Abstract
Purpose
To describe the clinical findings and long-term outcome of patients with chronic central serous chorioretinopathy (cCSC).
Materials and methods
This was a retrospective case series in 52 eyes of 36 patients with a follow-up period of at least 1 year. Extensive ophthalmic examination and a validated questionnaire concerning vision-related quality of life (National Eye Institute Visual Function Questionnaire [NEI-VFQ]-39) were analyzed.
Results
Mean visual acuity showed a significant decline over time of 0.16 logarithm of minimum angle of resolution ([logMAR] range: −0.22 to 1.3; P=0.009) after a mean follow-up period of 10.6 years. Also, patients reported lower vision-related quality of life based on the NEI-VFQ-39 for almost all categories compared to healthy controls. Macular atrophy was diagnosed more often on optical coherence tomography compared to other diagnostic entities. Retinal pigment epithelium detachments in the macula were documented on optical coherence tomography in 56% of the patients. A significant thinning of foveal thickness was measured over time compared to unaffected fellow eyes (P=0.002). On long-term follow-up, 13 eyes (37%) showed an increase in number of hot spots on fluorescein angiography.
Conclusion
This study indicates that cCSC is a progressive disease in many patients, causing a progressive decline in visual acuity, accompanied by lower reported vision-related quality of life. In deciding whether or not to treat, the progressive nature of cCSC should be taken into account in this relatively young and often still professionally active patient group.
Introduction
Central serous chorioretinopathy (CSC) is characterized by fluid accumulation between the neuroretina and retinal pigment epithelium (RPE).Citation1,Citation2 It often affects the macula, leading to central vision loss, scotoma, metamorphopsia, and/or micropsia.Citation1,Citation2 The accumulation of subretinal fluid (SRF) results from leakage through a dysfunctional RPE with a disrupted outer blood–retina barrier. Dilation, congestion, and hyperpermeability of the choriocapillaris and larger choroidal vessels appear to play a pivotal role in the pathogenesis of CSC.Citation3–Citation5 The etiology of CSC is unknown. Risk factors for CSC include the use of corticosteroids, Cushing’s disease, pregnancy, and male sex.Citation2,Citation6 Recent studies have also found genetic associations.Citation7,Citation8
There are two main subtypes of CSC. Acute CSC is characterized by sudden vision loss, due to fluid leakage through a focal pinpoint leak in the RPE, often in association with a small RPE detachment.Citation1,Citation2 In most acute CSC patients, the SRF resolves spontaneously within 2–3 months.Citation1 Visual acuity (VA) in these patients generally returns to (near-) normal levels.Citation1 In the other main subtype, chronic CSC (cCSC), patients have more widespread atrophic RPE abnormalities, as well as more extensive choroidal changes on fluorescein angiography (FA) and indocyanine green angiography (ICGA).Citation1,Citation2 In contrast to acute CSC, most patients with cCSC have more pronounced central vision loss, and often do not show a spontaneous resolution of SRF.Citation2
Although cCSC is one of the most common forms of macular degeneration,Citation1 relatively little is known about the long-term visual consequences in these patients. The aim of this study was to evaluate the clinical outcome of patients with cCSC and assess their vision-related quality of life to provide a more accurate prognosis to patients.
Materials and methods
Participants
Patients with cCSC and a follow-up of at least 1 year were included in this retrospective observational study. Subjects were recruited from the Department of Ophthalmology of Radboud University Medical Center (Nijmegen, the Netherlands) and the Oxford Eye Hospital (Oxford, UK). All patients gave written informed consent for the use of their data for this retrospective study. The diagnosis of cCSC was defined as the presence of SRF longer than 3 months in at least one eye on optical coherence tomography (OCT), RPE window defects on FA with at least 1 “hot spot”, defined as indistinct hyperfluorescent area of leakage and/or diffuse leakage in the affected eye(s), and corresponding hyperfluorescent zones on ICGA when available. Patients with evidence of other retinal diagnoses at the first visit were excluded. This study adhered to the tenets of the Declaration of Helsinki, and approval for the study in Oxford was obtained from the Integrated Research Authority, Essex 2 Research Ethics Committee. The ethical approval for the study in Nijmegen was waived by Commissie Mensgebonden Onderzoek (CMO) of the Radboud University Medical Center in Nijmegen, as all clinical data was obtained retrospectively and the questionnaire was found to be non-invasive and not personally sensitive for the study objects.
The following parameters, when available, were collected from the medical records: VA, disease activity, duration of visual symptoms, (prior) use of corticosteroids, number of treatments, color fundus photography, OCT, FA, and ICGA. Only visits for which VA and information about disease activity, defined as subfoveal fluid on OCT or (when OCT was not available) as stated by the ophthalmologist in the medical records, were included. Additionally, patients were asked to complete a validated questionnaire (Visual Function Questionnaire [VFQ]-39).Citation9 For all Dutch patients, a validated Dutch translation of this questionnaire was used.Citation10
Clinical evaluation
Submacular RPE detachments, subfoveal SRF, change in submacular SRF accumulation, and retinal atrophy were scored on OCT. The number of hot spots, defined as indistinct hyperfluorescent areas of leakage, seen on FA and ICGA were registered. Evidence of RPE atrophy was assessed on FA and color fundus photography.
Central foveal thickness (CFT), defined as the distance between the outer part of the internal limiting membrane and the outer part of the external limiting membrane at the central fovea, of the first and the last available OCT scans was measured by two independent graders. Only patients who had follow-up imaging with spectral domain OCT (Spectralis™; Heidelberg Engineering, Heidelberg, Germany) were included in CFT measurements. The mean of the measurements of both graders was used for further analysis after no significant difference between the graders was confirmed by Student’s independent t-test (P>0.05). In selected patients, unaffected fellow eyes, defined as eyes in which no central lesions suspect for cCSC were present, were included. The difference in CFT over time was compared between the affected and unaffected eyes using Student’s independent t-test.
Visual acuity
The VA of all first visits was compared to the VA of all last known visits using an independent-sample t-test. For the VA of the last visit, only patients with inactive disease, defined as absence of SRF on OCT, were included. Additionally, a Pearson’s correlation test was performed to determine if the number of episodes of active disease or the number of treatments affected the overall change in VA when comparing the first visit to the last visit.
Vision-related quality of life
The scores of every individual subcategory of the VFQ-39 were compared to the reference group, which was used for assessment in the original validation of the VFQ-39,Citation9,Citation21 using the independent-sample t-test. The original validation of the VFQ-39 analyzed the data of the reference group as being normally distributed; therefore, this study also chose this approach. A Pearson’s correlation test was performed to analyze if the VA at the end of follow-up was associated with VFQ-39 score. P<0.05 was considered statistically significant for all tests.
Results
Demographics
Demographic information is presented in . The mean number of episodes of active disease per eye was 1.7 (range: 0–6). Each episode was defined as a presence of documented SRF followed by a period of OCT-proven absence of SRF.
Table 1 Demographics of the study population
Clinical course
When the VA of the first and last visits was compared, excluding the 21 eyes (40%) with persistent subfoveal SRF at the final visit, a mean decline of 0.16 logarithm of minimum angle of resolution (logMAR; range: −0.22 to 1.3, P=0.009) was found after a mean follow-up period of 10.6 (range: 1.5–24) years. In the 21 eyes (40%) with persistent subfoveal SRF at the final visit, a mean decline of 0.22 logMAR (range: −0.35 to 0.66, P=0.003) with a mean follow-up period of 4.5 (range: 1–11) years was seen. In 11 of these 21 eyes (52%), the SRF was continuously present during follow-up. Neither the number of episodes of active disease nor number of treatments had a significant effect on overall change in VA.
Optical coherence tomography
An overview of OCT findings is displayed in and . In the 13 eyes with a decrease in SRF, eight (62%) had received treatment. The seven eyes with an increase of SRF had not received treatment. In the eyes showing fluctuating SRF accumulation, 18 (82%) had received treatment. The following treatments were performed: 41 micropulse therapy, 23 photodynamic therapy (PDT), one argon-laser treatment, one anecortave acetate treatment, and nine anti-VEGF treatments.
Figure 1 Imaging of two chronic central serous chorioretinopathy (cCSC) patients.
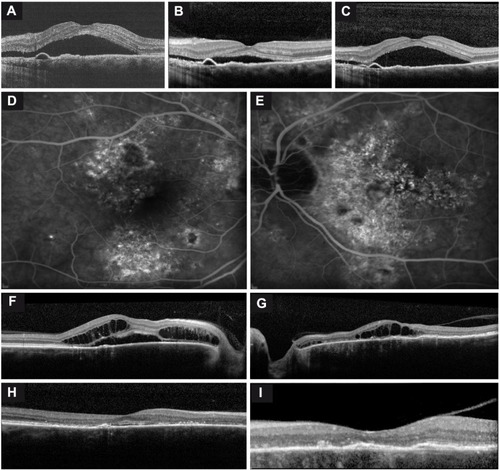
Table 2 Overview of findings on optical coherence tomography and fluorescein angiography
In 14 eyes (24%), SRF was continuously present on OCT until the last follow-up. In this group, this was also the case in five eyes (36%) despite receiving treatment (micropulse therapy [52%], PDT [29%], anti-VEGF [14%], and argon-laser treatment [5%]).
Posterior cystoid retinal degeneration, described previously by Piccolino et al, was seen in seven eyes of four patients (8%; ).Citation11 In three of these seven eyes, a lesion suspected to be choroidal neovascularization developed during follow-up. In three of seven eyes, the posterior cystoid retinal degeneration disappeared completely during follow-up, in two eyes this resolution occurred spontaneously, and in one eye it occurred after PDT (). The onset of posterior cystoid retinal degeneration was 2, 10, and 16 years after the reported start of the cCSC in three of the four patients in whom this was recorded ().
In 21 eyes (41%) with SRF and six eyes without SRF, spectral domain OCT was available for evaluation of CFT on follow-up. CFT showed a mean decrease of 15.1 μm (standard deviation: 17.7 μm) in eyes with SRF, whereas no significant change was seen in eyes without SRF. This difference in CFT was statistically significant (P=0.002).
Fluorescein angiography, indocyanine green angiography, and color fundus photography
Characteristics of hot spots of leakage on FA on follow-up are shown in . Of the nine eyes that showed a decrease in number of hot spots during follow-up, in seven the original hot spots disappeared after treatment, whereas the number of hot spots decreased spontaneously in the remaining two eyes. Overall, the mean number of hot spots at the first visit was 1 (range: 0–4) compared to 1.7 (range: 0–7) at the last visit (). An increase in atrophic RPE changes on FA was seen in 14 eyes (41%) during a mean follow-up of 9.7 years (range: 2–24 years). A classic “gravitational tract” was seen in eight eyes (15%), and was already present at the first visit in five eyes (). The other three eyes developed the gravitational tract after approximately 2, 4, and 8 years, respectively.
Figure 2 Abnormalities on fluorescein angiography (FA), indocyanine green angiography (ICGA), and fundus autofluorescence in chronic central serous chorioretinopathy.
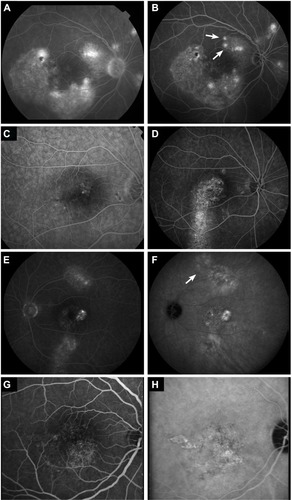
When ICGA was available (in 28 eyes [54%]), hot spots and hyperfluorescent areas on ICGA in 27 eyes (96%), was compared with characteristics on FA that was performed on the same date. The overall hyperfluorescent area was larger on ICGA in eight eyes (30%), in eleven eyes (41%) the hyperfluorescent area had comparable size, in one eye (4%) the hyperfluorescent area was smaller on ICGA, and in eight eyes (30%) no clear hyperfluorescence was seen on ICGA to be compared (). In eleven eyes (39%), ICGA showed multiple small punctate hyperfluorescent spots, without clear evidence of a leaking hot spot focus ().
Color fundus photography was available at baseline in 42 eyes (81%), and mild RPE atrophy in the macula was seen in 18 eyes (43%). Follow-up images were available for 31 eyes (74%), with a mean follow-up of 45 months (range: 1.5–87 months), and evidence of development and/or progression of RPE atrophy was present in 14 eyes (45%).
Quality of life
Patients of this cCSC cohort scored significantly less on the VFQ-39 questionnaire compared to the reference group on eight of the 12 subcategories (). Patients with a lower last recorded VA scored significantly lower on the following categories of the VFQ-39: general vision, near activities, distance activities, social functioning, mental health, role difficulties, dependence, and driving ().
Table 3 VFQ-39 questionnaire scores of the study population and controls, and Pearson’s correlation with last visual acuity
Discussion
This study shows that cCSC is a progressive chorioretinopathy with a significant impact on VA and vision-related quality of life. Patients in this study cohort generally showed a progressive decline in VA, as well as a gradual decrease in CFT on OCT. Wang et al demonstrated in the pre-OCT era that persistence of SRF for more than 4 months can result in foveal atrophy.Citation12 This permanent damage to photoreceptors and the RPE can explain the often-persistent visual complaints, even after resolution of SRF. The progressive nature of cCSC was demonstrated on FA, characterized by an increase of hyperfluorescent atrophic RPE changes and an increased number of hot spots during follow-up.
An interesting finding in this study were the small punctate hyperfluorescent spots on ICGA (). Similar lesions were described by Tsujikawa et al.Citation13 This group described small clusters, which were located within the center of the focal hyperfluorescent area on ICGA and thus thought to be very small leakage spots. In this study, no clear abnormalities corresponding to these lesions on ICGA could be identified on OCT. However, Lehmann et al identified similar hyperfluorescent lesions on ICGA that correlated with vascular dilations on en face enhanced-depth OCT.Citation14 In contrast to the lesions seen in this study, the lesions were always located under an area of SRF accumulation and/or RPE detachment. Additionally, a clinical and pathogenetic overlap between CSC and polypoidal choroidal vasculopathy may exist. Punctate hyperfluorescent spots on ICGA have also been identified in polypoidal choroidal vasculopathy, in which these lesions correlated with a thicker choroid.Citation15 We thus hypothesize that these lesions may be focal vascular dilations in the choriocapillaris that fill on ICGA.
The blood flow in the choroid is among the highest in the human body, and the macular choriocapillaris is thicker, has a distinct lobular architecture, and higher blood flow compared to the peripheral choroid.Citation16,Citation17 These anatomical and functional choroidal differences, as well as anatomical differences between central and peripheral RPE and Bruch’s membrane,Citation18,Citation19 probably explain why CSC principally affects the posterior pole. However, it is currently unclear why some individuals develop active CSC in the case of a dysfunctional thickened choroid (“pachychoroid”) unlike others,Citation20 and why in the same individual the disease can remain subclinical in the fellow eye despite the presence of similar choroidal abnormalities.Citation5 It is likely that additional factors play a role in determining an individual’s likelihood to develop CSC, for instance, patient-specific differences in genetic background,Citation7,Citation8 local differences in RPE outer blood–retina function, and possibly the interphotoreceptor matrix.Citation21
This studies data showed a marked decrease in reported vision-related quality of life in this cCSC cohort when compared to healthy individuals. Nevertheless, the impact of cCSC on vision-related quality of life seems to be less pronounced than in other common maculopathies, such as neovascular age-related macula degeneration and diabetic retinopathy.Citation22,Citation23 In this cCSC cohort, social functioning and dependence seemed to be most affected. Differences between the vision-related quality of life impact of the different diseases may not only be due to the variable effects on macular anatomy and visual function but may also be partly explained by the difference in the mean age of the different study populations during which the VFQ-39 questionnaire was taken (present study 55 years, neovascular age-related macular degeneration 77 years, diabetic retinopathy 68 years, and diabetic macular edema 62 years).Citation23 Also, mean years since diagnosis was far shorter in the neovascular age-related macular degeneration studies compared to the present study (0.6 and 7.4 years). A possible explanation could also be that younger individuals are more flexible and capable of coping with newly developed visual impairment than older individuals.Citation24 Also, the negative impact on reported vision-related quality of life appears to be higher when evaluated closer to the onset of visual impairment.
Surprisingly, a significant difference in the reported quality of color vision was not found, although impaired color vision has been previously reported in CSC.Citation25 It is still possible that disturbed color vision exists in this cohort, but that it remains subclinical, for example, due to a better-seeing fellow eye, and does not interfere with daily activities.
Conclusion
cCSC is a progressive chorioretinopathy, with many cCSC patients experiencing significant vision loss, lower vision-related quality of life, and lower rating for overall health compared to healthy individuals. Despite these observations, it is not uncommon for ophthalmologists to adopt a conservative approach in these patients, who are often relatively young. The findings of this study demonstrate that the possible impact of cCSC should not be underestimated, and thus an active treatment approach may be advocated. However, few high-quality randomized controlled treatment trials have been reported in cCSC, with a current lack of a gold standard for treatment of cCSC. We are currently performing a multicenter prospective randomized controlled treatment trial, comparing half-dose PDT with high-density subthreshold micropulse-laser treatment in cCSC (EudraCT 2012-004555-36, NCT01797861) in an attempt to identify the most suitable treatment strategy in cCSC.
Acknowledgments
The authors thank Joannes MM Groenewoud (PhD) for his statistical support and input to this manuscript. This study was supported by the Macula Vision Research Foundation, MD Fonds, Landelijke Stichting voor Blinden en Slechtzienden, Gelderse Blindenstichting, Stichting Nederlands Oogheelkundig Onderzoek, Stichting Blindenhulp, Stichting AF Deutman Oogheelkunde Researchfonds, Nijmeegse Oogonderzoek Stichting, Janivo Stichting, and Oogfonds. The sponsor or funding organization had no role in the design or conduct of this research. Dr Camiel JF Boon is now employed by the Department of Ophthalmology, Acedemic Medical Center Amsterdam.
Disclosure
Dr Camiel JF Boon was supported by a Niels Stensen Fellowship. The authors report no conflicts of interest in this work.
References
- LiewGQuinGGilliesMFraser-BellSCentral serous chorioretinopathy: a review of epidemiology and pathophysiologyClin Exp Ophthalmol201341220121422788735
- NicholsonBNobleJForooghianFMeyerleCCentral serous chorioretinopathy: update on pathophysiology and treatmentSurv Ophthalmol201358210312623410821
- GuyerDRYannuzziLASlakterJSSorensonJAHoAOrlockDDigital indocyanine green videoangiography of central serous chorioretinopathyArch Ophthalmol19941128105710628053819
- PrünteCFlammerJChoroidal capillary and venous congestion in central serous chorioretinopathyAm J Ophthalmol1996121126348554078
- KimYTKangSWBaiKHChoroidal thickness in both eyes of patients with unilaterally active central serous chorioretinopathyEye (Lond)201125121635164022020172
- BouzasEAKaradimasPPournarasCJCentral serous chorioretinopathy and glucocorticoidsSurv Ophthalmol200247543144812431693
- MikiAKondoNYanagisawaSBesshoHHondaSNegiACommon variants in the complement factor H gene confer genetic susceptibility to central serous chorioretinopathyOphthalmology201412151067107224365176
- de JongEKBreukinkMBSchellevisRLChronic central serous chorioretinopathy is associated with genetic variants implicated in age-related macular degenerationOphthalmology2015122356257025439433
- MangioneCMLeePPGutierrezPRSpritzerKBerrySHaysRDDevelopment of the 25-item National Eye Institute Visual Function QuestionnaireArch Ophthalmol200111971050105811448327
- Van der SterreGVan der GraafEVerezenCVisual Function Questionnaire – 25: National Eye Institute Nederlandse Consensus Vertaling versie 2001 (VFQ-25/NL)2013 Available from: http://www.erasmusmc.nl/mage/publicaties/aanvullingen/3503529Accessed October 9, 2016
- PiccolinoFCDe La LongraisRRManeaMCicinelliSPosterior cystoid retinal degeneration in central serous chorioretinopathyRetina20082871008101218698305
- WangMSSanderBLarsenMRetinal atrophy in idiopathic central serous chorioretinopathyAm J Ophthalmol2002133678779312036670
- TsujikawaAOjimaYYamashiroKPunctate hyperfluorescent spots associated with central serous chorioretinopathy as seen on indocyanine green angiographyRetina201030580180920094008
- LehmannMWolffBVasseurVRetinal and choroidal changes observed with ‘En face’ enhanced-depth imaging OCT in central serous chorioretinopathyBr J Ophthalmol20139791181118623823080
- ParkSJKimBHParkKHWooSJPunctate hyperfluorescence spot as a common choroidopathy of central serous chorioretinopathy and polypoidal choroidal vasculopathyAm J Ophthalmol201415861155116325127698
- NicklaDLWallmanJThe multifunctional choroidProg Retin Eye Res201029214416820044062
- AlmABillAOcular and optic nerve blood flow at normal and increased intraocular pressures in monkeys (Macaca irus): a study with radioactively labelled microspheres including flow determinations in brain and some other tissuesExp Eye Res197315115294630581
- BoultonMDayhaw-BarkerPThe role of the retinal pigment epithelium: topographical variation and ageing changesEye (Lond)200115Pt 338438911450762
- RamrattanRSvan der SchaftTLMooyCMde BruijnWCMulderPGde JongPTMorphometric analysis of Bruch’s membrane, the choriocapillaris, and the choroid in agingInvest Ophthalmol Vis Sci1994356285728648188481
- WarrowDJHoangQVFreundKBPachychoroid pigment epitheliopathyRetina20133381659167223751942
- HagemanGSMarmorMFYaoXYJohnsenLVThe interphotoreceptor matrix mediates primate retinal adhesionArch Ophthalmol199511356556607748138
- SunerIJKokameGTYuEWardJDolanCBresslerNMResponsiveness of NEI VFQ-25 to changes in visual acuity in neovascular AMD: validation studies from two phase 3 clinical trialsInvest Ophthalmol Vis Sci20095083629363519255158
- LloydAJLoftusJTurnerMLaiGPleilAPsychometric validation of the Visual Function Questionnaire-25 in patients with diabetic macular edemaHealth Qual Life Outcomes2013111023347793
- ChengCLauHPChanMPCoping flexibility and psychological adjustment to stressful life changes. A meta-analytic reviewPsychol Bull201414061582160725222637
- MaaranenTHTuppurainenKTMäntyjärviMIColor vision defects after central serous chorioretinopathyRetina200020663363711131417
