Abstract
Introduction
The Argus II retinal prosthesis is composed of an epiretinal electrode array positioned over the macula and connected to an extrascleral electronics case via a silicone cable, running through a sclerotomy. During implantation, the manufacturer recommends to cover the sclerotomy site with a patch of processed human pericardium to prevent postoperative hypotony and conjunctival erosion by the underlying electronics case. Due to biomedical regulations prohibiting the use of this material in France, we developed an alternative technique combining a scleral flap protecting the sclerotomy and an autogenous graft of superior temporalis fascia overlying the electronics case.
Methods
The purpose of this study is to describe the 4-year outcomes of this modified procedure in three subjects who underwent Argus II Retinal Prosthesis System implantation. Clinical data consisting of intraocular pressure measurements and tolerance in terms of conjunctival erosion or inflammation were retrospectively assessed over a 4-year postoperative follow-up.
Results
None of the three patients implanted with the modified technique developed ocular hypotony over 4 years. A normal, transient conjunctival inflammation occurred during the first postoperative month but conjunctival erosion was not observed in any of the three patients over 4 years. Four years after implantation, the autogenous temporalis fascia graft remained well tolerated and the retinal prosthesis was functional in all three patients.
Conclusion
The combination of an autograft of superficial temporalis fascia and a scleral flap efficiently prevented leakage through the sclerotomy site, ocular hypotony, and conjunctival erosion by the extrascleral electronics case. This modified technique is suitable for the implantation of existing and forthcoming retinal prostheses. Superficial temporalis fascia may also be used as alternative to commercial tectonic tissues for scleral wound repair in clinical settings where they are not available.
Introduction
The Argus II Retinal Prosthesis System (Second Sight Medical Products Inc., Sylmar, CA, USA) has received marketing authorizations by the US Food and Drug Administration and the European regulatory authorities. In several countries, including Germany, the Netherlands, UK, Spain, Italy, France, Switzerland, Saudi Arabia, the US, and Canada, patients presenting end-stage retinitis pigmentosa but maintaining a functional inner retinal circuitry are potential candidates for implantation.Citation1 A 30-participant prospective studyCitation2,Citation3 and additional investigations demonstrated visual and behavioral improvement in a majority of implanted subjects after visual rehabilitation.Citation4–Citation8 The retinal prosthesis consists of an electrode array placed on the macular area, connected via a silicone cable across the sclera to an extraocular electronics case. According to recommendations for the standard implantation procedure, the surgeon must perform a 5 mm direct pars plana sclerotomy, across which will run the cable connecting the macular electrode array to the extraocular electronics case, and finally to cover efficiently both sclerotomy site and electronics case with a biocompatible patch material to seal the wound and prevent conjunctival erosion by the extraocular case.Citation9 The manufacturer recommends as patch graft material the use of processed human pericardium. However, in several countries including France, processed pericardium is not commercially available due to biomedical regulations prohibiting its use, which prompted us to adapt the implantation procedure. We present an alternative surgical technique for retinal prosthesis implantation that overcomes these legal restrictions on processed human cadaveric pericardium by using an autograft of superficial temporalis fascia.
Methods
Adapted surgical technique
The modified procedure incorporated two main changes. First, instead of a direct sclerotomy, a 5 mm wide scleral flap was performed in order to protect the sclerotomy site. Second, instead of processed human cadaveric pericardium, or polytetrafluoroethylene, an alternative patch graft material initially recommended by the tissue bank at our institution, the sclerotomy site was covered by an autogenous graft of temporalis fascia (), sampled from the patient’s temporal scalp at the beginning of the implantation procedure. lists the recommended and adapted surgical steps. Briefly, under general anesthesia, a 3 cm cutaneous and subcutaneous incision was performed in the right temporal scalp, posterior and superior to the ear, after local hair shaving. A 3×1 cm fragment of superficial temporalis fascia was excised ( and ), corresponding to the first musculotendinous structure accessed below the subcutaneous adipose tissue. After achieving careful hemostasis, the temporal wound was then sutured using a 4-0 polyglactin 910 absorbable suture (Vicryl; Ethicon, Somerville, NJ, USA) for the subcutaneous tissues and a 5-0 polypropylene intradermic suture for the skin (Prolene; Ethicon). A 5×7 mm fragment of temporalis fascia was prepared and preserved in a gentamicin antibiotics solution (Gentamicin, 20 mg/mL; Panpharma, Fougères, France). After intraocular implantation of the retinal prosthesis and positioning of the extraocular electronics case fixed onto the sclera by its silicone band, according to the manufacturer’s instructions,Citation9 the temporalis fascia autograft was positioned onto the sclera in order to cover both the sclerotomy site and the extraocular case. It was sutured using a 6-0 absorbable suture (Vicryl; Ethicon), before closure of the overlying Tenon’s capsule, and finally the conjunctiva were closed above the fascia autograft using a 8-0 absorbable suture (Vicryl; Ethicon). As recommended by the manufacturer, the patients received daily oral levofloxacine (500 mg; Biogaran, Colombes, France) from 48 hours before, until 7 days after the procedure, and a single dose of intravenous cephazolin (1 g; Mylan, Saint Priest, France) at the start of the procedure.
Figure 1 (A) Structure of the Argus II Retinal Prosthesis System showing the electrode array, its silicone cable connected to the extraocular electronics case, and the band used for extrascleral fixation. (B) Fundus photograph showing the position of the electrode array over the macula in a patient with retinitis pigmentosa. (C) Schematic adapted technique for retinal prosthesis implantation with a scleral flap at the sclerotomy site (1) and an autograft of superior temporalis fascia (2). Before closure of Tenon’s capsule and conjunctiva, the temporalis fascia covers both the scleral flap and the extraocular electronics case.
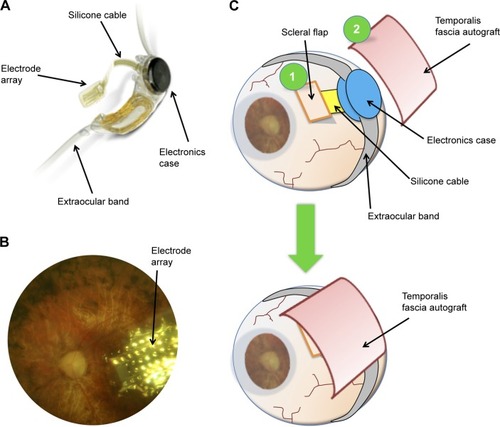
Figure 2 Schematic drawing of the surgical anatomy of the temporal scalp region.
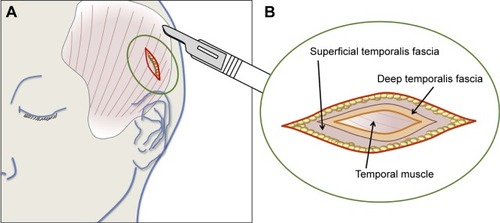
Figure 3 Excision of the temporalis fascia after surgical incision of the temporal scalp.
Table 1 Manufacturer’s protocol and adapted steps for Argus II retinal prosthesis implantation
Subjects
Three patients who underwent Argus II retinal prosthesis implantation between January and May 2009 at our institution during an international clinical trialCitation2,Citation3 and who benefited from this modified surgical technique were retrospectively included. This study describing surgical procedures and outcomes in human participants was designed in accordance with the Tenets of the 1964 Declaration of Helsinki and its later amendments. The study followed the ethical standards from the local ethics committee (CPP Ile-de-France 5, Saint-Antoine Hospital, Paris) and the need of formal informed consent was waived by the Quinze-Vingts Hospital internal review board due to the retrospective nature of the study.
Intraocular pressure (IOP) measurements were performed by noncontact tonometer (Tonoref II; Nidek, Gamagori, Japan). Three measures were performed by the device and the mean IOP was recorded. IOP values below 10 or above 20 mmHg were verified using Goldman aplanation tonometry. Conjunctival inflammation was assessed qualitatively by estimating the degree of conjunctival hyperemia and swelling. Conjunctival integrity was evaluated by fluorescein staining, and erosion was diagnosed when loss of conjunctival tissue was observed above subconjunctival material (electronics case, fascia temporalis, sutures).
To evaluate, in patients receiving the Argus II retinal prosthesis, the efficacy and tolerability of autogenous temporalis fascia as alternative patch graft material, IOP, signs of conjunctival inflammation, and conjunctival integrity were retrospectively assessed at regular interval during a 4-year follow-up.
Results
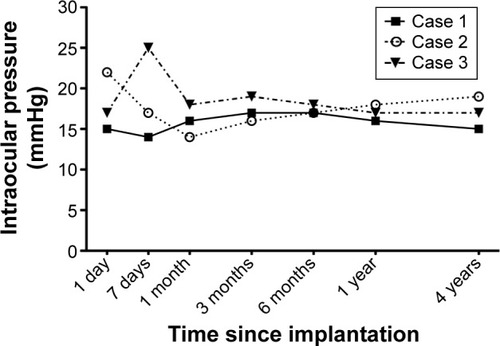
The modified procedure was performed in three patients (patients 1–3). No patient developed hypotony, neither during the immediate postoperative period nor during the 4-year follow-up. In patients 1 and 2, IOP measurements were between 14 and 22 mmHg during the first postoperative month. Patient 3 developed a mild postoperative IOP elevation 1 week after surgery (25 mmHg) that responded to topical beta-blocker therapy (timolol 1%, twice daily; Sandoz, Holzkirchen, Germany), and all ulterior IOP measurements were within the normal range. Over the next 4 years, all IOP values were measured between 10 and 19 mmHg in the three patients, with mean IOP values of 16.0 mmHg (range, 14–18 mmHg) at 1 month, 17.3 mmHg (16–19 mmHg) at 3 months, 17.0 mmHg (16–18 mmHg) at 1 year, and 17.0 mmHg (15–19 mmHg) at 4 years ().
Figure 4 Intraocular pressure trends over the 4-year follow-up in three patients who received the Argus II Retinal Prosthesis System with the adapted technique using a scleral flap and a superficial temporalis fascia autograft.
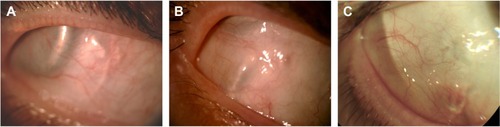
All three patients presented transient postoperative conjunctival inflammation related to the surgical de- and re-insertion of the conjunctiva, which resolved over 1 month under topical dexamethasone/trobramycin drops (four times daily; Alcon, Rueil-Malmaison, France). Over the 4-year follow-up period, neither conjunctival erosion nor abnormal conjunctival inflammation was observed in any of the three patients. Photographs showing the temporal conjunctiva of patients 1–3 at the 4-year follow-up visit, corresponding to the site of the implanted electronics case covered by temporalis fascia, are provided in .
Figure 5 Color photographs of the temporal conjunctiva overlying the electronics case 4 years after Argus II retinal prosthesis implantation.
At the excision site on the temporal scalp, the scar was no longer visible after a normal healing process of a few months, in all three cases ().
No adverse events were observed after implantation of the retinal prosthesis with this alternative technique. In particular, there was no donor-site morbidity, no functional or esthetic complications in the temporal area, and the Argus II system was functional and well tolerated 4 years after implantation.
Discussion
A modified Argus II retinal prosthesis implantation procedure, with a scleral flap and a patch of autogenous temporalis fascia, efficiently prevented ocular hypotony and conjunctival erosion in three subjects. Moreover, these favorable outcomes were prolonged over a 4-year follow-up period, and the graft had a favorable tolerance profile, as expected from an autogenous tissue.
Autogenous grafts of temporalis fascia are frequently used for ophthalmological, maxillofacial, or otorhinolaryngological procedures, such as orbit and eyelid reconstructions following complex traumas or malignancy,Citation10,Citation11 tympanoplasty,Citation12 nasal septal perforation,Citation13,Citation14 or cerebrospinal fluid leak repair.Citation15 Fascia sampled from other sites, such as fascia lata, are also often employed for eyelid surgery.Citation16–Citation18 Temporalis fascia is a double layer of loose connective tissue. The superficial fascia lies directly below the fatty layer and hair follicles of the scalp; removal of a fragment of superficial fascia is not reversible but is devoid of any functional consequences.Citation19 It overlies the deep fascia that covers the temporalis muscle and its aponeurosis (). Both lamina are supplied by the middle temporal artery, a branch of the superficial temporal artery that arises below the zygomatic arch.Citation19,Citation20 The two layers are similar in terms of collagen content and elasticity, and tend to lose their elasticity upon dehydration.Citation21 Thus, keeping the autogenous graft hydrated in an antibiotics solution before reimplantation, as performed in our protocol, helped preserve its mechanical properties.
Since the retinal prosthesis implantation procedure is systematically performed under general anesthesia, the two additional steps had limited consequences in terms of discomfort or additional risk for the patient. The extra time required was ~30 minutes (25 minutes for the temporalis fascia sampling and 5 minutes for the scleral flap), for a total duration of ~3–5 hours. At the excision site on the temporal scalp, esthetical consequences were limited since hair growth dissimulated the scar ().
Current and future retinal prosthesis devices are intended to remain implanted for decades. Therefore, a robust wound seal as well as an efficient coverage of the extraocular part of the device, preventing conjunctival erosion, are critical steps. Early and delayed conjunctival erosions may occur after Argus II system implantation. Among possible mechanisms, implantation of subconjunctival material and conjunctival surgery may lead to tear film instability, reduced corneal adhesiveness and delayed tear turnover.Citation22 The long-term safety analysis of the 30-patient international study reported a rate of conjunctival erosions of 10% after 1 year (three patients) and 13% after 3 years (four patients).Citation3 Among reported complications, ocular hypotony was 6.7% (two patients) through the first year and 13.3% (four patients) through the 3-year study period.Citation3
Processed human or bovine cadaveric pericardium is employed in ocular surgery for scleral wound repair after ocular perforationsCitation23 or glaucoma surgery with bleb-related complications and excessive filtration.Citation24,Citation25 It serves as tectonic support, epithelialization substrate or superficial patch graft and presents a favorable safety profile. Yet, the advantages of autograft over allogenous biological material are multiple. It eliminates the risks of histocompatibility mismatch, of infectious pathogen transmission, either known (human immunodeficiency virus, viral hepatitis, etc) or unknown (prions, etc), and of allergic reaction to products employed for the processing of cadaveric material. Moreover, it is independent from local legislation on human tissue and is cost-effective, since it can be performed with basic surgical instruments and sutures.
In the 30-patient international prospective study evaluating the Argus II retinal prosthesis, the experience from the first 15 implanted subjects proved beneficial, since fewer adverse events were observed with the 15 later enrollees.Citation2 Therefore, reports of early surgical and clinical experience, including adapted surgical techniques with this pioneering strategy,Citation26–Citation29 should contribute to optimize implantation procedures for Argus II and other forthcoming retinal prosthetic devices. We believe that sharing the lessons learned from initial cases with the growing number of vitreoretinal surgeons who will be implanting these devices, may improve patient safety.
This study has several limitations, including the low number of patients related to the rarity of the procedure, the lack of a control group due to the unavailability of processed pericardium, and the lack of advanced anterior segment imaging to follow scleral and conjunctival changes after temporalis fascia autograft.
Assuming that ocular hypotony and conjunctival erosion would occur at the frequency reported during the international clinical trial,Citation3 the study population would need at least 44 patients (based on the lowest frequency, 6.7%) to detect adverse events with a 95% confidence interval. Moreover, observing zero adverse events in a study population of three indicates that the 95% confidence interval for this specific event frequency is 0%–63%.Citation30 Therefore, these results should be confirmed on an extended study cohort. As part of a temporary reimbursement authorization for the Argus retinal prosthesis by the French public health authority, the modified implantation technique is currently employed on a larger scale, which will allow this issue to be addressed.
Conjunctival modifications after retinal prosthesis implantation were evaluated clinically, but future studies may also include conjunctival monitoring by anterior segment optical coherence tomography. This noninvasive, high resolution imaging modality allows the exploration of subsurface areas of conjunctival tissue. It enables the postsurgical evaluation of anterior segment features, such as filtering blebs,Citation31 and may provide useful knowledge about the adhesive scarring process following implantation of subconjunctival material.
Future developments of retinal prosthesis systems should attempt to reduce the silicone cable caliber to reduce the risk of leak, minimize the size of the extrascleral electronics case to reduce the risk of conjunctival erosion, and develop wireless transmission systems between the intra- and extraocular parts of the devices.
To summarize, superficial temporalis fascia autograft has been employed safely for retinal prosthesis implantation. It may also serve for scleral repair procedures as a substitute to processed cadaveric tectonic tissues, in clinical settings where they are unavailable or not allowed by local regulations. This technique is applicable to all retinal prosthesis devices, and should contribute in minimizing the use of exogenous material for these complex procedures.
Author contributions
AM: acquisition, analysis, and interpretation of data; drafting the manuscript. NA: conception and design of the study; took part in revising the manuscript. SM-S: conception and design of the study, acquisition of data; took part in revising the manuscript. J-AS: con ception and design of the study, analysis and interpretation of data; took part in revising the manuscript. P-OB: conception and design of the study, acquisition, analysis and interpretation of data; took part in revising the manuscript. All authors have agreed to be account able for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved, and have given final approval of the version to be published and take public responsibility for appropriate portions of the content.
Acknowledgments
The authors thank Michel Tazartes (Quinze-Vingts Hospital, Paris, France) for surgical counseling, Jessy Dorn and Grégoire Cosendai (Second Sight Medical Products, Sylmar, CA, USA) for technical assistance in reviewing the manuscript.
Disclosure
AM, NA, and SM-S declare that they have no conflicts of interests in this work. J-AS: stock ownership, consultancies (Pixium Vision). P-OB: reimbursements (Second Sight Medical Products, Inc.).
References
- AhujaAKBehrendMRThe Argus™ II retinal prosthesis: factors affecting patient selection for implantationProg Retin Eye Res20133612323500412
- HumayunMSDornJDda CruzLInterim results from the international trial of Second Sight’s visual prosthesisOphthalmology2012119477978822244176
- HoACHumayunMSDornJDLong-term results from an epiretinal prosthesis to restore sight to the blindOphthalmology201512281547155426162233
- DornJDAhujaAKCaspiAThe detection of motion by blind subjects with the epiretinal 60-electrode (Argus II) retinal prosthesisJAMA Ophthalmol2013131218318923544203
- LauritzenTZHarrisJMohand-SaidSReading visual braille with a retinal prosthesisFront Neurosci2012616823189036
- LuoYH-LZhongJJda CruzLThe use of Argus® II retinal prosthesis by blind subjects to achieve localisation and prehension of objects in 3-dimensional spaceGraefes Arch Clin Exp Ophthalmol2015253111907191425547618
- KotechaAZhongJStewartDda CruzLThe Argus II prosthesis facilitates reaching and grasping tasks: a case seriesBMC Ophthalmol2014147124885164
- SabbahNAuthiéCNSandaNMohand-SaidSSahelJ-ASafranABImportance of eye position on spatial localization in blind subjects wearing an Argus II retinal prosthesisInvest Ophthalmol Vis Sci201455128259826625414187
- Second Sight Medical Products IncArgus® II Retinal Prosthesis System – Surgeon ManualSylmar: CASecond Sight Medical Products, Inc2013
- BladenJCMoosajeeMTumuluriKOlverJMThe use of a pleated strip of autogenous temporalis fascia graft for frontalis suspension in recurrent poor levator function ptosis in adult patientsOrbit201231211411822489854
- CopcuESivriogluNThe new reconstruction technique in the treatment of the skin cancers located on the eyelid: Posterior temporalis fascia composite graftInt Semin Surg Oncol200411515306033
- KhanMMParabSRComparative study of sliced tragal cartilage and temporalis fascia in type I tympanoplastyJ Laryngol Otol20151291162225602596
- JeonE-JChoiJLeeJ-HThe role of temporalis fascia for free mucosal graft survival in small nasal septal perforation repairJ Craniofac Surg2014252e164e16624621759
- KayaECingiCOlgunYSokenHPinarbasliÖThree layer interlocking: a novel technique for repairing a nasal septum perforationAnn Otol Rhinol Laryngol2015124321221525225212
- Martín-MartínCMartínez-CapoccioniGSerramito-GarcíaREspinosa-RestrepoFSurgical challenge: endoscopic repair of cerebrospinal fluid leakBMC Res Notes2012545922925201
- SebastiáRFallicoEFallicoMFortunaEHerzog NetoGLessaSBilateral lid/brow elevation procedure for severe ptosis in Kearns-Sayre syndrome, a mitochondrial cytopathyClin Ophthalmol20149253125565765
- RoshdyMElsamkaryMClinical trial comparing autogenous fascia lata sling and Gore-Tex suspension in bilateral congenital ptosisClin Ophthalmol20161040540927022237
- AsamuraSKakizakiHEnjyoMHashimotoTIsogaiNFrontalis sling procedure for ocular myasthenia gravisClin Ophthalmol2012657557722553417
- Abul-HassanHSvon Drasek AscherGAclandRDSurgical anatomy and blood supply of the fascial layers of the temporal regionPlast Reconstr Surg198677117283941846
- DavidgeKMvan FurthWRAgurACusimanoMNaming the soft tissue layers of the temporoparietal region: unifying anatomic terminology across surgical disciplinesNeurosurgery2010673 Suppl Operativeons120129 discussion ons129–13020679939
- WormaldPJAlun-JonesTAnatomy of the temporalis fasciaJ Laryngol Otol199110575225241875131
- NapoliPECoronellaFSattaGMFossarelloMA novel technique of contrast-enhanced optical coherence tomography imaging in evaluation of clearance of lipids in human tearsPLoS One2014911e10984325369027
- AlioJLRodriguezAEMartinezLMBovine pericardium membrane (tutopatch) combined with solid platelet-rich plasma for the management of perforated corneal ulcersCornea201332561962422929158
- PapaconstantinouDGeorgalasITaliantzisSKoutsandreaCLadasIGeorgopoulosGHuman pericardium graft in the management of bleb’s complication performed in childhood: a case reportBMC Ophthalmol2011112721933427
- RavivTGreenfieldDSLiebmannJMSidotiPAIshikawaHRitchRPericardial patch grafts in glaucoma implant surgeryJ Glaucoma19987127329493112
- SeiderMIHahnPArgus II retinal prosthesis malrotation and repositioning with intraoperative optical coherence tomography in a posterior staphylomaClin Ophthalmol201592213221626648688
- GregoriNZDavisJLRizzoSBimanual technique for retinal tacking of epiretinal prosthesisRetina201636119920226441271
- MontezumaSRTangPHvan KuijkFJGMDraynaPKoozekananiDDImplantation of the Argus II retinal prosthesis in an eye with short axial lengthOphthalmic Surg Lasers Imaging Retina201647436937127065379
- TranB-KWolfensbergerTJRelation between implant position and perceptual threshold in a patient with epiretinal prosthesis systemKlin Monbl Augenheilkd2016233449349527116517
- CarterREWoolsonRFStatistical design considerations for pilot studies transitioning therapies from the bench to the bedsideJ Transl Med2004213715511289
- NapoliPEZuccaIFossarelloMQualitative and quantitative analysis of filtering blebs with optical coherence tomographyCan J Ophthalmol201449221021624767231
- System Overview [webpage on the Internet]Sylmar, CASecond Sight Medical Products, Inc2016 Available from: http://www.secondsight.com/system-overview-en.htmlAccessed August 2, 2016
