Citation10
IOP reduction in glaucoma patients can be achieved with medical, laser, or surgical therapy.Citation11 Medical therapy is generally the first step, due to the low rate of side effects. However, when target IOP is not achievable, laser and/or surgery should be chosen.Citation12 Trabeculectomy, which was first described by Cairns in 1968,Citation13 is considered the surgical “gold standard” for primary open-angle glaucoma and primary angle-closure glaucoma. Variable success rates for trabeculectomy have been reported in literature. Although success rates are high in the first few years after surgery (70%–92%),Citation14–Citation16 they tend to decrease with time (42%–90%),Citation17–Citation20 especially in secondary glaucomas.Citation21–Citation24 Moreover, as trabeculectomy involves anterior subconjunctival space, it is largely influenced by healing processes, and healing modulation with intraoperative and postoperative antimetabolites (ie, mitomycin C [MMC] and 5-fluorouracil [5FU]) is a fundamental part of the procedure.
Aqueous shunts are a reliable alternative to trabeculectomy.Citation25 Conceptually, shunting aqueous humor (AH) to the posterior subconjunctival space may avoid healing issues, especially in patients who have already undergone previous glaucoma surgeries or conjunctival manipulation. The first successful prototype of aqueous shunt was the Molteno implant, followed by Krupin, Ahmed, and Baerveldt implant. While in the past, these devices were usually reserved as a second choice surgery, today mounting experience has encouraged their use also as a primary surgery.Citation26 The aim of this review is to evaluate surgical technique and complications of Ahmed glaucoma valve (AGV) implant.
AGV: device description and technical data
First attempts at developing a glaucoma drainage implant were published in 1906,Citation27 even if the first device currently in use was developed by Molteno et al only in 1976.Citation28 Molteno device offers no resistance to AH outflow and initially was burdened by a high rate of complications, such as hypotony, shallow anterior chamber (AC), choroidal effusion, and choroidal detachment.Citation26 Implants with an embedded valve mechanism were developed as an attempt at overwhelming these complications. In 1976, Krupin designed a pressure-sensitive unidirectional valve to provide filtration restriction.Citation29 Its passive mechanism contemplates a silastic tube, whose distal end is sealed and contains several horizontal and vertical slits. Krupin implant is designed to open when IOP is >11 mmHg.Citation29,Citation30
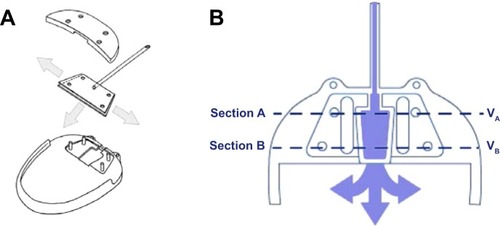
AGV provides a more complex mechanism to control AH outflow. It was developed by Mateen Ahmed and was approved by the Food and Drug Administration in 1993.Citation31 It consists of 3 parts (): 1) a plate, in medical grade silicone, polypropylene, or porous polyethylene, depending on the model; 2) a drainage tube in medical grade silicone; and 3) a valve mechanism in medical grade silicone. Polypropylene is a rigid plastic, not flexible and highly resistant to torsional forces, whereas silicone is a flexible rubber.
Figure 1 Ahmed glaucoma valve implant.
The adult model (S2) of AGV provides 180 mm2 of total plate area, whereas the pediatric one (S3) has a total area of 96 mm2. Obviously, a smaller plate facilitates positioning in infants and subjects with a small eye. A variant of the device with 2 plates (total filtration area: 360 mm2), and one with a clip for pars plana tube insertion have been also designed ().
Table 1 Ahmed glaucoma valve implant available models
The M4 AGV model, recently introduced, is a modified AGV S2 containing identical valve mechanism, but with a case made of porous high-density polyethylene (Medpor; Porex, Atlanta, GA, USA; subsequently, Stryker Corp., Kalamazoo, MI, USA).Citation32 Total plate area is 160 mm2, not including the surface area of pores. The pores may facilitate fibrotic and vascular ingrowth and increase resistance to infection, exposure, extrusion, and mechanical deformation.Citation32–Citation34 In animal models, this new implant behaved as a variable resistor with higher resistance at low flow rates and lower resistance at high flow rates.Citation35 These results support the idea that porous biomaterial may improve hydraulic conductivity of the capsule.
Valve mechanism of AGV consists of thin silicone elastomer membranes, which are 8 mm long and 7 mm wide and create a Venturi-shaped chamber. The membranes are pretensioned to open and close in response to IOP variations, in the range of 8–12 mmHg.Citation31 After implantation, AH flows slowly and continuously into the trapezoidal chamber of the valve (). As the pressure reaches the preset threshold value, the valve opens, thus decreasing the IOP. As the inlet cross-section of the chamber is wider than the outlet, a pressure differential is created across the chamber. This pressure differential enables the valve to remain open even with a small pressure differential between the AC and the subconjunctival spaces surrounding the device. In order for Bernoulli’s equation to be satisfied (fluid flowing into section A = fluid flowing out of section B, ), the fluid’s velocity has to increase as it leaves the chamber through the drainage tube. This increased velocity and the nonobtrusive flow account for better evacuation and smaller valve friction. The tension in the silicone membranes helps to reduce hypotony by closing after the pressure has decreased to normal level again.
Surgical technique
AGV is designed to guarantee no manipulation during the surgical procedure (ie, no restrictive methods to limit AH filtration). The implant should be examined for integrity and primed before implantation. Priming is performed by using a 26G cannula, injecting ~1 cc of balanced salt solution (BSS) or sterile water through the drainage tube. Functionality of the implant is demonstrated by BSS flow through the plate.Citation31
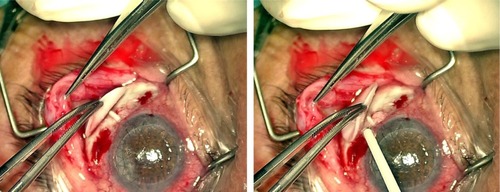
Surgical technique for AGV implantation consists of a fornix-based or limbal-based conjunctival incision to create a conjunctival flap between 2 recti muscles, generally in the superotemporal quadrant. As much as possible, Tenon’s capsule is dissected from the episclera and episcleral vessels are gently cauterized. The technique does not require recti muscles isolation.
Body implant is positioned 8–10 mm from the limbus, outside limbal healing space. The plate is then sutured to the sclera with a 9.0 or 10.0 nylon suture. The drainage tube is trimmed to permit a 2–3 mm insertion in the AC and is bevel cut to an angle of 30°, to facilitate AC entering. An AC paracentesis is performed, and viscoelastic substance is injected to increase spaces. The AC is then entered 1–3 mm posteriorly to the corneoscleral limbus with a 22–23G needle. The needle tract is anterior and parallel to the plane of the iris. The tube, which is trimmed so that the bevel faces to the corneal endothelial surface, is inserted into the AC through the needle tract. Care must be taken at this point to ensure that the drainage tube does not contact iris or corneal endothelium after insertion.
An “hangback” technique has been recently proposed to reduce tissue manipulation and facilitate plate insertion during AGV implantation.Citation36 According to this technique, the plate is not sutured to the sclera and is allowed to “hang” from the tube, which is anchored to the sclera 6–7 mm from the limbus, using a 6.0 vicryl suture. Despite initial results for this technique are promising, more extensive research is needed.Citation36
In patients with a previous vitrectomy, implant’s tube can be inserted in vitreous cavity, avoiding complications that can arise from the presence of the tube in the AC. In this case, a pars plana clip is used to secure the tube to the sclera and to give the tube a suitable angle. The clip is anchored to the sclera with a 9.0 or 10.0 nylon suture.
The drainage tube and eventually the pars plana clip are covered with a piece of preserved, donor sclera, pericardium, cornea, or other suitable patch graft material, which is sutured to the sclera. Alternatively, a two-third thickness limbus scleral flap is created, and the tube is inserted into the AC through a needle tract under the flap. The flap should be closely sutured to avoid peritubular leakage of AH.
In the final step, conjunctiva is anchored to the limbus with adsorbable/nonadsorbable sutures. Optionally, a sub-conjunctival injection of corticosteroids and antibiotics is performed at the end of the procedure. Postoperatively, a regimen of topical corticosteroids and antibiotics is introduced.
A sutureless variant for tube covering has been recently proposed. The tube is covered with human donor scleral graft and kept in place with fibrin glue (Tissue Coll®).Citation37 Tissue Coll is a biologic glue with adhesive properties derived from the formation of fibrin polymers. However, costs, commercial availability, poor uniformity, and quality of donor sclera may limit the widespread use of this procedure. The sutureless technique has been further modified with the use of bovine pericardial graft patch (Tuttopatch, Tutogen Medical GmbH, Neunkirken am Brand, Germany) instead of human sclera, with good results in the medium term ().Citation38 The relative high costs of fibrin glue are in this way counterbalanced by the low cost of Tuttopatch, when compared with human scleral donor patch. The absence of sutures is advantageous in the postoperative, because sutures can promote inflammation and provide a nidus for infections or neovascularization. Written informed consent was received from the patients for this review including publication of their medical data and images.
Antimetabolite and Ahmed valve surgery
The use of antimetabolites in AGV surgery is debated. In 2004, Costa et al randomized 60 patients affected by neovacular glaucoma to receive intraoperative MMC (0.5 mg/mL for 5 minutes; n=34) or BSS (n=26) during surgery for AGV implantation.Citation39 Kaplan–Meier survival analysis showed a 59% and 61% probability of success at 18 months for the MMC and control groups, respectively, with no statistically significant difference. Similar results were obtained by Kurnaz et al in a study on 48 patients affected with refractory glaucoma.Citation40 Authors found no difference in success rates between the MMC and no-antimetabolite groups at 1 year (86.36% versus 80.76%, respectively), but 3 cases of tube exposure were encountered in the MMC group.
Alvarado et al obtained high success rates at 6-year follow-up in patients who underwent AGV implantation (either alone or in combination with cataract surgery) with both intraoperative MMC and postoperative 5FU injections.Citation41 MMC-soaked sponges were placed subconjuntivally for ~5–8 minutes during surgery (median: 8 minutes, MMC concentration: 0.5 mg/mL). 5FU subconjunctival injections were performed postoperatively, for 4 consecutive weeks, starting at 1 week, with a fifth injection at week 6. Kaplan–Meier estimates of the cumulative probability of implant success at the sixth follow-up year were 0.72 and 0.84 for eyes that underwent AGV implantation (n=88) and AGV implantation + cataract extraction (n=42), respectively. Comparing results at 2- and 4-year follow-up with those from other studies in which antimetabolites were not used, authors concluded that there was a potential benefit associated with the use of MMC and 5FU during the intraoperative and early postoperative period.
The way antimetabolites are administered could be important in determining the efficacy of the surgical procedure. Zhou et al proposed a new technique for MMC administration, in which the valve plate was first encompassed with a thin layer of cotton soaked with MMC and then positioned on the sclera.Citation42 After 2–5 minutes, the cotton and the encompassed AGV were removed and irrigated with 200 mL BSS. In comparison with the traditional MMC administration procedure (ie, sponges soaked with MMC and applied on the sclera), the new technique obtained better results in the short and long terms. The new-technique group had only 1 case of encapsulated cyst over the plate out of 38 surgeries (2.6%), whereas the traditional-technique group had 8 cases of encapsulated cyst out of 41 surgeries (19.5%) (P=0.030). However, it should be noted that MMC concentrations in this study were considerably smaller than in other studies (0.25–0.33 mg/mL, 2–5 minutes), and this could have biased the results.
AGV complications
A report from the American Academy of Ophthalmology has reported the major short-term (up to 5 years after surgery) to medium-term (5–10 years after surgery) complications of aqueous shunt devices.Citation43 They include immediate and late hypotony after surgery, excessive capsule fibrosis and clinical failure, erosion of the tube or plate edge, and very rarely infection.
Actually, there is no evidence in literature about different rates of complications with 1 AGV model than another.Citation44–Citation48 Although an higher IOP reduction with the silicone-plate model than the polyethylene one has been described in the short term,Citation44,Citation45,Citation48 long-term results are not conclusive.Citation47
Hypotony
AGV valve mechanism was designed with the aim of preventing postoperative hypotony, allowing for AH drainage when IOP is in the range of 8–12 mmHg. Studies have demonstrated that the mechanism is effective in reducing, but not abolishing, postoperative hypotony, in comparison with other nonvalve implants.Citation49–Citation55 The Ahmed versus Baerveldt Study (AVB Study) was a prospective, multicenter, randomized clinical trial comparing AGV (model FP7) and Baerveldt 350 mm2 implant in patients affected by refractory glaucoma.Citation56 In this study, 7 of 114 patients (6.1%) in the Baerveldt group experienced vision-threatening complications related to hypotony at 3-year analysis (3 developed suprachoroidal hemorrhage, 3 had retinal/choroidal detachments, and 1 had refractory hypotony), against 0 of 124 AGV patients.Citation50 Similarly, in the Ahmed–Baerveldt Comparison (ABC) study, a multicenter clinical trial designed to prospectively compare safety and efficacy of these two commonly implanted devices, 1 eye (2%) in the AGV group experienced failure due to persistent hypotony, against 6 eyes (13%) in the Baerveldt group after 5-year follow-up.Citation52 In comparison with Molteno implant (both single and double plates), AGV demonstrated lower risk of hypotony-related complications, both in retrospective and prospective studies.Citation53–Citation55 Besides these results, postoperative hypotony following the placement of AGV (S2 and FP7) has been reported up to 3% of cases.Citation44
The reason of persistent hypotony after AGV implantation is not completely clear. Attention should be taken during surgical procedure to not over-prime the tube and to not excessively manipulate the valve housing, as these actions could damage the valve mechanism embedded in the implant.Citation57,Citation58 Importance has been placed on the utilization of a 22 or 23G needle when creation of the sclerostomy is undertaken in order to avoid the egress of AH around the silicone tube in the immediate post-operatory.Citation57 In addition, ciliary body function may fail or decrease after surgery in complicated eyes in which glaucoma drainage implants are used.Citation59
In a study by Prata et al, pressure flow characteristics at physiologic flow rates, in vitro and in vivo rabbits, were evaluated for valve (Ahmed and Krupin) and nonvalve (Baerveldt and Molteno) implants.Citation60 Authors concluded that none of the implants tested maintained advertised pressure levels during in vitro tests, when immersed and while being perfused at flow rates close to those expected in normal human eyes. AGV and Krupin implants functioned as flow restriction devices or regulators, rather than as valves that truly open and close in response to pressure change after immersion in fluid. In vivo, conjunctival tissue reaction surrounding the explant portion of the device contributed significantly to the outflow resistance, increasing the restriction effect due to the valve mechanism.
Besides persistent and long-term hypotony, transient hypotony in the immediate postoperatory has been frequently described after AGV implantation. An “hypotensive” phase was recorded in 13% and 15% of patients in the AVB and ABC studies, respectively.Citation50,Citation61 Choroidal effusion may also be present. In these cases, hypotony resolves spontaneously as soon as encapsulation of the plate increases outflow resistance, within days or weeks from the surgery. Observation should be the choice, monitoring AC depth and extension of choroidal detachment. Prompt intervention should be taken if shallow AC, hypotony maculopathy, or near-kissing choroidal detachment is present.
IOP increase and excessive capsule fibrosis
An “hypertensive” phase after glaucoma drainage implantation is quite common and has been frequently described in patients with AGV. Typically, this phase peaks at 1 or 2 months postoperatively and resolves within 6 months.Citation31,Citation62–Citation64 The hypertensive phase could be less frequent in patients who have been implanted with the silicone than with the polypropylene AGV, probably because silicone is less inflammatory than polypropylene.Citation44 The primary reason for elevated IOP in the postoperative period is from capsular fibrosis. Attempts have been made to modulate the fibrotic reaction around the plate, varying plate size, shape, flexibility, and materials.Citation26 Initial data showed that a mitigation of the early postoperative hypertensive phase may be achievable with the new M4 AGV model, in comparison with the FP7 and S2 model.Citation32
An option in the management of the hypertensive phase is, similar to trabeculectomy, digital massage.Citation65,Citation66 The purpose of digital massage is to force AH through the tube, opening the valve mechanism, and reducing scar formation. Caution should be placed in this maneuver in order to avoid repeated tube-corneal endothelial touch.
Late IOP increase (>6 months) is the main cause of long-term failure of AGV surgery. If encapsulation of the plate is evident, a needling revision of the bleb may be attempted, with the aim of reducing outflow resistance. An encapsulated bleb is recognizable as a bleb that has been walled-off by the Tenon’s capsule, resulting in a rigid elevation of the mobile conjunctival tissue. Quaranta et al performed bleb needling with 5FU at the slit lamp in 36 consecutive patients implanted with an S2 AGV, noncontrolled IOP, and encapsulated bleb over the plate.Citation67 Qualified success (IOP ≤18 mmHg with or without medications) was achieved at 1, 3, 6, 12, 18, and 24 months in 100%, 97.8%, 86.1%, 75%, 75%, and 72.2%, respectively. Complications were encountered in 14 eyes (38.8%) and resolved spontaneously in 12 out of 14 cases.
If medical therapy and needling revision are not successful, surgical revision of the implant should be performed. Conjunctiva is dissected over the encapsulated bleb, and the cyst wall is excised. After excision, the conjunctiva is closed with a nylon or vicryl suture. In a retrospective study by Eibschitz-Tsimhoni et al, surgical revision was effective in achieving adequate IOP control in 8 of 11 patients, with or without medications.Citation68 However, in 3 patients, cyst excision was not successful, and further surgical interventions were needed.
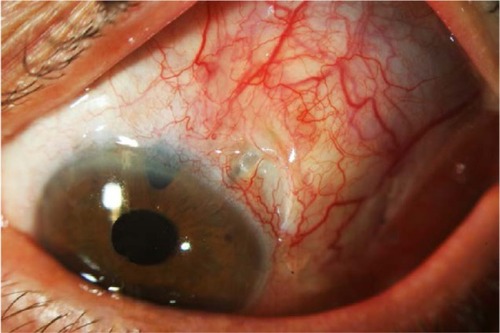
Tube exposure
Tube exposure is a well-known complication of glaucoma drainage implants (). Erosion of the conjunctiva and of the covering patch graft has been described in the late postoperative period in 2%–7% of eyes after implantation of glaucoma devices.Citation62,Citation69–Citation74 With regard to AGV, frequency of tube exposure varies from 5% to 14.3% of cases.Citation63,Citation75,Citation76 In a recently published study on 12 patients, tube exposure has been encountered in up to 30.8% of cases.Citation77 However, these high frequencies are not in accordance with data deriving from multicenter studies.
Tube exposure can lead to ocular inflammation, hypotony, poor vision, and phthisis. Most of all, tube exposure represents a major risk factor for the development of late endophthalmitis, as the exposed tube provides a way for microorganisms to migrate into the eye from ocular surface and conjunctiva.Citation78,Citation79 Although covering the tube with a patch graft material has markedly reduced the rate of tube exposure,Citation80 no significant difference in patch graft survival has been found when sclera, dura, and pericardium were compared.Citation73
The mechanism responsible for tube exposure is not completely clear. A high grade, immune-mediated process could be responsible for rapid melting (<6 months) of the patch, via cell- or noncell immune-mediated process.Citation73 A mechanical process could be involved in patch erosion as well. If tube is not fixed on the sclera, continuous and minimum movements may produce tube-graft tension, resulting in gradual patch atrophy.Citation73,Citation81 Finally, patch melting could occur as a result of a low grade, possibly immune-mediated, long-term, atrophy process, with consequent gradual patch thinning.Citation73
Chaku et al evaluated risk factors for tube exposure in a comparative, retrospective study of 64 eyes, including 32 eyes with tube exposure and 32 control eyes.Citation81 All patients had a glaucoma drainage device implanted, including 35 AGVs and 29 Baerveldt implants. Patients developed tube exposure at a mean of 1.43±1.5 years from surgery, and no difference was found in the rate of exposure between AGV and Baerveldt implant. In both univariate and multivariate analyses, younger age (P<0.01 and P=0.02) and inflammation prior to tube exposure (P<0.01) were significant risk factors for tube exposure. Diabetes was a significant factor only in the univariate analysis (P=0.02).
In a meta-analysis by Stewart et al, 38 previously published studies describing conjunctival erosion in patients with a glaucoma device (16 AGVs, 12 Baerveldt, and 17 Molteno implants) were evaluated.Citation82 A total of 3,105 patients and 3,255 eyes with an average follow-up of 26.1±3.3 months were included in the analysis. The incidence of tube exposure from these studies was 2.0%±2.6% (n=64), with an average exposure rate per month of 0.09%±0.14%. No difference was found among AGV, Baerveldt, and Molteno implant. Although the correlation between study length and incidence of exposure was not significant, there appeared to be a little increase in exposure incidence for studies up to 2-year follow-up.
Implant positioning may be important in determining the risk of tube exposure. In a study by Pakravan et al, 58 eyes underwent AGV implantation in the superotemporal quadrant and 48 eyes in the inferior quadrants.Citation83 Although no difference was found between the 2 groups in terms of IOP reduction, the inferior implant group had a higher rate of complications (P<0.01). One eye (1.7%) in the superior group and 4 eyes (8.3%) in the inferior group required AGV explantation because of conjunctival erosion and implant exposure, unresponsive to conservative measures and surgical intervention (P=0.173). Other studies, comprising different models of glaucoma implants than AGV, confirmed that the location of the implant may be important to prevent tube exposure.Citation84,Citation85
In a report by the American Academy of Ophthalmology, authors suggest that the observation of the loss of conjunctival capillaries over the tube, usually 1–3 mm from the corneoscleral junction, is an indication of impending erosion through the surface.Citation43 Before exposure of the tube, repatching can be accomplished in these cases by redissection from the posterior aspect of the patch and placement of a new patch, ideally donor-preserved sclera, under intact conjunctiva. If exposure of the tube is already present, surgical repairing may be more difficult. Extensive lateral dissection of the conjunctiva should be performed to obtain sufficient mobility to recover the implant. However, in eyes with previous multiple surgeries, covering the new patch with conjunctiva may be difficult, due to the fragile nature of the tissue and firm adhesion of the conjunctiva to underlying scar tissue. In these cases, many solutions have been undertaken, such as the use of buccal membrane,Citation86 amniotic membrane,Citation87 or autologous conjunctiva.Citation88 As a general rule, as long as the edges of the patch graft are covered by conjunctiva, healing usually occurs by migrating conjunctival epithelium over the central defect.
Corneal complications
The presence of the silicone tube in the AC is known to disturb corneal endothelium and may induce corneal decompensation and edema.Citation89 The exact frequency of corneal issues in patients implanted with AGV is not known, but it has been reported to be 9%–27% in the long term.Citation89–Citation91
Topouzis et al, after a mean follow-up of 30.5 months, found a 27% incidence of corneal decompensation in patients who underwent AGV implantation.Citation91 However, in this study, 16 of the 60 eyes enrolled had prior or concurrent corneal grafts and 9 of these 16 eyes had corneal graft failure during the follow-up. In the ABC study, persistent corneal edema was recorded in 20.1% of patients in the AGV group, at 5 years from surgery.Citation51 However, edema was attributed to nonimplant causes in 50% of cases, so that the real percentage of subjects with persistent corneal edema due to the implant was 12%. No difference was found between AGV and Baerveldt implant in terms of persistent corneal edema incidence (20.1% and 20.4%, respectively). In the AVB study, corneal edema affected 7% of patients in the AGV group and 14% of patients in the Baerveldt group at 3-year follow-up (P=0.08).Citation50 Authors hypothesized that the higher rate of corneal edema in the Baerveldt group was the result of greater IOP variability in the early postoperative phase.
The exact mechanism causing endothelial damage in patients with a drainage implant is unknown. Jet flow around the end of the tube caused by heartbeat, AC inflammation, intermittent tube-corneal touch, tube-uveal touch, and foreign body reaction to the silicon tube are all potential mechanisms of endothelial damage in these patients.Citation92 However, factors independent of tube existence in the AC may contribute to corneal decompensation. High IOP and long duration of elevated IOP before surgery, toxicity of eye-drop preservatives, duration of surgery, and changes in the composition of AH may influence endothelial cell functions and take a part in corneal decompensation.Citation93,Citation94
Endothelial cell loss in patients implanted with AGV has been prospectively and retrospectively evaluated. Kim et al found a mean percentage decrease in corneal endothelial cell density of 3.5% at 1 month, 7.6% at 6 months, and 10.5% at 12 months from surgery.Citation95 Lee et al recorded an average decrease in corneal endothelial cell count of 5.8% at 1 month, 11.5% at 6 months, 15.3% at 12 months, 16.6% at 18 months, and 18.6% at 24 months from surgery.Citation96 In both studies, the superotemporal area, which was closest to the site of the tube, showed the greatest decrease in endothelial cell count, whereas the central area showed the smallest decrease. Besides these data, in the study by Lee et al, Kaplan–Meier analysis showed an alarming 36.6% cumulative rate of failure (ie, patients with a decrease in central corneal endothelial cell density >20%) at 24 months.Citation96
In a recent study, medical records of 127 patients who underwent AGV implantation were retrospectively reviewed to evaluate changes in corneal endothelial cell density and rates of corneal decompensation.Citation97 Mean follow-up was 43.1±20.5 months, with 53% of patients followed-up to 60 months. Corneal decompensation occurred only in 3 cases and the cumulative risk of corneal decompensation was 3.3% at 5 years from surgery. Seventy-two eyes were evaluated for annual change in endothelial cell count and compared with 31 control eyes. Although a more rapid loss of endothelial cell count was found in these 72 eyes compared with controls (−7.0% and −0.1%/year, respectively; P<0.01), the rate of loss decreased over time and statistical significance compared with control eyes disappeared after 2 years postoperatively.
Apart from corneal decompensation due to direct tube-corneal touch, tube position could be important in determining corneal complications. Koo et al measured various AH parameters in 39 eyes with previous superotemporal AGV implantation.Citation98 Parameters measured included tube distance from the cornea, tube angle, and AC tube length. All parameters were obtained by anterior segment optical coherent tomography. In univariate analysis, tube-cornea angle and the closest distance from the tip of the tube to the cornea were statistically significant predictors of superotemporal corneal endothelial cell loss after glaucoma surgery. In multivariate analysis, only the distance from the tip of the tube to the cornea was significant; each millimeter that the tube was closer to the endothelial surface was associated with 353.1 (95% CI, 56.1–650.1; P=0.02) fewer endothelial cells.
In patients with corneal decompensation and AGV, Descemet stripping endothelial keratoplasty (DSAEK) has been attempted, with survival rates at 1 year similar to full-thickness keratoplasty.Citation99 Surgical procedure can be challenging in these cases. The length and location of the glaucoma drainage tube within the AC may need to be modified for DSAEK to be successful, and eventually the glaucoma implant posteriorly repositioned.Citation100,Citation101 Moreover, air management is often difficult because injected air may escape through the implant to the subconjunctival space, thereby making it difficult to obtain a firm intraoperative fill.Citation100–Citation102 This is consistent with a potential high rate of early postoperative graft detachment.Citation99,Citation101 Finally, a higher rate of endothelial cell loss has been described in eyes with AGV and previous DSAEK, compared with nonglaucomatous eyes. In a study by Kim et al, mean reduction in graft endothelial cell count was 69% over a 20-month follow-up period,Citation101 whereas Schoenberg et al found an average endothelial cell loss of 40.7% at 12 months from surgery.Citation99
Infection and endophthalmitis
Endophthalmitis or infections associated with grafting material covering the tube is a rare complication of glaucoma drainage implants. For this reason, recurrent blebitis after trabeculectomy can be a reasonable indication for shunt implantation, according to a report by the American Academy of Ophthalmology.Citation43 Several retrospective studies about glaucoma drainage implants included few cases of endophthalmitis, resulting in rates ranging from 0.8% to 6.3% (mean: 2.0%).Citation78 There appears to be no significant difference in reported rates of endophthalmitis among various glaucoma drainage implants.Citation78,Citation103
In a retrospective study, Al-Torbak et al reviewed records of patients implanted with AGV from 1994 to 2003 in a single eye hospital in Saudi Arabia and found an incidence of endophthalmitis of 1.7% (9 patients on 542 implanted).Citation78 Five cases of endophthalmitis were detected in pediatric patients (ie, age <18 years) and 4 in adults. Median interval between AGV implantation and diagnosis of endophthalmitis was 260 days and delayed onset endophthalmitis developed in 8 of 9 eyes (88.8%). Conjunctival erosion over the tube was found in 6 of 9 patients (66.6%) who developed endophthalmitis and 4 of 6 eyes (66.6%) had a Seidel-positive leak. Multiple regression analysis revealed younger age (<18 years; P<0.05) and conjunctival erosion over the tube (P<0.01) as significant risk factors for endophthalmitis development.
Conjunctival erosion and tube exposure appear to be a major risk factor for infection development in eyes with glaucoma drainage implants. Several case reports in literature describe episodes of late endophthalmitis in patients with AGV and tube exposure.Citation104–Citation107 It has been postulated that the exposed tube may act as a direct channel for the intraocular passage of the conjunctival flora from the ocular surface.Citation103 Younger age is another major risk factor. Case series of pediatric patients implanted with glaucoma drainage devices have shown an incidence of endophthalmitis ranging from 2.9% to 5%.Citation103,Citation108,Citation109 This could be related to the higher rate of conjunctival erosion and implant exposure in children.Citation109,Citation110 Interestingly, younger age has been advocated as a significant risk factor for endophthalmitis also in patients who underwent trabeculectomy, with an incidence of late bleb-related endophthalmitis as high as 8.3% in the pediatric population.Citation103
Diplopia and strabismus
Diplopia and strabismus are well-known postsurgical complications of glaucoma drainage devices.Citation43 The cause of diplopia is likely a restrictive strabismus, either from the plate itself or from the plate impinging on the muscle insertion.Citation111 Manipulation of the rectus muscles during surgery may induce strabismus as well, which usually resolves spontaneously in weeks or months.Citation63 A systematic review of literature by Hong et al found an higher incidence of diplopia in patients with Baerveldt implant compared with patients with other glaucoma devices.Citation111 However, the ABC study found an equal, ~12% cumulative risk of persistent diplopia in both the AGV and Baerveldt groups.Citation52 Authors suggested that the end-plate fenestrations of the new designed Baerveldt implant (applied in the ABC study) could reduce the height of the bleb and consequently minimize restrictive strabismus. For this reason, an equal risk of diplopia may have been found in both groups of the ABC study.
In a retrospective study, including 159 eyes implanted with AGV, Huang et al found diplopia in 4 patients (2.5%), 3 requiring extraocular muscle surgery and 1 who had removal of the device.Citation62 However, Ayyala et al reported an incidence of transient diplopia of 4.7% (4 of 85) in patients who underwent AGV surgery.Citation63 Fifty percent cases of diplopia occurred within 3 months of surgery.
Conclusion
AGV implantation is an effective and relatively safe surgical procedure, which allows to manage particular phenotypes of glaucoma (ie, secondary glaucoma) and glaucoma refractory to previous filtration surgeries (ie, second choice surgery). Despite an apparently “user-friendly” technique, many surgical tips are to be acquired by the surgeon, and a long learning curve is always needed. In comparison with other nonvalve glaucoma drainage devices, AGV has the great advantage of an easier postoperative management. Nevertheless, early postoperative hypotony is still a dangerous complication that can affect this type of surgery.
Acknowledgments
The contribution of the IRCCS Fondazione GB Bietti per l’Oftalmologia in this paper was supported by Ministry of Health and Fondazione Roma.
Disclosure
The authors report no conflicts of interest in this work.
References
- ThamYCLiXWongTYQuigleyHAAungTChengCYGlobal prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysisOphthalmology2014121112081209024974815
- LeskeMCHeijlAHusseinMBengtssonBHymanLKomaroffEEarly Manifest Glaucoma Trial GroupFactors for glaucoma progression and the effect of treatment: the early manifest glaucoma trialArch Ophthalmol20031211485612523884
- GordonMOBeiserJABrandtJDThe ocular hypertension treatment study: baseline factors that predict the onset of primary open-angle glaucomaArch Ophthalmol2002120671472012049575
- GordonMOTorriVMigliorSOcular Hypertension Treatment Study G, European Glaucoma Prevention StudyValidated prediction model for the development of primary open-angle glaucoma in individuals with ocular hypertensionOphthalmology20071141101917095090
- Nouri-MahdaviKHoffmanDColemanALPredictive factors for glaucomatous visual field progression in the advanced glaucoma intervention studyOphthalmology200411191627163515350314
- GreenCMKearnsLSWuJAdvanced Glaucoma Intervention StudyHow significant is a family history of glaucoma? Experience from the glaucoma inheritance study in TasmaniaClin Exp Ophthalmol200735979379918173405
- PillunatLEStodtmeisterRMarquardtRMatternAOcular perfusion pressures in different types of glaucomaInt Ophthalmol1989131–237422744953
- QuarantaLManniGDonatoFBucciMGThe effect of increased intraocular pressure on pulsatile ocular blood flow in low tension glaucomaSurv Ophthalmol1994Suppl 38S177S1817940140
- QuarantaLKatsanosARussoARivaI24-hour intraocular pressure and ocular perfusion pressure in glaucomaSurv Ophthalmol2013581264123217586
- Garway-HeathDFCrabbDPBunceCLatanoprost for open-angle glaucoma (UKGTS): a randomised, multicentre, placebo-controlled trialLancet201538599751295130425533656
- RulliEBiagioliERivaIEfficacy and safety of trabeculectomy vs nonpenetrating surgical procedures: a systematic review and meta-analysisJAMA Ophthalmol2013131121573158224158640
- PrumBEJrRosenbergLFGeddeSJPrimary Open-Angle Glaucoma Preferred Practice Pattern((R)) GuidelinesOphthalmology20161231P41P11126581556
- CairnsJETrabeculectomy. Preliminary report of a new methodAm J Ophthalmol19686646736794891876
- LawSKShihKTranDHColemanALCaprioliJLong-term outcomes of repeat vs initial trabeculectomy in open-angle glaucomaAm J Ophthalmol20091485685695.e68119596220
- EdmundsBThompsonJRSalmonJFWormaldRPThe National Survey of Trabeculectomy. II. Variations in operative technique and outcomeEye (Lond)200115Pt 444144811767016
- ScottIUGreenfieldDSSchiffmanJOutcomes of primary trabeculectomy with the use of adjunctive mitomycinArch Ophthalmol199811632862919514480
- WilenskyJTChenTCLong-term results of trabeculectomy in eyes that were initially successfulTrans Am Ophthalmol Soc1996941471598981694
- MoltenoACBosmaNJKittelsonJMOtago glaucoma surgery outcome study: long-term results of trabeculectomy – 1976 to 1995Ophthalmology199910691742175010485545
- BevinTHMoltenoACHerbisonPOtago Glaucoma Surgery Outcome Study: long-term results of 841 trabeculectomiesClin Exp Ophthalmol200836873173719128377
- LandersJMartinKSarkiesNBourneRWatsonPA twenty-year follow- up study of trabeculectomy: risk factors and outcomesOphthalmology2012119469470222196977
- TakiharaYInataniMFukushimaMIwaoKIwaoMTaniharaHTrabeculectomy with mitomycin C for neovascular glaucoma: prognostic factors for surgical failureAm J Ophthalmol2009147591291819195639
- IwaoKInataniMSetoTLong-term outcomes and prognostic factors for trabeculectomy with mitomycin C in eyes with uveitic glaucoma: a retrospective cohort studyJ Glaucoma2014232889422895522
- BettisDIMorshediRGChayaCGoldsmithJCrandallAZabriskieNTrabeculectomy with mitomycin C or Ahmed valve implantation in eyes with uveitic glaucomaJ Glaucoma201524859159925393037
- SinghDChandraASihotaRKumarSGuptaVLong-term success of mitomycin-augmented trabeculectomy for glaucoma after vitreoretinal surgery with silicone oil insertion: a prospective case seriesRetina201434112312823615348
- GeddeSJSchiffmanJCFeuerWJHerndonLWBrandtJDBudenzDLTube versus Trabeculectomy Study GroupTreatment outcomes in the tube versus trabeculectomy (TVT) study after five years of follow-upAm J Ophthalmol2012153578980322245458
- PatelSPasqualeLRGlaucoma drainage devices: a review of the past, present, and futureSemin Ophthalmol2010255–626527021091010
- RolletMLe drainage au irin de la chambre anterieure contre l’hypertonie et al douleurRev Gen Ophthalmol190625481
- MoltenoACStraughanJLAnckerELong tube implants in the management of glaucomaS Afr Med J1976502710621066951630
- KrupinTPodosSMBeckerBNewkirkJBValve implants in filtering surgeryAm J Ophthalmol1976812232235814819
- Krupin eye valve with disk for filtration surgeryThe Krupin Eye Valve Filtering Surgery Study GroupOphthalmology199410146516588152759
- ColemanALHillRWilsonMRInitial clinical experience with the Ahmed glaucoma valve implantAm J Ophthalmol1995120123317611326
- KimJAllinghamRRHallJKlitzmanBStinnettSAsraniSClinical experience with a novel glaucoma drainage implantJ Glaucoma2014232e91e9723689073
- KlawitterJJBagwellJGWeinsteinAMSauerBWAn evaluation of bone growth into porous high density polyethyleneJ Biomed Mater Res19761023113231254618
- SpectorMHarmonSLKreutnerACharacteristics of tissue growth into proplast and porous polyethylene implants in boneJ Biomed Mater Res1979135677692479215
- DeCroosFCKondoYMordesDIn vitro fluid dynamics of the Ahmed glaucoma valve modified with expanded polytetrafluoroethyleneCurr Eye Res201136211211721281065
- PandavSBangerAIchpujaniPRajSKaushikSResults of Ahmed glaucoma valve implantation with “hangback” techniqueWorld Glaucoma Congress2011Paris
- ZeppaLRomanoMRCapassoLTortoriAMajoranaMACostagliolaCSutureless human sclera donor patch graft for Ahmed glaucoma valveEur J Ophthalmol201020354655120037905
- QuarantaLRivaIFlorianiICOutcomes of using a sutureless bovine pericardial patch graft for Ahmed glaucoma valve implantationEur J Ophthalmol201323573874223483494
- CostaVPAzuara-BlancoANetlandPALeskMRArcieriESEfficacy and safety of adjunctive mitomycin C during Ahmed glaucoma valve implantation: a prospective randomized clinical trialOphthalmology200411610711076
- KurnazEKubalogluAYilmazYKoytakAOzerturkYThe effect of adjunctive mitomycin C in Ahmed glaucoma valve implantationEur J Ophthalmol20051512731
- AlvaradoJAHollanderDAJusterRPLeeLCAhmed valve implantation with adjunctive mitomycin C and 5-fluorouracil: long-term outcomesAm J Ophthalmol2008146227628418538300
- ZhouMWangWHuangWZhangXUse of mitomycin C to reduce the incidence of encapsulated cysts following Ahmed glaucoma valve implantation in refractory glaucoma patients: a new techniqueBMC Ophthalmol20141410725194218
- MincklerDSFrancisBAHodappEAAqueous shunts in glaucoma: a report by the American Academy of OphthalmologyOphthalmology200811561089109818519069
- IshidaKNetlandPACostaVPShiromaLKhanBAhmedIIComparison of polypropylene and silicone Ahmed glaucoma valvesOphthalmology200611381320132616877071
- LawSKNguyenAColemanALCaprioliJComparison of safety and efficacy between silicone and polypropylene Ahmed glaucoma valves in refractory glaucomaOphthalmology200511291514152016005977
- BrasilMVRockwoodEJSmithSDComparison of silicone and polypropylene Ahmed glaucoma valve implantsJ Glaucoma2007161364117224747
- MackenziePJSchertzerRMIsbisterCMComparison of silicone and polypropylene Ahmed glaucoma valves: two-year follow-upCan J Ophthalmol200742222723217392844
- HinkleDMZurakowskiDAyyalaRSA comparison of the polypropylene plate Ahmed glaucoma valve to the silicone plate Ahmed glaucoma flexible valveEur J Ophthalmol2007175696701
- ChristakisPGKalenakJWZurakowskiDThe Ahmed versus Baerveldt study: one-year treatment outcomesOphthalmology2011118112180218921889801
- ChristakisPGTsaiJCKalenakJWThe Ahmed versus Baerveldt study: three-year treatment outcomesOphthalmology2013120112232224023796764
- BudenzDLBartonKGeddeSJAhmed Baerveldt Comparison Study GroupFive-year treatment outcomes in the Ahmed Baerveldt comparison studyOphthalmology2015122230831625439606
- BudenzDLFeuerWJBartonKAhmed Baerveldt Comparison Study GroupPostoperative complications in the Ahmed Baerveldt comparison study during five years of follow-upAm J Ophthalmol201616375.e7382.e7326596400
- AyyalaRSZurakowskiDMonshizadehRComparison of double-plate Molteno and Ahmed glaucoma valve in patients with advanced uncontrolled glaucomaOphthalmic Surg Lasers20023329410111942556
- NassiriNKamaliGRahnavardiMAhmed glaucoma valve and single-plate Molteno implants in treatment of refractory glaucoma: a comparative studyAm J Ophthalmol2010149689390220451896
- TagliaDPPerkinsTWGangnonRHeatleyGAKaufmanPLComparison of the Ahmed glaucoma valve, the Krupin eye valve with disk, and the double-plate molteno implantJ Glaucoma200211434735312169973
- ChristakisPGTsaiJCZurakowskiDKalenakJWCantorLBAhmedIIThe Ahmed Versus Baerveldt study: design, baseline patient characteristics, and intraoperative complicationsOphthalmology2011118112172217921906813
- BaileyAKSarkisianSRJrComplications of tube implants and their managementCurr Opin Ophthalmol201425214815324463417
- SarkisianSRJrTube shunt complications and their preventionCurr Opin Ophthalmol200920212613019240545
- KrupinTRitchRCamrasCBA long Krupin-Denver valve implant attached to a 180 degrees scleral explant for glaucoma surgeryOphthalmology1988959117411803211495
- PrataJAJrMermoudALaBreeLMincklerDSIn vitro and in vivo flow characteristics of glaucoma drainage implantsOphthalmology199510268949047777296
- BudenzDLBartonKFeuerWJAhmed Baerveldt Comparison Study GroupTreatment outcomes in the Ahmed Baerveldt Comparison Study after 1 year of follow-upOphthalmology2011118344345220932583
- HuangMCNetlandPAColemanALSiegnerSWMosterMRHillRAIntermediate-term clinical experience with the Ahmed glaucoma valve implantAm J Ophthalmol1999127127339932995
- AyyalaRSZurakowskiDSmithJAA clinical study of the Ahmed glaucoma valve implant in advanced glaucomaOphthalmology199810510196819769787371
- Nouri-MahdaviKCaprioliJEvaluation of the hypertensive phase after insertion of the Ahmed glaucoma valveAm J Ophthalmol200313661001100814644209
- McIlraithIBuysYCampbellRJTropeGEOcular massage for intraocular pressure control after Ahmed valve insertionCan J Ophthalmol2008431485218219346
- SmithMGeffenNAlasbaliTBuysYMTropeGEDigital ocular massage for hypertensive phase after Ahmed valve surgeryJ Glaucoma2010191111420075672
- QuarantaLFlorianiIHollanderLPoliDKatsanosAKonstasAGNeedle revision with 5-fluorouracil for the treatment of Ahmed glaucoma valve filtering blebs: 5-fluoruracil needling revision can be a useful and safe tool in the management of failing Ahmed glaucoma valve filtering blebsJ Glaucoma2016254e367e37126766399
- Eibschitz-TsimhoniMSchertzerRMMuschDCMoroiSEIncidence and management of encapsulated cysts following Ahmed glaucoma valve insertionJ Glaucoma200514427627915990607
- ByunYSLeeNYParkCKRisk factors of implant exposure outside the conjunctiva after Ahmed glaucoma valve implantationJpn J Ophthalmol200953211411919333694
- WishartPKChoudharyAWongDAhmed glaucoma valves in refractory glaucoma: a 7-year auditBr J Ophthalmol20109491174117919965829
- MillsRPReynoldsAEmondMJBarlowWELeenMMLong-term survival of Molteno glaucoma drainage devicesOphthalmology199610322993058594518
- VuoriMLMolteno aqueous shunt as a primary surgical intervention for uveitic glaucoma: long-term resultsActa Ophthalmol2010881333619900205
- SmithMFDoyleJWTicrneyJWJrA comparison of glaucoma drainage implant tube coverageJ Glaucoma200211214314711912362
- SiegnerSWNetlandPAUrbanRCJrClinical experience with the Baerveldt glaucoma drainage implantOphthalmology19951029129813079097766
- MontanezFJLasoESunerMAmayaCAhmed drainage device implant. Our experience between 1995 and 2003Arch Soc Esp Oftalmol200580423924415852165
- ChenHZhangSXLiuLIntermediate-term and long-term clinical evaluation of the Ahmed glaucoma valve implantationZhonghua Yan Ke Za Zhi2005419796802 Chinese16191345
- KayaMOzbekZYamanADurakILong-term success of ahmed glaucoma valve in refractory glaucomaInt J Ophthalmol20125110811222553766
- Al-TorbakAAAl-ShahwanSAl-JadaanIAl-HommadiAEdwardDPEndophthalmitis associated with the Ahmed glaucoma valve implantBr J Ophthalmol200589445445815774923
- GeddeSJScottIUTabandehHLate endophthalmitis associated with glaucoma drainage implantsOphthalmology200110871323132711425695
- HeuerDKBudenzDColemanAAqueous shunt tube erosionJ Glaucoma200110649349611740221
- ChakuMNetlandPAIshidaKRheeDJRisk factors for tube exposure as a late complication of glaucoma drainage implant surgeryClin Ophthalmol20161054755327099461
- StewartWCKristoffersenCJDemosCMFsadniMGStewartJAIncidence of conjunctival exposure following drainage device implantation in patients with glaucomaEur J Ophthalmol201020112413019927268
- PakravanMYazdaniSShahabiCYaseriMSuperior versus inferior Ahmed glaucoma valve implantationOphthalmology2009116220821319062098
- LevinsonJDGiangiacomoALBeckADGlaucoma drainage devices: risk of exposure and infectionAm J Ophthalmol2015160351652126032191
- TrubnikVZangalliCMosterMREvaluation of risk factors for glaucoma drainage device-related erosions: a retrospective case-control studyJ Glaucoma201524749850224326968
- LowSARootmanDBRootmanDSTropeGERepair of eroded glaucoma drainage devices: mid-term outcomesJ Glaucoma201221961962222828001
- AinsworthGRotchfordADuaHSKingAJA novel use of amniotic membrane in the management of tube exposure following glaucoma tube shunt surgeryBr J Ophthalmol200690441741916547316
- GodfreyDGMerrittJHFellmanRLStaritaRJInterpolated conjunctival pedicle flaps for the treatment of exposed glaucoma drainage devicesArch Ophthalmol2003121121772177514662599
- WilsonMRMendisUPaliwalAHaynatzkaVLong-term follow-up of primary glaucoma surgery with Ahmed glaucoma valve implant versus trabeculectomyAm J Ophthalmol2003136346447012967799
- KookMSYoonJKimJLeeMSClinical results of Ahmed glaucoma valve implantation in refractory glaucoma with adjunctive mitomycin COphthalmic Surg Lasers200031210010610743919
- TopouzisFColemanALChoplinNFollow-up of the original cohort with the Ahmed glaucoma valve implantAm J Ophthalmol1999128219820410458176
- McDermottMLSwendrisRPShinDHJuzychMSCowdenJWCorneal endothelial cell counts after Molteno implantationAm J Ophthalmol1993115193968420384
- SetalaKCorneal endothelial cell density after an attack of acute glaucomaActa Ophthalmol (Copenh)197957610041013545996
- FiorePMRichterCUArzenoGThe effect of anterior chamber depth on endothelial cell count after filtration surgeryArch Ophthalmol198910711160916112818280
- KimCSYimJHLeeEKLeeNHChanges in corneal endothelial cell density and morphology after Ahmed glaucoma valve implantation during the first year of follow upClin Experiment Ophthalmol200836214214718352870
- LeeEKYunYJLeeJEYimJHKimCSChanges in corneal endothelial cells after Ahmed glaucoma valve implantation: 2-year follow-upAm J Ophthalmol2009148336136719505676
- KimKNLeeSBLeeYHLeeJJLimHBKimCSChanges in corneal endothelial cell density and the cumulative risk of corneal decompensation after Ahmed glaucoma valve implantationBr J Ophthalmol Epub20151027
- KooEBHouJHanYKeenanJDStamperRLJengBHEffect of glaucoma tube shunt parameters on cornea endothelial cells in patients with Ahmed valve implantsCornea2015341374125393097
- SchoenbergEDLevinKHSavetskyMJMcIntireLUAyyalaRSSurgical outcomes of DSAEK in patients with prior Ahmed glaucoma drainage device placementEur J Ophthalmol201323680781323787454
- RiazKMSugarJTuEYEarly results of Descemet-Stripping and Automated Endothelial Keratoplasty (DSAEK) in patients with glaucoma drainage devicesCornea200928995996219724221
- KimPAmiranMDLichtingerAYeungSNSlomovicARRootmanDSOutcomes of Descemet stripping automated endothelial keratoplasty in patients with previous glaucoma drainage device insertionCornea201231217217522146552
- IdeTYooSHLengTO’BrienTPSubconjunctival Air Leakage After Descemet’s Stripping Automated Endothelial Keratoplasty (DSAEK) in a Post-Trabeculectomy EyeOpen Ophthalmol J200931219554220
- AngGSVargaZShaarawyTPostoperative infection in penetrating versus non-penetrating glaucoma surgeryBr J Ophthalmol201094121571157619897472
- RaoAWallangBPadhyTRMittalRSharmaSDual infection by streptococcus and atypical mycobacteria following Ahmed glaucoma valve surgerySemin Ophthalmol201328423323523627305
- StewartMWBollingJPBendelRENocardia brasiliensis endophthalmitis in a patient with an exposed Ahmed glaucoma drainage implantOcul Immunol Inflamm2013211697023323584
- RanganathAHashimALate-onset endophthalmitis secondary to exposed glaucoma tube implant in a rare case of paediatric glaucomaCase Rep Ophthalmol Med2011201118364722611505
- Gutierrez-DiazEMontero-RodriguezMMencia-GutierrezEFernandez-GonzalezMCPerez-BlazquezEPropionibacterium acnes endophthalmitis in Ahmed glaucoma valveEur J Ophthalmol200111438338511820313
- DjodeyreMRPeralta CalvoJAbelairas GomezJClinical evaluation and risk factors of time to failure of Ahmed glaucoma valve implant in pediatric patientsOphthalmology2001108361462011237918
- MoradYDonaldsonCEKimYMAbdolellMLevinAVThe Ahmed drainage implant in the treatment of pediatric glaucomaAm J Ophthalmol2003135682182912788122
- ChenTCBhatiaLSWaltonDSAhmed valve surgery for refractory pediatric glaucoma: a report of 52 eyesJ Pediatr Ophthalmol Strabismus200542527428316250216
- HongCHArosemenaAZurakowskiDAyyalaRSGlaucoma drainage devices: a systematic literature review and current controversiesSurv Ophthalmol2005501486015621077