Abstract
Aim
Our specific aim was to document the pathoetiologic importance of thrombophilia among females presenting with severe ischemic retinal vein (RVO) or retinal artery (RAO) occlusion, without typical risk factors, and to emphasize that the ophthalmologists’ diagnosis of thrombophilia has important diagnostic and therapeutic downstream ramifications for nonocular thrombosis, including reproductive outcomes.
Methods
We evaluated familial and acquired thrombophilia in 60 females with RVO (central RVO, n=52; branch RVO, n=8) and 16 with RAO (central RAO, n=11; branch RAO, n=5). They were referred by retinologists, without typical risk factors for RVO/RAO and/or severe ocular ischemic presentation. We focused on extraocular thrombotic events, particularly pregnancy complications, including unexplained spontaneous abortion, pre-eclampsia–eclampsia. Thrombophilia measurements in the 76 females were compared with 62 healthy normal females without ocular vascular occlusions (OVOs).
Results
The 76 females with OVO were more likely than 62 normal female controls to have high homocysteine (24% vs 0%, P<0.0001), high anticardiolipin antibody (immunoglobulin M, 17% vs 3%, P=0.012), high (>150%) factor VIII (42% vs 11%, P<0.0001), and high (>150%) factor XI (22% vs 4%, P=0.004). Of the 76 females, 26 (34%) had ≥1 spontaneous abortion; 17 (22%) had ≥2 spontaneous abortions and/or pre-eclampsia–eclampsia. Compared to 62 healthy female controls, these 17 females with pregnancy complications had high homocysteine (29% vs 0%, P=0.0003), high anticardiolipin antibody immunoglobulin M (24% vs 3%, P=0.02), high factor VIII (38% vs 11%, P=0.02), and were marginally more likely to be heterozygous for the factor V Leiden mutation (19% vs 3%, P=0.058).
Conclusion
In females lacking typical risk factors for retinal vascular occlusion or severely ischemic presentation, by diagnosing thrombophilia as an etiology for OVO, the ophthalmologist opens a window to family screening and preventive therapy, with particular relevance to pregnancy outcomes and venous thromboembolism.
Introduction
Widely recognized, but neither sensitive nor specific risk factors for ocular vascular occlusion (OVO) include age, history of smoking, hypertension, hyperlipidemia, and diabetes mellitus.Citation1–Citation11 Rare systemic risk factors for OVO include hyperviscosity, myelo-proliferative disorders, retro-orbital mass effect, and vasculitis such as Behcet’s.Citation12,Citation13 Open-angle glaucoma decreases venous outflow via increased intraocular pressure, thus creating vascular stasis and increased risk of OVO.Citation2,Citation14–Citation16 In the absence of a cardioembolic etiology for OVO, thrombophilia is a common, major cause of ocular thrombotic events.Citation12,Citation17–Citation25 In particular, thrombophilia should be carefully assessed in younger patients, <65 years old, or in patients with a personal or family history of thrombosis.Citation25
ThrombophiliaCitation20 can be heritable – such as hyperhomocysteinemia, factor V Leiden (FVL), prothrombin G20210A (PTG) mutation,Citation18 antithrombin III deficiency, protein C deficiency or protein S deficiency – or acquired, particularly the antiphospholipid syndrome-lupus anticoagulant.Citation26 Of the thrombophilias that are risk factors for OVO,4,8,9,13,17,24,27–37 hyperhomocysteinemia is the most common disorder.Citation8,Citation13,Citation17,Citation24,Citation33–Citation37 Homocysteine is also a risk factor for systemic vascular thrombosis, including ischemic heart disease and deep venous thrombosis.Citation38,Citation39 In addition to hyperhomocysteinemia, FVL and PTG heterozygosity have been shown to be major risk factors for both OVOCitation18 and large vein thrombosis.Citation33,Citation40–Citation43
Thrombophilia is only one of many causes of spontaneous abortion.Citation44–Citation47 Familial and acquired thrombophilia are important risk factors for spontaneous pregnancy loss,Citation48,Citation49 interacting with the physiologic thrombophilia of pregnancyCitation50,Citation51 or the postpartum periodCitation52 to promote thrombosis of the spiral arteries of the placenta, facilitating development of placental insufficiency, with resultant spontaneous abortion; pre-eclampsia, eclampsia; and hemolysis, elevated liver enzymes, and low platelet count syndrome.Citation51
Our specific aim was to document the pathoetiologic importance of thrombophilia among females presenting with severe ischemic retinal vein (RVO) or retinal artery (RAO) occlusion, without typical risk factors, and to emphasize that the ophthalmologists’ diagnosis of thrombophilia has important diagnostic and therapeutic downstream ramifications for nonocular thrombosis, including reproductive outcomes.
Methods
The study was approved by the Jewish Hospital Institutional Review Board (ID 12-03), Cincinnati, OH, USA. Written informed consent was obtained from patients after the nature of the study was fully explained. The study was conducted in accordance with the principles of the Declaration of Helsinki.
In a consecutive case series of females referred to vitreoretinal specialists at the Cincinnati Eye Institute with severe ischemiaCitation18,Citation53 (OVO without typical risk factors), studies of thrombophilia were carried out in parallel with assessment of other organ systems affected by thrombosis, with special focus on reproductive outcomes, known to be affected by thrombophilia.
The analysis cohort was divided into patients with RVO (low pressure, low velocity), including branch and central RVO (BRVO and CRVO), and those with RAO (high pressure and velocity), including central RAO (CRAO) and branch RAO. Patients were referred and evaluated by vitreoretinal specialists at the Cincinnati Eye Institute. The ocular diagnoses were established by complete ophthalmological evaluations during which the patients’ histories, visual deficits, and fundus abnormalities were ascertained. Patients were referred to our outpatient thrombosis research center between January 1, 2014 and January 1, 2016, for thrombophilia/hypofibrinolysis evaluation based on lack of typical risk factors for OVO, or severely ischemic presentation. The 16 patients referred with RAO had normal carotid ultrasound and cardiac echocardiograms without any evidence for emboli causing RAO.
Patients with RVO showed dilation of retinal veins (all veins if a CRVO, and not all the veins if a BRVO) associated with intraretinal hemorrhages, retinal edema, and cotton wool spots limited in area by the drainage bed of the affected veins.Citation18 Patients with RAO demonstrated retinal arterial narrowing, segmentation of the arterial blood column in some cases, and whitening of the retina due to opacification and thickening of the inner retina.Citation18 In CRAO cases, a cherry-red spot was seen in the macula. Fluorescein angiography and optical coherence tomography were performed to corroborate the diagnosis depending on the preference of the referring ophthalmologist.
During the patients’ initial visit to our center, a detailed history, obstetrical–gynecological history, and physical examination were completed. The number of pregnancies, live births, spontaneous abortions, and elective abortions were recorded. When there was a history of spontaneous abortion, information was systematically obtained on maternal age, gravidity, smoking, alcohol, and cocaine use, in addition to whether there had been prior investigations of risk factorsCitation54,Citation55 for spontaneous abortion. Information was gathered regarding any studies for chromosomal abnormalities,Citation44 uterine structural issues (including septate uterus),Citation47 maternal thyroid status, and maternal trauma. Maternal and family histories of previous thrombotic events were obtained. Serologic coagulation assays were done and polymerase chain reaction (PCR) analyses for thrombophilia and hypofibrinolysis were performed. Atherosclerotic risk factors were measured, including age, body mass index (BMI), smoking history, blood pressure, hemoglobin A1c, glucose, homocysteine, and triglyceride and cholesterol levels, including high-density lipoprotein (HDL) and low-density lipoprotein (LDL) cholesterol.Citation17
PCR measuresCitation56 were used to measure G1691A FVL, PTG, methylene tetrahydrofolate reductase C677T and A1298C mutations, and the plasminogen activator inhibitor-1 4G4G mutation. PCR measures for the thrombomodulin gene mutation were not obtained. In addition, serologic measures of thrombophiliaCitation20 were done, including anticardiolipin antibodies (ACLA) IgG and IgM, antigenic protein C, total and free protein S, antithrombin III, lupus anticoagulant, factors VIII and XI, and homocysteine. All PCR and serologic measures were done as previously described.Citation17,Citation18
After signed informed consent was obtained, healthy normal female volunteers (n=62) served as controls. They were documented by interview and physical examination to be free of acute and chronic disease, including any history or evidence of OVO.
Statistical methods
All statistical analyses were performed using SAS V9.4 (SAS institute Inc., Cary, NC, USA). Cases were compared to controls by Fisher’s exact test. Sample size was estimated based on our recent studies of 265 patients, 191 with ocular vein occlusion, and 74 with ocular artery occlusion, with comparison to 110 normal controls,Citation20 where 50% of RVO patients had at least one of seven thrombophilias (FVL, prothrombin gene heterozygosity, low free protein S, high homocysteine, high factor VIII, factor XI, ACLA IgM high) vs 20% in controls. At least 39 subjects were required in each group to detect the difference at significance level alpha =0.05 with power 80%.
Results
As an entire cohort, the 76 females with RVO were more likely than the 62 normal female controls to have high homocysteine (24% vs 0%, P<0.0001), ACLA IgM (17% vs 3%, P=0.012), high factor VIII (>150%) (42% vs 11%, P<0.0001), and high factor XI (>150%) (22% vs 4%, P=0.004) ( and ).
Figure 1 Factor V Leiden heterozygosity, high homocysteine,a and high ACLA IgMb in 16 patients with RAO (five branch, eleven central), 60 with RVO (eight branch, 52 central), all 76 patients with OVO, and 17 of the OVO patients with ≥2 spontaneous abortions or eclampsia, compared with 62 healthy normal females.
Abbreviations: ACLA, anticardiolipin antibody; IgM, immunoglobulin M; MPL, IgM phospholipid units; OVO, ocular vascular occlusion; RAO, retinal artery occlusion; RVO, retinal vein occlusion.
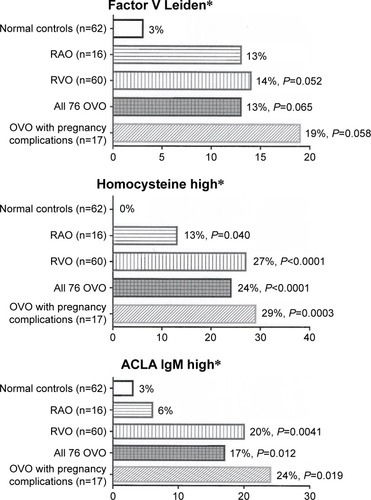
Figure 2 High factors VIII,a XI,b and low free protein Sc in 16 patients with RAO (five branch, eleven central), 60 with RVO (eight branch, 52 central), all 76 patients with OVO, and 17 of the OVO patients with ≥2 spontaneous abortions or eclampsia, compared with 62 healthy normal females.
Abbreviations: OVO, ocular vascular occlusion; RAO, retinal artery occlusion; RVO, retinal vein occlusion.
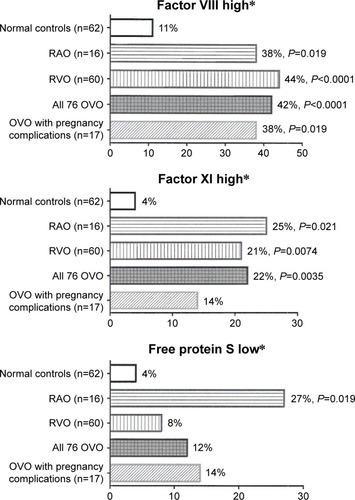
Of the 76 females, 16 presented with RAO (eleven CRAO and five branch RAO), and 60 presented with RVO (52 CRVO and eight BRVO). Of the 76 women, 37 (49%) were ≤60 years of age and 48 (63%) ≤ 65 years of age. Homocysteine was high in 13% with RAO (P=0.04) and 27% with RVO (P<0.0001) compared to 0% in 62 healthy normal female controls (). Factors VIII and XI were higher in 16 females with RAO (38% and 25%, respectively, [P=0.019, 0.021]) and in 60 RVO females (44% and 21%, respectively, [P=<0.0001, 0.0074]) than in normal female controls (11% and 4%, respectively) (). The 16 females with RAO were more likely than normal female controls (27% vs 4%, P=0.019) to have low free protein S (<66%) ( and ). The 60 females with RVO were more likely to have high ACLA IgM than normal female controls (20% vs 3%, P=0.0041) ( and ).
Of the 76 females, 73 had a total of 244 pregnancies, 180 live births, and 57 spontaneous abortions. Five females (6.8%) had ≥3 consecutive pregnancy losses before 20 weeks (recurrent pregnancy lossCitation57,Citation58), ten (13.9%) experienced two consecutive pregnancy losses, and 26 (35.6%) had ≥1 pregnancy loss. None of the 57 spontaneous miscarriages in the 73 females could be attributed to alcohol or cocaine use, maternal infection or endocrine abnormalities, uterine structural abnormalities, poorly controlled maternal diabetes, maternal thyroid disorders, or maternal trauma. Cytogenetic studies of the postmiscarriage products of conception samples were done in only three cases, where eight, six, and four spontaneous unexplained abortions had occurred, without demonstration of cytogenetic abnormalities.Citation54,Citation55
Of the 76 females, 17 (23%) had ≥2 spontaneous abortions or eclampsia. Of these 17 females, ten had two spontaneous abortions, three had four, one had six, one had eight spontaneous abortions, and two had eclampsia. Compared to 62 normal female controls, these 17 females had high factor VIII (38% vs 11%, P=0.02), high homocysteine (29% vs 0%, P=0.0003), high ACLA IgM (24% vs 3%, P=0.02), and were marginally more likely to be heterozygous for the FVL mutation (19% vs 3%, P=0.058) ( and ).
Discussion
Since thrombophilia plays a significant role in the development of OVO,Citation18,Citation20,Citation24,Citation59,Citation60 particularly in younger patients, and in patients without atherosclerosis but with insulin resistance syndrome risk factors, the ophthalmologist is often the first medical diagnostician to initiate steps to diagnose thrombop hilia.Citation8,Citation12,Citation20,Citation21,Citation24,Citation30,Citation53,Citation61–Citation69 Although all 76 females in the current study were referred to retinologists because of OVO, subsequent thrombophilia studies revealed not only an enrichment of familial thrombophilia in an OVO cohort but also emphasized the potential diagnostic and therapeutic payoffs obtained when the retinologist looks for thrombophilia in otherwise unexplained OVO. In the current study, congruent with our recent evaluation of 191 patients with RVO and 74 with RAO,Citation20 the group of 76 females (16 with RAO, 60 with RVO) differed from 62 healthy female controls by having high homocysteine, high ACLA IgM, and high factors VIII and XI. RAO and RVO patients both were more likely than healthy normal controls to have high homocysteine, high factor VIII and XI.
Thrombophilia has been reported to play an important pathoetiologic role in RAO and RVO,Citation18 as previously documented in this article, which is congruent with findings in our’sCitation18,Citation21,Citation53 and other’sCitation70–Citation74 studies. Thrombophilia is a known pathoetiology for adverse obstetrical outcomes, including, as in the current study, miscarriage,Citation75–Citation80 recurrent miscarriage,Citation48,Citation49,Citation57,Citation81 pre-eclampsia,Citation82,Citation83 and eclampsia.Citation84,Citation85 Unique to this article is our emphasis on the often central diagnostic role of the ophthalmologist, who, by revealing the pathologic importance of thrombophilia in OVO, opens the gates to primary and secondary prevention and therapy of thrombosis in other vascular beds, including the uterus and placenta, as in this report.
A growing body of evidence highlights thrombophilia as an important cause for spontaneous miscarriage.Citation48,Citation49,Citation75–Citation80,Citation86 Thrombophilia should be assessed in all females with recurrent fetal death,Citation87 but thrombophilia is only one of multiple causesCitation54,Citation55 for spontaneous abortion, including fetal cytogenetic abnormalities,Citation44 congenital abnormalities, illicit drug use, teratogens, maternal trauma, infection, uterine structural issues,Citation45–Citation47 maternal disease, including infection and endocrinopathies. In our cohort, where 73 females had one or more pregnancies with 57 spontaneous abortions, excepting a thrombophilic etiology in 12% to 42% of females, the varied other causes of spontaneous abortionCitation44,Citation54,Citation55 were not identified.
In our cohort, referred solely by OVO, pregnancy loss was much greater than in the general population,Citation57 recurrent pregnancy loss (three or more spontaneous unexplained abortions before 20 weeks’ gestation) occurred in 6.8% vs 0.4% to 1% in general populations, two consecutive losses in 12.3% vs 2% in general population, and 35.6% had ≥1 spontaneous pregnancy loses vs 15% in general population.
Normal pregnancy is characterized by an increase in thrombophilia,Citation88 where a physiologic hyperestrogenic hypercoagulable state appears to be a physiological adaptive mechanismCitation51 to prevent postpartum hemorrhage.Citation50 Thrombophilia,Citation89–Citation94 producing placental insufficiency via thrombosis of the placental spiral arteries, causes spontaneous abortion; pre-eclampsia; eclampsia; and hemolysis, elevated liver enzymes, and low platelet count syndrome.Citation95–Citation100 Within this frame of reference, our current study revealed the 17 females, originally evaluated for RVOs and with two or more unexplained spontaneous pregnancy losses or eclampsia, as a group had high homocysteine, high factor VIII, high ACLA IgM, and increased FVL rates compared with normal female controls. We speculate that thrombophilia associated with RAO and RVO, amplified by the thrombophilia of pregnancy, contributes to placental insufficiency and spontaneous abortion. This finding is congruent with our recent reportCitation18 that, of 17 females with FVL or PTG mutations, seven (41%) experienced at least one unexplained spontaneous abortion.
Although prospective with regard to the study of the etiology of OVO, our study is limited by being retrospective with regard to assessment of adverse pregnancy outcomes (spontaneous abortion, eclampsia). A second limitation of the study involves the expensive nature of laboratory assessment of thrombophilia, which often is not fully covered by private health insurance or Medicare or Medicaid.
Conclusion
In females lacking typical risk factors for RVO or severe ischemic presentation, by diagnosing thrombophilia as an etiology for OVO, the ophthalmologist opens a window to family screening and preventive therapy, with particular relevance to pregnancy outcomes and venous thromboembolism. The diagnosis of an underlying thrombophilia is important not only for the management of OVO but also for the success of the pregnancy, allowing timely thromboprophylaxisCitation101–Citation103 to prevent maternal thrombosis and pregnancy loss.
Disclosure
The authors report no conflicts of interest in this work.
References
- MohamedQMcIntoshRLSawSMWongTYInterventions for central retinal vein occlusion: an evidence-based systematic reviewOphthalmology2007114350751917324695
- Group EDC-CSRisk factors for central retinal vein occlusionArch Ophthalmol199611455455548619763
- StemMSTalwarNComerGMSteinJDA longitudinal analysis of risk factors associated with central retinal vein occlusionOphthalmology2013120236237023177364
- WegerMRennerWPinterORole of factor V Leiden and prothrombin 20210A in patients with retinal artery occlusionEye (Lond)200317673173412928685
- HayrehSSZimmermanBMcCarthyMJPodhajskyPSystemic diseases associated with various types of retinal vein occlusionAm J Ophthalmol20011311617711162981
- PriscoDMarcucciRBertiniLGoriAMCardiovascular and thrombophilic risk factors for central retinal vein occlusionEur J Intern Med200213316316912020623
- RecchiaFMBrownGCSystemic disorders associated with retinal vascular occlusionCurr Opin Ophthalmol200011646246711141642
- BackhouseOParapiaLMahomedILeeDFamilial thrombophilia and retinal vein occlusionEye (Lond)200014Pt 1131710755093
- RehakMRehakJMüllerMThe prevalence of activated protein C (APC) resistance and factor V Leiden is significantly higher in patients with retinal vein occlusion without general risk factorsThromb Haemost200899592592918449423
- CheungNKleinRWangJTraditional and novel cardiovascular risk factors for retinal vein occlusion: the multiethnic study of atherosclerosisInvest Ophthalmol Vis Sci200849104297430218539932
- O’MahoneyPWongTRayJRetinal vein occlusion and traditional risk factors for atherosclerosisArch Ophthalmol2008126569269918474782
- BickRLAlfarHGoedeckeCThrombophilic causes of retinal vascular thrombosis: etiology and treatment outcomesClin Appl Thromb Hemost20028431531812516681
- KariaNRetinal vein occlusion: pathophysiology and treatment optionsClin Ophthalmol2010480981620689798
- BeaumontPEKangHKClinical characteristics of retinal venous occlusions occurring at different sitesBr J Ophthalmol200286557258011973257
- HitchingsRASpaethGLChronic retinal vein occlusion in glaucomaBr J Ophthalmol197660106946991009041
- DrydenRMCentral retinal vein occlusions and chronic simple glaucomaArch Ophthalmol19657365966314281983
- GlueckCJWangPHutchinsRPetersenMRGolnikKOcular vascular thrombotic events: central retinal vein and central retinal artery occlusionsClin Appl Thromb Hemost200814328629418160589
- SchockmanSGlueckCJHutchinsRKPatelJShahPWangPDiagnostic ramifications of ocular vascular occlusion as a first thrombotic event associated with factor V Leiden and prothrombin gene heterozygosityClin Ophthalmol2015959160025897198
- GlueckCJGoldenbergNBellHGolnikKWangPAmaurosis fugax: associations with heritable thrombophiliaClin Appl Thromb Hemost200511323524116015408
- GlueckCJHutchinsRKJuranteeJKhanZWangPThrombophilia and retinal vascular occlusionClin Ophthalmol201261377138422969282
- GlueckCJPingWHutchinsRPetersenMRGolnikKOcular vascular thrombotic events: central retinal vein and central retinal artery occlusionsClin Appl Thromb Hemost200814328629418160589
- GlueckCJWangPBellHRangarajVGoldenbergNNonarteritic anterior ischemic optic neuropathy: associations with homozygosity for the C677T methylenetetrahydrofolate reductase mutationJ Lab Clin Med2004143318419215007309
- GlueckCJWangPBellHRangarajVGoldenbergNAssociations of thrombophilia, hypofibrinolysis, and retinal vein occlusionClin Appl Thromb Hemost200511437538916244763
- SottilottaGOrianaVLatellaCRole of hyperhomocystinemia in retinal vascular occlusive diseaseClin Appl Thromb Hemost200713110410717164500
- YauJWLeePWongTYBestJJenkinsARetinal vein occlusion: an approach to diagnosis, systemic risk factors and managementIntern Med J2008381290491019120547
- BickRLAntiphospholipid thrombosis syndromesClin Appl Thromb Hemost20017424125811697705
- WilliamsonTHRumleyALoweGDBlood viscosity, coagulation, and activated protein C resistance in central retinal vein occlusion: a population controlled studyBr J Ophthalmol19968032032088703856
- LarssonJOlafsdottirEBauerBActivated protein C resistance in young adults with central retinal vein occlusionBr J Ophthalmol19968032002028703855
- Ben-AmiRZeltserDLeibowitzIBerlinerSARetinal artery occlusion in a patient with factor V Leiden and prothrombin G20210A mutationsBlood Coagul Fibrinolysis2002131575911994569
- ArasSYilmazGAlpasIBaltaciVTayançEAydinPRetinal vein occlusion and factor V Leiden and prothrombin 20210 G:A mutationsEur J Ophthalmol200111435135511820306
- DemirciFYKGüneyDBAkarçayKPrevalence of factor V Leiden in patients with retinal vein occlusionActa Ophthalmol Scand199977663163310634553
- LarssonJHillarpAThe prothrombin gene G20210A mutation and the platelet glycoprotein IIIa polymorphism PIA2 in patients with central retinal vein occlusionThromb Res199996432332710593436
- JanssenMCHHeijerMDCruysbergJRMWollersheimHBredieSJHRetinal vein occlusion: A form of venous thrombosis or a complication of atherosclerosis?J Thromb Haemost200593610211026
- FeganCDCentral retinal vein occlusion and thrombophiliaEye (Lond)20021619810611913903
- TurelloMPascaSDaminatoRRetinal vein occlusion: evaluation of “classic” and “emerging” risk factors and treatmentJ Thromb Thrombolysis201029445946419669864
- CahillMTStinnettSSFekratSMeta-analysis of plasma homocysteine, serum folate, serum vitamin B(12), and thermolabile MTHFR genotype as risk factors for retinal vascular occlusive diseaseAm J Ophthalmol200313661136115014644226
- BiousseVNewmanNJSternbergPJRetinal vein occlusion and transient monocular visual loss associated with hyperhomocystinemiaAm J Ophthalmol199712422572609262559
- McCullyKSHomocysteine and vascular diseaseNat Med1996243863898597939
- WaldDSLawMMorrisJKHomocysteine and cardiovascular disease: evidence on causality from a meta-analysisBMJ20023257374120212446535
- SimsekEYesilyurtAPinarliFEyerciNUlusATCombined genetic mutations have remarkable effect on deep venous thrombosis and/or pulmonary embolism occurrenceGene2014536117117624334115
- SeligsohnULubetskyAGenetic susceptibility to venous thrombosisN Engl J Med2001344161222123111309638
- PoortSRRosendaalFRReitsmaPHBertinaRMA common genetic variation in the 3′-untranslated region of the prothrombin gene is associated with elevated plasma prothrombin levels and an increase in venous thrombosisBlood1996881369837038916933
- KosterTRosendaalFRRondeHdBriëtEVandenbrouckeJPBertinaRMVenous thrombosis due to poor anticoagulant response to activated protein C: Leiden Thrombophilia StudyLancet19933428886–8887150315067902898
- LevyBSigurjonssonSPettersenBGenomic imbalance in products of conception: single-nucleotide polymorphism chromosomal microarray analysisObstet Gynecol20141242 Pt 120220925004334
- MolloADe FranciscisPColacurciNHysteroscopic resection of the septum improves the pregnancy rate of women with unexplained infertility: a prospective controlled trialFertil Steril20099162628263118571168
- ShokeirTAbdelshaheedMEl-ShafieMSherifLBadawyADeterminants of fertility and reproductive success after hysteroscopic septoplasty for women with unexplained primary infertility: a prospective analysis of 88 casesEur J Obstet Gynecol Reprod Biol20111551545721185112
- TomazevicTBan-FrangezHVirant-KlunIVerdenikIPozlepBVrtacnik-BokalESeptate, subseptate and arcuate uterus decrease pregnancy and live birth rates in IVF/ICSIReprod Biomed Online201021570070520864409
- ReyEKahnSRDavidMShrierIThrombophilic disorders and fetal loss: a meta-analysisLancet2003361936190190812648968
- PrestonFERosendaalFRWalkerIDIncreased fetal loss in women with heritable thrombophiliaLancet199634890329139168843809
- JamesAHThrombosis in pregnancy and maternal outcomesBirth Defects Res C Embryo Today2015105315916626383185
- EldorAThrombophilia, thrombosis and pregnancyThromb Haemost200186110411111486995
- KamelHNaviBBSriramNHovsepianDADevereuxRBElkindMSRisk of a thrombotic event after the 6-week postpartum periodN Engl J Med2014370141307131524524551
- GlueckCJWangPOcular vascular thrombotic events: a diagnostic window to familial thrombophilia (compound factor V Leiden and prothrombin gene heterozygosity) and thrombosisClin Appl Thromb Hemost2009151121818796459
- RischHAWeissNSClarkeEAMillerABRisk factors for spontaneous abortion and its recurrenceAm J Epidemiol198812824204303273482
- RaschVCigarette, alcohol, and caffeine consumption: risk factors for spontaneous abortionActa Obstet Gynecol Scand200382218218812648183
- GlueckCJFreibergRAWangPHeritable thrombophilia-hypofibrinolysis and osteonecrosis of the femoral headClin Orthop Relat Res200846651034104018350351
- Salat-BarouxJRecurrent spontaneous abortionsReprod Nutr Dev1988286B155515683073445
- FordHBSchustDJRecurrent pregnancy loss: etiology, diagnosis, and therapyRev Obstet Gynecol200922768319609401
- RehakMKrcovaVSlavikLThe role of thrombophilia in patients with retinal vein occlusion and no systemic risk factorsCan J Ophthalmol201045217117520379305
- ChapinJCarlsonKChristosPJDeSanchoMTRisk factors and treatment strategies in patients with retinal vascular occlusionsClin Appl Thromb Hemost201521767267724335246
- RehakJDusekLChrapekOFricERehakMInitial visual acuity is an important prognostic factor in patients with branch retinal vein occlusionOphthalmic Res201045420420921079410
- WegerMStangerODeutschmannHHyperhomocyst(e) inemia and MTHFR C677T genotypes in patients with central retinal vein occlusionGraefes Arch Clin Exp Ophthalmol2002240428629011981642
- PriscoDMarcucciRRetinal vein thrombosis: risk factors, pathogenesis and therapeutic approachPathophysiol Haemost Thromb2002325–630831113679663
- RehakMKrcovaVFricEDisturbances of the plasma coagulation defects in retinal venous occlusionsCesk Slov Oftalmol200864310811118630161
- DemirciFYKucukkayaRAkarcayKOcular involvement in primary antiphospholipid syndrome. Ocular involvement in primary APSInt Ophthalmol199822632332910937845
- LarssonJCentral retinal artery occlusion in a patient homozygous for factor V LeidenAm J Ophthalmol2000129681681710927002
- LarssonJHillarpAOlafsdottirEBauerBActivated protein C resistance and anticoagulant proteins in young adults with central retinal vein occlusionActa Ophthalmol Scand199977663463710634554
- CahillMKarabatzakiMMeleadyRRaised plasma homocysteine as a risk factor for retinal vascular occlusive diseaseBr J Ophthalmol200084215415710655190
- GreinerKHafnerGDickBPeetzDPrellwitzWPfeifferNRetinal vascular occlusion and deficiencies in the protein C pathwayAm J Ophthalmol19991281697410482096
- DesaiSRaiNKulkarniPNatarajanSCombined CRVO with CRAO in a patient with protein C deficiencyRetin Cases Brief Rep20148214514925372333
- KolarPRisk factors for central and branch retinal vein occlusion: a meta-analysis of published clinical dataJ Ophthalmol2014201472478025009743
- JaulimAAhmedBKhanamTChatziralliIPBranch retinal vein occlusion: epidemiology, pathogenesis, risk factors, clinical features, diagnosis, and complications. an update of the literatureRetina201333590191023609064
- IncorvaiaCBandelloFParmeggianiFD’AngeloSCostagliolaCSebastianiARecurrent central retinal vein occlusion in a young thrombophilic patient with factor V Leiden mutationEur J Ophthalmol200212213113412022286
- LercheRCWilhelmCEifrigBRichardGThrombophilia factors as inducers of retinal vascular occlusionOphthalmologe200198652953411450475
- Dizon-TownsonDMillerCSibaiBThe relationship of the factor V Leiden mutation and pregnancy outcomes for mother and fetusObstet Gynecol2005106351752416135581
- SaidJMHigginsJRMosesEKInherited thrombophilia polymorphisms and pregnancy outcomes in nulliparous womenObstet Gynecol2010115151320027027
- MurphyRPDonoghueCNallenRJProspective evaluation of the risk conferred by factor V Leiden and thermolabile methylenetetrahydrofolate reductase polymorphisms in pregnancyArterioscler Thromb Vasc Biol200020126627010634828
- ClarkPWalkerIDGovanLWuOGreerIAThe GOAL study: a prospective examination of the impact of factor V Leiden and ABO(H) blood groups on haemorrhagic and thrombotic pregnancy outcomesBr J Haematol2008140223624018028481
- LindqvistPGSvenssonPJMarsaalKGrennertLLuterkortMDahlbackBActivated protein C resistance (FV:Q506) and pregnancyThromb Haemost199981453253710235434
- SilverRMZhaoYSpongCYProthrombin gene G20210A mutation and obstetric complicationsObstet Gynecol20101151142020027028
- FordESGilesWHDietzWHPrevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination SurveyJAMA2002287335635911790215
- BerksDDuvekotJJBasalanHDe MaatMPSteegersEAVisserWAssociations between phenotypes of preeclampsia and thrombophiliaEur J Obstet Gynecol Reprod Biol201519419920526444331
- SimcoxLEOrmesherLTowerCGreerIAThrombophilia and pregnancy complicationsInt J Mol Sci20151612284182842826633369
- DehkordiMASoleimaniAHaji-GholamiAVardanjaniAKDehkordiSAAssociation of deficiency of coagulation factors (Prs, Prc, ATIII) and FVL positivity with preeclampsia and/or eclampsia in pregnant womenInt J Hematol Oncol Stem Cell Res20148451125774262
- WangXBaiTLiuSPanHWangBAssociation between thrombophilia gene polymorphisms and preeclampsia: a meta-analysisPLoS One201496e10078924967675
- KjellbergUvan RooijenMBremmeKHellgrenMFactor V Leiden mutation and pregnancy-related complicationsAm J Obstet Gynecol20102035469e1e821055512
- BarrosVIIgaiAMAndres MdePFranciscoRPZugaibMPregnancy outcome and thrombophilia of women with recurrent fetal deathRev Bras Ginecol Obstet2014362505524676012
- CernecaFRicciGSimeoneRMalisanoMAlbericoSGuaschinoSCoagulation and fibrinolysis changes in normal pregnancy. Increased levels of procoagulants and reduced levels of inhibitors during pregnancy induce a hypercoagulable state, combined with a reactive fibrinolysisEur J Obstet Gynecol Reprod Biol199773131369175686
- GlueckCJAwadallaSGPhillipsHCameronDWangPFontaineRNPolycystic ovary syndrome, infertility, familial thrombophilia, familial hypofibrinolysis, recurrent loss of in vitro fertilized embryos, and miscarriageFertil Steril200074239439710927066
- MekajYLulajSDaciFPrevalence and role of antithrombin III, protein C and protein S deficiencies and activated protein C resistance in Kosovo women with recurrent pregnancy loss during the first trimester of pregnancyJ Hum Reprod Sci20158422422926752858
- WangYLinXWuQThrombophilia markers in patients with recurrent early miscarriageClin Lab201561111787179426732006
- GlueckCJGogeniniSMunjalJTracyTPranikoffJWangPFactor V Leiden mutation: a treatable etiology for sporadic and recurrent pregnancy lossFertil Steril200889241041617582408
- GlueckCJPranikoffJAregawiDThe factor V Leiden mutation, high factor VIII, and high plasminogen activator inhibitor activity: etiologies for sporadic miscarriageMetabolism200554101345134916154434
- O’DonnellCIGlueckCJFingerlinTEGlueckDHA likelihood model that accounts for censoring due to fetal loss can accurately test the effects of maternal and fetal genotype on the probability of miscarriageHum Hered2009671576518931510
- BesharatMTabandehAKeshtkarAMobasheriEBesharatSJoshaghaniHEvaluation of some plasma coagulation factors in women with spontaneous miscarriageInt J Fertil Steril20159330931226644853
- BouvierSCochery-NouvellonELavigne-LissaldeGComparative incidence of pregnancy outcomes in thrombophilia-positive women from the NOH-APS observational studyBlood2014123341442124200686
- de JongPGGoddijnMMiddeldorpSAntithrombotic therapy for pregnancy lossHum Reprod Update201319665667323766357
- GlueckCJWangPGoldenbergNSieveLPregnancy loss, polycystic ovary syndrome, thrombophilia, hypofibrinolysis, enoxaparin, metforminClin Appl Thromb Hemost200410432333415497018
- LinoFLTrainaEBarretoJAMoronAFMattarRThrombophilic mutations and polymorphisms, alone or in combination, and recurrent spontaneous abortionClin Appl Thromb Hemost201521436537224463599
- MazzucconiMGDe SanctisVAlfoMMaternal thrombophilia and adverse pregnancy outcome: a case-control studyActa Haematol2015133224224825401392
- GrandoneEDe StefanoVRossiECappucciFColaizzoDMargaglioneMAntithrombotic prophylaxis during pregnancy in women with deficiency of natural anticoagulantsBlood Coagul Fibrinolysis200819322623018388503
- RamidiGKhanNGlueckCJWangPGoldenbergNEnoxaparin-metformin and enoxaparin alone may safely reduce pregnancy lossTransl Res20091531334319100956
- BrennerBThrombophilia and pregnancy loss in first intended pregnancyJ Thromb Haemost20053102176217716194195
