Abstract
Purpose
To investigate the clinical characteristics and therapeutic outcome of patients with neovascular age-related macular degeneration (nAMD) in 1 eye, without drusen in the fellow eye.
Patients and methods
Medical records of 381 patients were analyzed to identify the cases. The main outcomes included Early Treatment Diabetic Retinopathy Study (ETDRS) best-corrected visual acuity (BCVA) and change in central retinal thickness (CRT). These parameters were reviewed at baseline, first follow-up visit, and after 6, 12, and 24 months.
Results
Out of 381 patients, 29 cases (8%) were included (of whom 3 had polypoidal choroidal vasculopathy [PCV]) who were treated with anti-vascular endothelial growth factor (anti-VEGF) therapy which was supplemented by photodynamic therapy (PDT) in the PCV patients. Overall, no statistically significant change in mean BCVA was observed during follow-up. BCVA improved or remained stable (defined as a gain in BCVA, a stable BCVA, or a loss of <5 ETDRS letters) in 22 patients (76%), and 7 patients (23%) had lost ≥5 ETDRS letters at final follow-up. A gain of ≥15 ETDRS letters at final follow-up was seen in 5 patients (17%). Mean CRT had decreased significantly with 99 µm (P<0.001) at 24 months after the initial visit.
Conclusion
There is a clinical spectrum of nAMD that is not associated with drusen in the fellow eye. Patients with nAMD without drusen in the fellow eye respond to anti-VEGF treatment and, in cases of PCV, to supplemental PDT. The pathophysiology of this spectrum of nAMD may be different from drusen-associated age-related macular degeneration.
Introduction
Age-related macular degeneration (AMD) is the most common cause of irreversible vision loss among elderly in the Western world. Marked vision loss can occur in late AMD, as a result of choroidal neovascularization (CNV) and/or profound chorioretinal atrophy (geographic atrophy).Citation1
Traditionally, macular drusen are characteristic for early and intermediate stages of this disease. However, the definition of AMD has varied among authors.Citation2–Citation4 Although most classifications consider drusen mandatory lesions in AMD, 1 or more small hard drusen with a diameter <63 µm can be detected in 85%–99% of the overall population aged ≥50 years.Citation5–Citation8
In cases of unilateral neovascular AMD (nAMD), the fellow eye is at high risk of developing CNV, with cumulative incidence rates of 10%, 22%, 28%, 37%, and 42% after 1, 2, 3, 4, and 5 years, respectively.Citation9,Citation10 Factors that contribute to these rates are the presence, number, location, and the individual and cumulative size of drusen in the fellow eye.Citation11 However, there is a clinical observation that some patients with nAMD in 1 eye with no drusen in the fellow eye. Little is known about the clinical characteristics and therapeutic outcome of this subgroup of patients with unilateral nAMD without drusen in the fellow eye. As drusen are often considered pivotal precursor lesions for advanced AMD such as nAMD, the question arises whether nAMD without drusen in the fellow eye is clinically distinct from drusen-associated nAMD. Therefore, this study analyzed the clinical spectrum, characteristics, and treatment outcome of a subgroup of patients with nAMD in 1 eye, without drusen in the fellow eye.
Patients and methods
Data collection
A retrospective review of charts was performed at the Department of Ophthalmology of Leiden University Medical Center, the Netherlands. This study adhered to the tenets of the Declaration of Helsinki, and approval for this study was obtained from the Medical Ethical Committee of Leiden University Medical Center. All participants gave their written informed consent.
All data were obtained from patients who were diagnosed with unilateral nAMD between January 2010 and July 2015. These cases were retrospectively analyzed to identify cases of unilateral nAMD without drusen in the macular region of the fellow eye at initial diagnosis. The grading of drusen was performed according to the International Classification for age-related maculopathy and AMD,Citation2 and the location of the lesions was determined using the Early Treatment Diabetic Retinopathy Study (ETDRS) grid. Fellow eyes with <5 small hard drusen with a diameter <63 µm in the macular region were not considered to have AMD, but rather a consequence of normal aging.Citation3 All images were obtained from routine eye examinations and were assessed by an experienced retina specialist (CJFB).
Patients had to meet all of the following inclusion criteria: 1) age ≥50 years; 2) AMD with signs of CNV confirmed on optical coherence tomography (OCT) (on which either subretinal or intraretinal fluid or both could be detected) and fluorescein angiography (FA) in 1 eye; 3) <5 small hard drusen in the macular region in the fellow eye as evidenced by fundus photography, FA, and OCT; 4) at least 1 follow-up visit after initial diagnosis. Exclusion criteria were any of the following: 1) multimodal imaging which was considered to be of insufficient quality; 2) a confirmed diagnosis of AMD with signs of CNV or fovea-involving atrophy in the fellow eye; 3) history of another diagnosis as a primary underlying cause/background of neovascularization, such as pseudoxanthoma elasticum, high myopia (ie, ≥6 diopters), central serous chorioretinopathy (CSC), macular telangiectasia, or multifocal choroiditis.
Image acquisition
All patients included in this study underwent color fundus photography (Topcon Corp., Tokyo, Japan), OCT with either the Cirrus OCT (Carl Zeiss Meditec, Dublin, CA, USA) or spectral-domain OCT device (Spectralis HRA + OCT; Heidelberg Engineering, Heidelberg, Germany), and FA was performed with either a Topcon fundus camera (Topcon Corp.) or the Spectralis HRA + OCT camera.
Study measurements
After patient selection, the following clinical parameters were collected at baseline: age at onset, best-corrected visual acuity (BCVA), the anatomical level of fluid accumulation on OCT (intraretinal, subretinal, or both), performed treatments (anti-vascular endothelial growth factor [anti-VEGF] treatment [bevacizumab, ranibizumab, and aflibercept] and/or photodynamic therapy [PDT]), central retinal thickness (CRT), and the type of CNV. The type of CNV was either “type 1” (sub-retinal pigment epithelium [RPE] neovascularization [occult neovascularization]), “type 2” (subretinal neovascularization [classic neovascularization]), or “type 3” (retinal angiomatous proliferation).Citation12,Citation13 In case of a mixed neovascularization, the CNV was considered to be “minimally classic” if the majority of the CNV was type 1. If the mixed neovascularization was predominantly type 2, it was referred to as “predominantly classic,”Citation13 The first choice of anti-VEGF treatment in treatment-naive patients was 3 monthly intravitreal injections of bevacizumab. Switching to another type of anti-VEGF treatment (either ranibizumab or aflibercept) was considered when insufficient reduction in subretinal and/or intraretinal fluid was observed on OCT at a control examination within 4 weeks after the third bevacizumab injection. Indocyanine green angiography was performed either in case of a suspicion of polypoidal choroidal vasculopathy (PCV) or when patients did not respond sufficiently to anti-VEGF treatment. Treatments were applied according to the pro re nata regimen, where patients had a follow-up every 4 weeks after initial treatment. Retreatment of 3 injections every 4 weeks was performed upon reoccurrence of subretinal and/or intraretinal fluid on OCT and/or on FA, or when hemorrhage was seen during fundoscopy.Citation14
Information regarding BCVA and CRT was collected at the first visit before initial treatment (baseline), at the first visit after initial treatment, which was usually scheduled after 3 intravitreal injections, and 6, 12, and 24 months after initial treatment. For patients for whom ETDRS BCVA was not available, a previously published formula was used to convert Snellen BCVA to ETDRS values.Citation15 The number and mode of anti-VEGF injections during follow-up, and changes in subretinal and/or intraretinal fluid on OCT were recorded. The used dosages of bevacizumab, ranibizumab, and aflibercept were 1.25, 0.5, and 2 mg, respectively. In order to quantify the number of anti-VEGF injections required to achieve a total resolution of subretinal and/or intraretinal fluid for ≥3 months without treatment, OCT images were used.
Statistical analysis
Statistical analysis was performed using IBM SPSS Statistics, version 23 (IBM Corporation, Armonk, NY, USA). The clinical parameters at baseline and during follow-up were analyzed in an autoregressive model within a linear mixed model with time as a factor. A P-value of <0.05 was considered to be statistically significant.
Results
Fundus photography, FA, and OCT of 381 patients were analyzed to identify cases within the spectrum of unilateral nAMD without drusen in the macular region of the fellow eye at initial diagnosis. Twenty-nine patients (8%) (16 male and 13 female patients) met the inclusion criteria. The mean age at onset of disease of these 29 patients was 75 years (range: 53–89 years). In 1 patient (3%), a few small hard drusen were found in the eye with nAMD (). A spectrum of neovascular abnormalities was observed (): 18 patients were diagnosed with type 1 neovascularization, 7 patients with type 2 neovascularization, and 4 patients with a mixed neovascularization. Three of these 29 patients (10%) were also diagnosed with PCV (of whom in 1 patient the lesion was an isolated polyp, and in 2 patients the lesions occurred together with a neovascularization/branching vascular network, confirmed on additional indocyanine green angiography) (). Patients received the first intravitreal injection at a mean of 7 days (range: 0–16 days) after being diagnosed with nAMD. Some patients were also scheduled for either half-dose or full-dose PDT (). In none of the patients, ophthalmic surgery had been performed in their study eye during follow-up. One patient received cataract surgery in the fellow eye during the study.
Figure 1 Spectrum of neovascular age-related macular degeneration without drusen in the fellow eye.
Abbreviations: FA, fluorescein angiography; OCT, optical coherence topography; PED, pigment epithelial detachment.
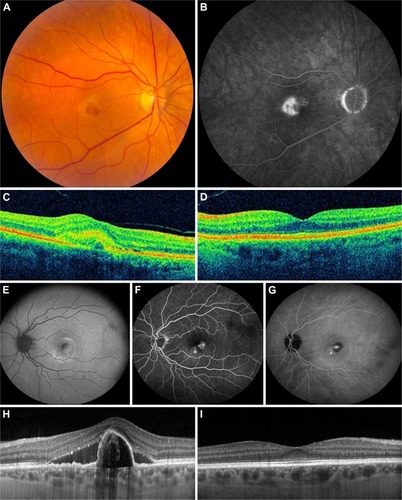
Table 1 Baseline characteristics of patients with neovascular age-related macular degeneration without drusen in the fellow eye
Table 2 Treatment and number of received intravitreal injections during follow-up
Overall, BCVA in all patients increased with a mean of 2.5 ETDRS letters at 24 months, which was not statistically significant as compared to baseline (P=0.456) (). The BCVA of 22 patients (76%) improved or remained stable (defined as a gain in BCVA, a stable BCVA, or a loss of <5 ETDRS letters), whereas BCVA decreased (defined as a loss of ≥5 ETDRS letters) in 7 patients (24%). A gain of ≥15 ETDRS letters from baseline was seen in 5 patients (17%). The BCVA of the 3 PCV patients during follow-up and the treatments performed in these patients are shown separately ().
Figure 2 Mean change in BCVA during follow-up, compared to baseline.
Abbreviations: BCVA, best-corrected visual acuity; ETDRS, Early Treatment Diabetic Retinopathy Study; SE, standard error.
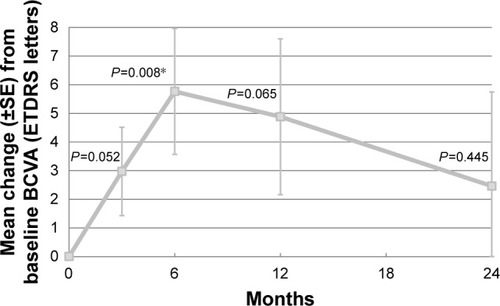
Table 3 Treatment and best-corrected visual acuity of patients with polypoidal choroidal vasculopathy during follow-up
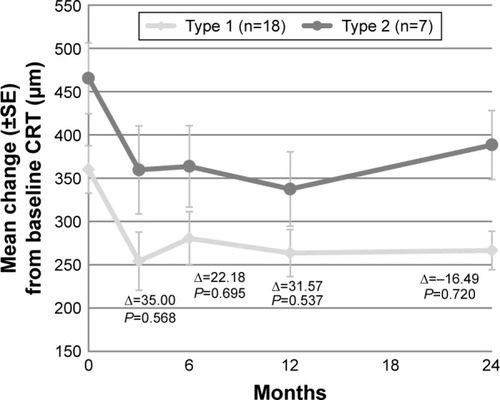
Compared to baseline, the mean CRT had significantly decreased with 99 µm (P<0.001) at final follow-up (). At all follow-up visits, the decrease in CRT was statistically significant (). Based on the type of neovascularization, no significant difference in CRT decrease between the 2 groups at several time points was found ().
Figure 3 Mean change in CRT during follow-up, compared to baseline.
Abbreviations: CRT, central retinal thickness; SE, standard error.
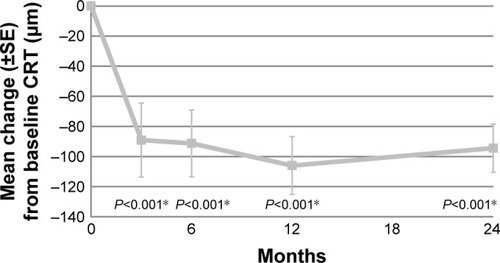
Figure 4 Mean CRT during follow-up per type of neovascularization (type 1 versus type 2).
Abbreviations: CRT, central retinal thickness; SE, standard error.
In 6 of 29 treated patients (21%), there were no signs of CNV activity on OCT for ≥3 months () after baseline. However, 1 patient needed full-dose PDT after 8 intravitreal bevacizumab injections and 3 ranibizumab injections before a complete resolution of subretinal and/or intraretinal fluid was achieved for these 3 months.
Table 4 Received treatments in several patients in whom subretinal fluid resolved for ≥3 months after treatment
Discussion
This study examined the clinical spectrum and therapeutic outcome of patients with unilateral nAMD without drusen in the macular region of their fellow eye. In these patients, no statistically significant improvement in BCVA occurred after a follow-up of 24 months after first treatment for nAMD. Moreover, in 7 patients (24%), the BCVA declined with ≥5 ETDRS letters. Overall, the mean decrease in CRT was statistically significant at the final follow-up visit. In all patients, the mean increase in BCVA in this study was 2.5 ETDRS letters at the last follow-up visit at 24 months after baseline, which is relatively low in comparison with other studies. In these studies, a statistically significant increase in BCVA of 14 and 6–7.9 ETDRS letters was found from baseline until the follow-up visits at 12 and 24 months, respectively, after using intravitreal injections according to the pro re nata regimen.Citation16,Citation17 However, the mean number of intravitreal injections used in our study over the period of 24 months, and the significant decrease in CRT at 24 months after baseline, is comparable to another study.Citation17 This may suggest that subgroups of AMD could have a different prognosis and that different treatment strategies may be needed to achieve a significant improvement in BCVA.
The correlation between nAMD and clinical characteristics of non-neovascular fellow eyes has been studied previously.Citation18–Citation20 Available studies indicate a correlation between the subtype of neovascularization, based on the anatomical classification, and phenotypic characteristics of the non-neovascular fellow eyes.Citation18,Citation19 Predominantly, intraretinal lesions are more likely to be accompanied by reticular pseudodrusen and/or a decreased subfoveal choroidal thickness compared to supra-RPE lesions.Citation19 Other studies have shown an association between the development of nAMD and reticular pseudodrusen in the fellow eye.Citation18 However, no studies of patients with unilateral nAMD without any drusen in the fellow eye have been performed thus far.
Patients with nAMD without drusen in the fellow eye could be argued to have overlapping phenotypes and/or phenocopies mimicking AMD, which share clinical and possibly pathophysiologic features with drusenoid AMD, such as idiopathic PCV, and neovascular CSC.Citation21,Citation22 At baseline only 1 patient (3%) had a few small hard drusen in the eye with nAMD. The remaining patients (97%) had no drusen in their eye with nAMD. A possible explanation for this finding is that drusen in earlier stages may regress before being converted to advanced nAMD.Citation23 The clinical observation that some patients can have this type of unilateral nAMD raises the question whether these patients truly have AMD in the strict sense, because early AMD is characterized by the appearance of drusen with a size of >63 µm in either eye.Citation3,Citation6 However, this study excluded patients with other diagnoses explaining the occurrence of CNV, such as pseudoxanthoma elasticum, high myopia, CSC, macular telangiectasia, or multifocal choroiditis. The mean age at onset of the nAMD patients in this study was 75 years, and CNV in the affected eye could be easily classified as type 1 or 2 (or mixed type) CNV, sometimes with a component of PCV. Apart from an absence of drusen in the fellow eye in the current patient group, any drusen in the eye affected by nAMD was also not observed although the absence of drusen in the case of neovascularization is not a definitive proof that these were not present prior to the onset of nAMD. It is therefore argued that the patient group that is described here truly has a form of nAMD although they do not have drusen in the fellow eye. This study seems to point out that for a subgroup of nAMD patients the presence of drusen is not a prerequisite to develop nAMD, as evidenced indirectly by an absence of drusen in the fellow eye.
Drusen are located between the RPE and Bruch’s membrane and consist of a broad spectrum of components, such as proteins that are involved in the complement system.Citation24 Drusen are considered to be by-products of chronic, local inflammatory events in Bruch’s membrane and play an important role in the pathogenesis and progression of AMD.Citation24 They also contain esterified and unesterified cholesterol which may be produced by the RPE.Citation25,Citation26 Moreover, strong evidence has been found that genes involved in lipid transport and processing are consistent with the composition of drusen.Citation27 It is possible that the current patient cohort had earlier, clinically invisible underlying pathophysiologic signs such as basal laminar deposits and basal linear deposits, and membranous debris in the fellow eye without visible drusen, which may then still cause a drusenoid environment with pathophysiological consequences such as CNV.Citation28 A better understanding of the development of drusen in nAMD, and the possible prerequisite of drusen or their precursor material to cause CNV, could provide insight in the pathogenesis, prognostic factors, and intervention strategies for CNV in all nAMD patients.
Although the number of Caucasian PCV patients (10%) involved in this study is too small to draw conclusions about the therapeutic outcome in this specific subgroup, these patients appeared to respond to a treatment combination of PDT and intravitreal anti-VEGF injections. The prevalence of PCV in Caucasians seems to be substantially lower than in Asians,Citation29 and Caucasian PCV patients may differ from PCV patients with an Asian ethnicity in terms of presenting clinical features and angiographic behavior.Citation29–Citation31 Larger studies are necessary to explore the differences in phenotypes, genotypes, and treatment response between the Caucasian and Asian population. It is currently unclear whether PCV can be seen as a specific pathogenetic and/or clinical entity within the spectrum of AMD in Caucasians, or as a variant of CNV.Citation32,Citation33 There is evidence that PCV shares characteristics with nAMD, but different phenotypic subtypes are also possible.Citation34
The findings of this study clearly indicate that there is an entity of unilateral AMD without drusen in the fellow eye. This is the first exploratory study assessing the clinical characteristics and therapeutic outcome of patients with nAMD and no drusen in the fellow eye. The retrospective aspect and non-comparative nature of this study gives its inherent shortcomings. In some cases, different OCT machines were used during follow-up for CRT measurements. Only 17 of the 29 (59%) included patients in the cohort had a follow-up of 24 months. Larger, preferably prospective clinical studies can provide a better understanding of this specific subgroup of AMD. It was demonstrated that therapeutic response in patients with unilateral nAMD without drusen in the fellow eye could differ from typical nAMD patients. These findings indicate that drusen as precursor lesions may not be a prerequisite to develop nAMD.
Acknowledgments
The authors thank MSc Ron Wolterbeek from the Department of Medical Statistics and Bioinformatics of the Leiden University Medical Center, for his statistical support. This research was supported by the Mathilde Grant, Stichting Leids Oogheelkundig Ondersteuningsfonds, Rotterdamse Stichting Blindenbelangen, and the Netherlands Organisation for Scientific Research (NWO). The funding organizations MD Fonds, Landelijke Stichting voor Blinden en Slechtzienden, Retina Netherlands, and BlindenPenning contributed through UitZicht. The aforementioned funding sources provided unrestricted grants and had no role in the design or conduct of this research.
Disclosure
The authors report no conflicts of interest in this work.
References
- de JongPTAge-related macular degenerationN Engl J Med20063551474148517021323
- BirdACBresslerNMBresslerSBAn international classification and grading system for age-related maculopathy and age-related macular degeneration. The International ARM Epidemiological Study GroupSurv Ophthalmol1995393673747604360
- FerrisFL3rdWilkinsonCPBirdAClinical classification of age-related macular degenerationOphthalmology201312084485123332590
- FreundKBZweifelSAEngelbertMDo we need a new classification for choroidal neovascularization in age-related macular degeneration?Retina2010301333134920924258
- BresslerNMBresslerSBWestSKFineSLTaylorHRThe grading and prevalence of macular degeneration in Chesapeake Bay watermenArch Ophthalmol19891078478522786408
- KleinRKleinBELintonKLPrevalence of age-related maculopathy. The Beaver Dam Eye StudyOphthalmology1992999339431630784
- MitchellPSmithWAtteboKWangJJPrevalence of age-related maculopathy in Australia. The Blue Mountains Eye StudyOphthalmology1995102145014609097791
- VingerlingJRDielemansIHofmanAThe prevalence of age-related maculopathy in the Rotterdam StudyOphthalmology19951022052107862408
- GroupMPSRisk factors for choroidal neovascularization in the second eye of patients with juxtafoveal or subfoveal choroidal neovascularization secondary to age-related macular degenerationArch Ophthalmol19971157417479194725
- SolomonSDJefferysJLHawkinsBSBresslerNMIncident choroidal neovascularization in fellow eyes of patients with unilateral subfoveal choroidal neovascularization secondary to age-related macular degeneration: SST report No 20 from the Submacular Surgery Trials Research GroupArch Ophthalmol20071251323133017923538
- KleinRMyersCELeeKESmall drusen and age-related macular degeneration: The Beaver Dam Eye StudyJ Clin Med20154425440
- AgarwalASaundersGass’ Atlas of Macular Diseases5th edPhiladelphiaElsevier201292100
- YannuzziLSaundersThe Retinal AtlasLondonElsevier2010568577
- MitchellPKorobelnikJFLanzettaPRanibizumab (Lucentis) in neovascular age-related macular degeneration: evidence from clinical trialsBr J Ophthalmol20109421319443462
- GregoriNZFeuerWRosenfeldPJNovel method for analyzing snellen visual acuity measurementsRetina2010301046105020559157
- BohniSCBittnerMHowellJPBachmannLMFaesLSchmidMKComparison of Eylea(R) with Lucentis(R) as first-line therapy in patients with treatment-naive neovascular age-related macular degeneration in real-life clinical practice: retrospective case-series analysisBMC Ophthalmol20151510926289356
- MartinDFMaguireMGFineSLRanibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: two-year resultsOphthalmology20121191388139822555112
- HoggREChakravarthyUVisual function and dysfunction in early and late age-related maculopathyProg Retin Eye Res20062524927616580242
- MarsigliaMBodduSChenCYCorrelation between neovascular lesion type and clinical characteristics of nonneovascular fellow eyes in patients with unilateral, neovascular age-related macular degenerationRetina20153596697425627089
- KangSWLeeHBaeKShinJYKimSJKimJMKorean Age-related Maculopathy Study (KARMS) GroupInvestigation of precursor lesions of polypoidal choroidal vasculopathy using contralateral eye findingsGraefes Arch Clin Exp Ophthalmol Epub201695
- HatzKPrunteCPolypoidal choroidal vasculopathy in Caucasian patients with presumed neovascular age-related macular degeneration and poor ranibizumab responseBr J Ophthalmol20149818819424246375
- SchatzHMadeiraDJohnsonRNMcDonaldHRCentral serous chorioretinopathy occurring in patients 60 years of age and olderOphthalmology19929963671741142
- SchlanitzFGBaumannBKundiMDrusen volume development over time and its relevance to the course of age-related macular degenerationBr J Ophthalmol2016
- AndersonDHRadekeMJGalloNBThe pivotal role of the complement system in aging and age-related macular degeneration: hypothesis re-visitedProg Retin Eye Res2010299511219961953
- CurcioCAPresleyJBMalekGMedeirosNEAveryDVKruthHSEsterified and unesterified cholesterol in drusen and basal deposits of eyes with age-related maculopathyExp Eye Res20058173174116005869
- CurcioCAPresleyJBMillicanCLMedeirosNEBasal deposits and drusen in eyes with age-related maculopathy: evidence for solid lipid particlesExp Eye Res20058076177515939032
- PikulevaIACurcioCACholesterol in the retina: the best is yet to comeProg Retin Eye Res201441648924704580
- SarksSCherepanoffSKillingsworthMSarksJRelationship of basal laminar deposit and membranous debris to the clinical presentation of early age-related macular degenerationInvest Ophthalmol Vis Sci20074896897717325134
- CiardellaAPDonsoffIMHuangSJCostaDLYannuzziLAPolypoidal choroidal vasculopathySurv Ophthalmol200449253714711438
- LafautBALeysAMSnyersBRasquinFDe LaeyJJPolypoidal choroidal vasculopathy in CaucasiansGraefes Arch Clin Exp Ophthalmol200023875275911045343
- ImamuraYEngelbertMIidaTFreundKBYannuzziLAPolypoidal choroidal vasculopathy: a reviewSurv Ophthalmol20105550151520850857
- BalaratnasingamCLeeWKKoizumiHDansinganiKInoueMFreundKBPolypoidal choroidal vasculopathy: a distinct disease or manifestation of many?Retina2016361826414957
- LaudeACackettPDVithanaENPolypoidal choroidal vasculopathy and neovascular age-related macular degeneration: same or different disease?Prog Retin Eye Res201029192919854291
- CoscasGLupidiMCoscasFToward a specific classification of polypoidal choroidal vasculopathy: idiopathic disease or subtype of age-related macular degenerationInvest Ophthalmol Vis Sci2015563187319526024102
