Abstract
Purpose
We aimed to evaluate the prevalence of abnormal peripheral fundus autofluorescence (FAF) in wet age-related macular degeneration (AMD) using wide-field imaging instrument.
Patients and methods
A retrospective, case-controlled study involving 66 eyes of 46 Japanese wet AMD patients and 32 eyes of 20 control patients was performed. Wide-field FAF images were obtained for typical AMD (37 eyes/28 patients), polypoidal choroidal vasculopathy (PCV) (22 eyes/20 patients), and retinal angiomatous proliferation (RAP) (seven eyes/four patients). Two masked ophthalmologists independently graded the images for mottled, granular, and nummular patterns. Main outcome measures were abnormal peripheral FAF frequencies and relative risks by disease subgroups and treatments.
Results
Abnormal peripheral FAF patterns were found in 51.5% of wet AMD eyes compared with 18.8% of control eyes (P<0.001). Mottled, granular, and nummular patterns were found in 45.5%, 31.8%, and 16.7%, respectively, of wet AMD eyes. Each disease subgroup (typical AMD, 54.1%; PCV, 36.4%; and RAP, 85.7%) showed significantly higher frequencies of peripheral FAF (P<0.001, P=0.03, and P<0.001, respectively) than control eyes (18.8%). There were no significant differences (P=0.76) between the frequencies in untreated and treated eyes.
Conclusion
Eyes of Japanese wet AMD patients had a higher abnormal FAF prevalence compared with control eyes. Among the three disease subtypes, abnormal patterns were least prevalent in PCV eyes.
Introduction
Age-related macular degeneration (AMD) is currently the leading cause of blindness in the industrialized world.Citation1–Citation5 Choroidal neovascularization often causes severe and rapid visual loss in the wet or neovascular form of AMD, while geographic atrophy gradually impairs the central vision in the dry or atrophic type of AMD. It is important to understand age-related changes in retinal pigment epithelial (RPE) cells under the sensory retina, because the changes predispose eyes to development of AMD.
Fundus autofluorescence (FAF) imaging is a noninvasive technique used to assess RPE abnormalities, which are observed often in aging eyes and eyes with AMD, retinitis pigmentosa, and other chorioretinal diseases. FAF signals increase with lipofuscin accumulation in RPE cells and decrease with RPE atrophy. Analysis of FAF is an effective method to observe the functions of the RPE cells, which are related closely to the pathogenesis of wet AMD.Citation6–Citation11
Previous research into AMD has focused primarily on the posterior pole (central 30°–50° around the macula). Currently, a wide-field imaging instrument, the Optos®200Tx (Optos plc, Dunfermline, Scotland, UK), allows noninvasive, nonmydriatic scanning of the fundus, including the periphery.Citation12–Citation17 Some studies have reported abnormal peripheral FAF in patients with AMD examined using the wide-field retinal imaging technique.Citation18–Citation21
The purpose of the current study was to characterize the frequencies of abnormal peripheral FAF patterns in the eyes of Japanese patients with AMD with respect to AMD disease types, treatments, and locations.
Patients and methods
Study model
This was a retrospective, case–control study conducted in Nagoya City University Hospital. The institutional review board of Nagoya City University Graduate School of Medical Sciences approved the retrospective data collection and analysis (University hospital Medical Information Network [UMIN] identification number: UMIN000018746).
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Informed consent
All patients provided written informed consent for use of their medical data in research.
Inclusion and exclusion criteria
Patients with wet AMD included in the study had been diagnosed with typical AMD, polypoidal choroidal vasculopathy (PCV), and retinal angiomatous proliferation (RAP). Control eyes included in the study were mainly normal eyes or eyes with mild cataract without other diagnosed abnormalities. Normal fellow eyes of patients with wet or dry AMD, eyes with central serous chorioretinopathy, and eyes with three or more obscure peripheral FAF quadrants were excluded from the study.
Patient characteristics
The mean patient ages of the three wet AMD disease subgroups, namely, typical AMD, PCV, and RAP, are shown in . There were no significant (P=0.07) differences between the mean ages of the patients with wet AMD (76±6.1 years) and those with the control eyes (72±7.6 years). There also were no significant (P=0.09, P=0.24, and P=0.19, respectively) differences among the mean patient ages in the typical AMD, PCV, and RAP subgroups compared with the ages of patients with control eyes. All patients were Japanese.
Table 1 Characteristics of the study group
Image analysis
Wide-field FAF images obtained using the Optos 200Tx were collected and analyzed. The central 30° region of the retina was covered in each image. Each 90° section of the peripheral images was divided into superior, inferior, temporal, and nasal quadrants (). Two masked ophthalmologists (MY, TY) graded the wide-field FAF images from the eyes with wet AMD and the control eyes in random order. The images were graded independently in each quadrant for the presence of three abnormal peripheral FAF patterns, ie, mottled, granular, and nummular (–), according to a previous report.Citation20 If the images were obscured, the results were reported as undeterminable. A third masked ophthalmologist grader (AK) resolved any discrepancies; in these cases, the matches between one of the first two graders and the third grader were adopted as the final gradings. If several types of abnormal peripheral FAF patterns were observed in the same quadrant, these were all recorded. If none of the three graders agreed, the image was graded as having no abnormal pattern.
Figure 1 Optos® image of an eye divided into four quadrants.
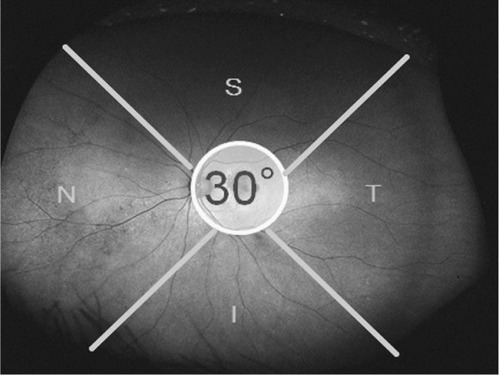
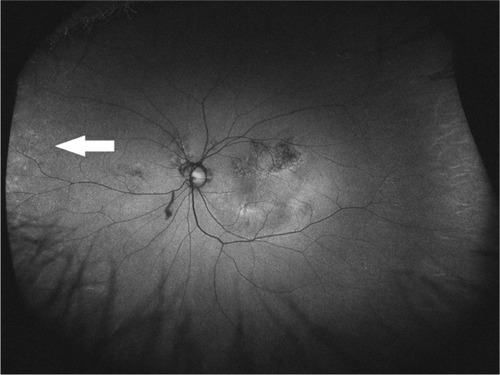
Figure 2 Optos® image of an eye with mottled pattern from a wet age-related macular degeneration patient.
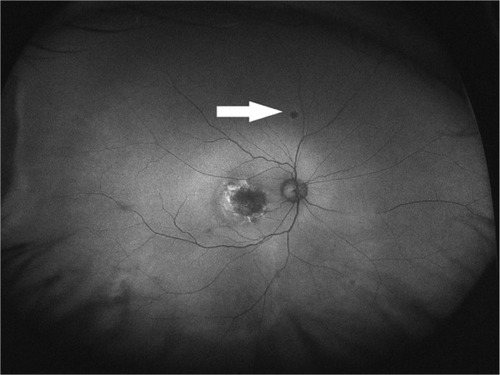
Main outcome measures
The main outcome measures were abnormal peripheral FAF frequencies and relative risks by disease subgroups and treatments.
Statistical analysis
Statistical analyses were conducted using Microsoft Excel 2010 (Microsoft Corporation, Redmond, WA, USA). Normal distribution of the mean ages in all groups was confirmed using the Jarque-Bera (JB) test after obtaining skewness and kurtosis values with Excel functions and calculating the JB values using its formula. The calculations were confirmed by MATLAB® (MathWorks, Natick, MA, USA) software, and the same results were obtained. F-tests for age distribution analyses, unpaired t-tests for mean age comparisons, and chi-square tests for frequency comparisons were performed. In all analyses, P<0.05 was considered significant.
Results
Prevalence of abnormal peripheral FAF
Abnormal peripheral FAF was evident in 34 (51.5%) eyes with wet AMD and six (18.8%) control eyes, a difference that reached significance (P<0.001). The prevalence rates of abnormal peripheral FAF patterns in all wet AMD and control groups are shown in . Abnormal patterns observed in descending order were mottled, granular, and nummular. The agreement between two graders was κ=0.17 for the types of abnormal FAF patterns. Table S1 shows the reasons for grading disagreements between any two ophthalmologists.
Table 2 Frequencies of abnormal peripheral FAF patterns in wet AMD eyes
Relative risk analyses by wet AMD disease types
The relative risk analyses of the frequencies of abnormal peripheral FAF patterns by wet AMD disease types are shown in . Abnormal FAF patterns were most prevalent in eyes with RAP, intermediate in eyes with typical AMD, and least prevalent in eyes with PCV.
Table 3 Relative risk analyses of abnormal peripheral FAF by wet AMD disease types
Prevalence of abnormal peripheral FAF patterns based on wet AMD disease types
The prevalence rates of abnormal peripheral FAF patterns in each wet AMD disease type are shown in . The mottled pattern was seen most often among all disease types. The granular pattern was seen most often in eyes with RAP.
Table 4 Frequencies of abnormal peripheral FAF in each wet AMD disease type
Relative risk analysis of abnormal peripheral FAF by wet AMD treatments
The relative risk analyses of the frequencies of abnormal peripheral FAF patterns based on the wet AMD treatments are shown in . Of the 66 eyes with wet AMD studied, 24 (36.4%) eyes were untreated and 42 eyes (63.6%) were treated with intravitreal aflibercept injections (Eylea®; Regeneron Pharmaceuticals Inc, Tarrytown, NY, USA) (22 eyes), intravitreal ranibizumab injections (Lucentis®; Genentech, South San Francisco, CA, USA) (29 eyes), and photodynamic therapy (PDT) with verteporfin injection (Visudyne®; Novartis International AG, Basel, Switzerland) (12 eyes). There were no significant differences in the frequencies of the abnormal FAF patterns between each treated group and the untreated group, ie, 50.0% in untreated eyes and 52.4% in treated eyes.
Table 5 Relative risk analyses of abnormal peripheral FAF by wet AMD treatments
Prevalence of abnormal peripheral FAF in each image quadrant
The prevalence rates of abnormal peripheral FAF in each image quadrant are shown in . Abnormal peripheral FAF was most prevalent in the nasal quadrant and least prevalent in the superior quadrant. Eight (12.1%) eyes with wet AMD had images that were obscured in the superior quadrant, six (9.1%) eyes in the inferior quadrant, two (3.0%) eyes in the temporal quadrant, and one (1.5%) eye in the nasal quadrant.
Table 6 Frequencies of abnormal peripheral FAF patterns in each image quadrant
Discussion
In this study, we observed abnormal peripheral FAF in 34 eyes (51.5%) in the group of eyes with wet AMD in Japanese patients. Compared with the prevalence (six eyes, 18.8%) in the healthy control eye group, the prevalence was significantly higher in eyes with wet AMD. The types of abnormal peripheral FAF observed were mottled (45.5%), granular (31.8%), and nummular (16.7%). The frequencies of abnormal peripheral FAF patterns were highest in eyes with RAP (85.7%), intermediate in eyes with typical AMD (54.1%), and lowest in eyes with PCV (36.4%). The treatments did not affect the frequencies of the abnormal peripheral FAF patterns in eyes with wet AMD. To the best of our knowledge, the previous findings regarding abnormal peripheral FAF patterns in patients with wet AMD were reported mainly in Caucasian patients,Citation19–Citation21 while only a few findings were described solely in Asian patients.Citation18
Tan et alCitation20 and Nomura et alCitation18 previously reported that the frequencies of abnormal peripheral FAF in patients with wet AMD were 86.0% and 60.4%, respectively. Although dry and wet AMD were not differentiated, Witmer et alCitation21 and Heussen et alCitation12 reported 63.6% and 73.9%, respectively, as the frequencies of the abnormal peripheral FAF patterns in eyes with AMD. The current report found lower incidence rates compared with these previous reports. However, in the current study, the increase of FAF abnormalities in eyes with wet AMD was significant compared with control eyes. The previous studies did not report differences in the frequencies among different AMD types or the effect of AMD treatments on the frequencies of abnormal FAF patterns.
Japanese populations are characterized by higher rates of PCV and lower rates of RAP compared with Western populations. The prevalence rates of PCV in Japanese populations with AMD range from 23% to about 55%,Citation22–Citation25 while they range from 9% to about 17% in Caucasian populations with AMD.Citation22,Citation26,Citation27 In contrast, the prevalence of RAP has been reported as 4.5% in a Japanese population with AMD,Citation25 while the rates range from 13.5% to about 15% in a Caucasian population with AMD.Citation22,Citation28 In the current study, more than a third of the eyes had PCV. Japanese populations have tended to have more eyes with PCV, and the frequency of abnormal peripheral FAF patterns is lower in PCV eyes than in eyes with other disease types. Such ethnic characteristics may have contributed to the lower total number of eyes with abnormal FAF observed in the current study.
Previous studies have reported three major patterns of the abnormal peripheral FAF.Citation18,Citation20 First, the mottled pattern of decreased FAF was characterized by diffuse areas of irregular hypoautofluorescence, which corresponded to RPE depigmentation in 76.5% of eyes with wet AMD, according to Tan et al.Citation20 In a study of the fellow eyes of Japanese patients with unilateral wet AMD, Sasaki et alCitation29 reported that retinal pigment abnormalities without drusen were critical in the development of PCV. In the current study, the mottled pattern was found in 31.8% of PCV eyes; this abnormality may be associated closely with RPE depigmentation.
Second, the granular pattern of increased FAF was characterized by small, interspersed areas of hyperautofluorescence that corresponded to peripheral drusen in 82.7% of eyes with wet AMD, according to Tan et al.Citation20 The drusen in Japanese eyes with wet AMD was reported to be associated more typically with RAP than with PCV, and these were observed in 77% to ~95% of eyes with RAPCitation25,Citation30 and in 19% to ~24% of eyes with PCV.Citation25,Citation31 The drusens were identified easily as increased autofluorescence in wide-field FAF images. The presence of drusen may have contributed to a higher frequency of abnormal FAF in eyes with RAP. In the current study, the drusen corresponding to the granular FAF pattern was observed in 42.9% of eyes with RAP.
Finally, the nummular pattern of decreased FAF was characterized by small-to-intermediate areas of homogeneous hypoautofluorescence that corresponded to RPE atrophy with a cobblestone appearance in 69.8% of eyes with wet AMD, according to Tan et al.Citation20
The current study also analyzed the frequency of abnormal peripheral FAF relative to treatments and locations. Intravitreal injections of anti-vascular endothelial growth factor and PDT treatments did not affect the rates of abnormal peripheral FAF patterns, perhaps because of the marginal effect of these therapies on the development of FAF abnormalities. The current study also showed that abnormal FAF developed less often in the superior quadrant, which may be due to disruption by artifacts such as eyelashes and eyelids, which result in more obscured images in the superior quadrant than in other quadrants.
The evaluation of abnormal peripheral FAF in eyes with wet AMD may lead to development of potent treatment strategies based on abnormal FAF patterns. Different types of treatments may be administered to treat mottled, granular, and nummular patterns in subsequent studies. The prognoses based on factors such as visual acuity, number of treatments, and quality of life also should be analyzed. The efficacy of the treatments may be analyzed by wide-field FAF images acquired at different time points. Studies of differential treatment strategies based on the abnormal peripheral FAF patterns may lead to novel treatment approaches for wet AMD.
The current study had some limitations. First, the data were collected retrospectively and did not consider the time period after the onset of wet AMD. The treatment types and periods during which they were administered varied among the patients. Second, we did not perform multivariate analyses of the effects of age, sex, smoking, time length after wet AMD onset, and number of treatments. Multivariate analyses may yield some valuable findings about the prevalence of abnormal peripheral FAF in patients with wet AMD. Third, we did not evaluate the healthy fellow eyes in patients with wet AMD. The fellow eyes of patients with wet AMD may have more abnormal peripheral FAF compared with the eyes of healthy control patients because a previous study reported a high concordance rate (90%) in both eyes of patients with wet AMD.Citation20 Finally, fewer patients were recruited compared with other similar studies. The results may have differed if we had recruited more patients with wet AMD.
Conclusion
The eyes of Japanese patients with wet AMD had higher prevalence rates of abnormal peripheral FAF in typical AMD, PCV, and RAP subgroups compared with control eyes. Mottled, granular, and nummular patterns were all observed in the eyes with wet AMD, and the mottled pattern was seen most frequently. Associations between abnormal peripheral FAF with the treatments and the prognostic factors should be investigated further in prospective clinical investigations.
Acknowledgments
No author has received any financial support.
Supplementary material
Table S1 Reasons for grading disagreements between two ophthalmologists
Disclosure
The authors report no conflicts of interest in this work.
References
- CongdonNO’ColmainBKlaverCCEye Diseases Prevalence Research GroupCauses and prevalence of visual impairment among adults in the United StatesArch Ophthalmol2004122447748515078664
- MaberleyDAHollandsHChuoJThe prevalence of low vision and blindness in CanadaEye (Lond)200620334134615905873
- WongTYLoonSCSawSMThe epidemiology of age related eye diseases in AsiaBr J Ophthalmol200690450651116547337
- XuLWangYLiYCauses of blindness and visual impairment in urban and rural areas in Beijing: the Beijing Eye StudyOphthalmology200611371134.e11134.e1116647133
- OwenCGJarrarZWormaldRCookDGFletcherAERudnickaARThe estimated prevalence and incidence of late stage age related macular degeneration in the UKBr J Ophthalmol201296575275622329913
- BindewaldABirdACDandekarSSClassification of fundus autofluorescence patterns in early age-related macular diseaseInvest Ophthalmol Vis Sci20054693309331416123434
- DandekarSSJenkinsSAPetoTAutofluorescence imaging of choroidal neovascularization due to age-related macular degenerationArch Ophthalmol2005123111507151316286612
- DeloriFCFlecknerMRGogerDGWeiterJJDoreyCKAutofluorescence distribution associated with drusen in age-related macular degenerationInvest Ophthalmol Vis Sci200041249650410670481
- HolzFGBellmanCStaudtSSchuttFVolckerHEFundus autofluorescence and development of geographic atrophy in age-related macular degenerationInvest Ophthalmol Vis Sci20014251051105611274085
- HolzFGBindewald-WittichAFleckensteinMDreyhauptJSchollHPSchmitz-ValckenbergSFAM-Study GroupProgression of geographic atrophy and impact of fundus autofluorescence patterns in age-related macular degenerationAm J Ophthalmol2007143346347217239336
- Schmitz-ValckenbergSBindewald-WittichADolar-SzczasnyJFundus Autofluorescence in Age-Related Macular Degeneration Study GroupCorrelation between the area of increased autofluorescence surrounding geographic atrophy and disease progression in patients with AMDInvest Ophthalmol Vis Sci20064762648265416723482
- HeussenFMTanCSSaddaSRPrevalence of peripheral abnormalities on ultra-widefield greenlight (532 nm) autofluorescence imaging at a tertiary care centerInvest Ophthalmol Vis Sci201253106526653122871828
- HeussenFMVasconcelos-SantosDVPappuruRRWalshACRaoNASaddaSRUltra-wide-field green-light (532-nm) autofluorescence imaging in chronic Vogt-Koyanagi-Harada diseaseOphthalmic Surg Lasers Imaging201142427227721553702
- OguraSYasukawaTKatoAWide-field fundus autofluorescence imaging to evaluate retinal function in patients with retinitis pigmentosaAm J Ophthalmol201415851093109825062603
- OishiAOginoKMakiyamaYNakagawaSKurimotoMYoshimuraNWide-field fundus autofluorescence imaging of retinitis pigmentosaOphthalmology201312091827183423631947
- ReznicekLSeidenstickerFStumpfCSystematic analysis of wide-field fundus autofluorescence (FAF) imaging in posterior uveitisCurr Eye Res201439216417124144279
- SeidenstickerFNeubauerASWasfyTWide-field fundus autofluorescence corresponds to visual fields in chorioretinitis patientsClin Ophthalmol201151667167122174575
- NomuraYTakahashiHTanXObataRYanagiYWidespread choroidal thickening and abnormal midperipheral fundus autofluorescence characterize exudative age-related macular degeneration with choroidal vascular hyperpermeabilityClin Ophthalmol2015929730425709392
- ReznicekLWasfyTStumpfCPeripheral fundus autofluorescence is increased in age-related macular degenerationInvest Ophthalmol Vis Sci20125342193219822410571
- TanCSHeussenFSaddaSRPeripheral autofluorescence and clinical findings in neovascular and non-neovascular age-related macular degenerationOphthalmology201312061271127723433790
- WitmerMTKozbialADanielSKissSPeripheral autofluorescence findings in age-related macular degenerationActa Ophthalmol2012906e428e43322578271
- CoscasGYamashiroKCoscasFComparison of exudative age-related macular degeneration subtypes in Japanese and French patients: multicenter diagnosis with multimodal imagingAm J Ophthalmol20141582309.e318.e24844973
- MoriRYuzawaMAkazaEHaruyamaMTreatment results at 1 year of ranibizumab therapy for polypoidal choroidal vasculopathy in eyes with good visual acuityJpn J Ophthalmol201357436537123665979
- ShoKTakahashiKYamadaHPolypoidal choroidal vasculopathy: incidence, demographic features, and clinical characteristicsArch Ophthalmol2003121101392139614557174
- MarukoIIidaTSaitoMNagayamaDSaitoKClinical characteristics of exudative age-related macular degeneration in Japanese patientsAm J Ophthalmol20071441152217509509
- HatzKPrunteCPolypoidal choroidal vasculopathy in Caucasian patients with presumed neovascular age-related macular degeneration and poor ranibizumab responseBr J Ophthalmol201498218819424246375
- Scassellati-SforzoliniBMariottiCBryanRYannuzziLAGiulianiMGiovanniniAPolypoidal choroidal vasculopathy in ItalyRetina200121212112511321137
- DonatiMCCarifiGVirgiliGMenchiniURetinal angiomatous proliferation: association with clinical and angiographic featuresOphthalmologica20062201313616374046
- SasakiMKawasakiRUchidaAEarly signs of exudative age-related macular degeneration in AsiansOptom Vis Sci201491884985324978864
- SawaMUenoCGomiFNishidaKIncidence and characteristics of neovascularization in fellow eyes of Japanese patients with unilateral retinal angiomatous proliferationRetina201434476176724100709
- MoriKHorie-InoueKGehlbachPLPhenotype and genotype characteristics of age-related macular degeneration in a Japanese populationOphthalmology2010117592893820132989