Abstract
Dry eye (DE) is a chronic ocular condition with high prevalence and morbidity. It has a complex pathophysiology and is multifactorial in nature. Chronic ocular surface inflammation has emerged as a key component of DE that is capable of perpetuating ocular surface damage and leading to symptoms of ocular pain, discomfort, and visual phenomena. It begins with stress to the ocular surface leading to the production of proinflammatory mediators that induce maturation of resident antigen-presenting cells which then migrate to the lymph nodes to activate CD4 T cells. The specific antigen(s) targeted by these pathogenic CD4+ T cells remains unknown. Two emerging theories include self-antigens by autoreactive CD4 T cells or harmless exogenous antigens in the setting of mucosal immunotolerance loss. These CD4 T cells migrate to the ocular surface causing additional inflammation and damage. Lifitegrast is the second topical anti-inflammatory agent to be approved by the US Food and Drug Administration for the treatment of DE and the first to show improvement in DE symptoms. Lifitegrast works by blocking the interaction between intercellular adhesion molecule-1 and lymphocyte functional associated antigen-1, which has been shown to be critical for the migration of antigen-presenting cells to the lymph nodes as well as CD4+ T cell activation and migration to the ocular surface. In four large multicenter, randomized controlled trials, lifitegrast has proven to be effective in controlling both the signs and symptoms of DE with minimal side effects. Further research should include comparative and combination studies with other anti-inflammatory therapies used for DE.
Introduction
Dry eye (DE) is a chronic ocular condition with high prevalence and morbidity.Citation1 It leads to symptoms of ocular pain, discomfort, and visual dysfunction which can greatly impact the quality of life (QOL).Citation2 DE is multifactorial in nature with a complex pathophysiology.Citation3 One important facet is chronic ocular surface inflammation that can perpetuate ocular surface damage.Citation4 Lifitegrast is the second topical anti-inflammatory agent to be approved by the US Food and Drug Administration (FDA) for the treatment of DE and the first to show improvement in DE symptoms.Citation5 This review discusses the contribution of inflammation in DE in both animal models and humans and highlights the place of lifitegrast in the treatment of the disease.
DE disease definition, classification, and epidemiology
As per the Tear Film and Ocular Surface Dry Eye WorkShop II, DE is a multifactorial ocular surface disease causing ocular symptoms.Citation3 It is characterized by a loss of tear film homeostasis as a result of tear film instability and hyperosmolarity, ocular surface inflammation and damage, or neurosensory pathology.Citation3
DE is classified into two major groups based on the effect on tear film stability: aqueous deficient (decreased production of the aqueous component of the tear film) and evaporative (increased evaporation of the tear film).Citation3 Aqueous deficient DE disease (ADDE) is the least common and accounts for 14% of DE.Citation6 ADDE is further classified as Sjögren’s syndrome DE and non-Sjögren’s syndrome DE.Citation3 Evaporative DE (EDE) is far more common and accounts for 50% of DE.Citation6 The most common cause is meibomian gland dysfunction (MGD) leading to meibum deficiency.Citation3 The remaining 36% of DE patients show evidence of both ADDE and EDE.Citation6
DE affects 5%–30% of the world population 50 years and older, with women representing approximately two-thirds of those affected.Citation1,Citation7 Many factors have been linked to DE, including hormonal alterations (eg, menopause),Citation8 medication use (eg, antihistamines),Citation9 and comorbidities (eg, depression).Citation10 DE can also be seen after ophthalmic procedures such as LASIK, where it has been reported in up to 95% of individuals immediately after surgery and 60% of individuals 1 month after surgery.Citation11 DE is also seen to a lesser degree following cataract surgery, with an estimated incidence of 9.8%.Citation12
DE has a significant impact on QOL due to chronic symptoms of ocular pain and discomfort in addition to functional vision impairment. Utility assessment is a tool for quantifying QOL and comparing QOL across different medical conditions. When applied to patients with moderate to severe DE, utility assessment scores showed a decrease in QOL for everyday and leisure activities by 60%, which was comparable to medical conditions such as severe angina, disabling hip fractures, and dialysis.Citation13,Citation14 Furthermore, DE can impact functional vision, interfering with everyday activities such as driving, reading, computer usage, watching television, and work performance.Citation15–Citation17 In addition, DE negatively impacts mental health as DE patients report higher rates of anxiety and depression.Citation18–Citation20 Financially, DE is estimated to cost $3.84 million per year to the health care system with an average annual cost of $783 per patient for treatment alone.Citation21,Citation22
DE symptoms and signs
Currently, there is no “gold standard” test to diagnose DE. Instead, a combination of presenting signs and symptoms is used in clinical judgment. Further complicating the diagnosis of DE is the poor correlation that exists between DE symptoms and signs.Citation23
DE symptoms
The evaluation of DE symptoms can be challenging due to varying presentations and course. For example, some individuals predominantly complain of spontaneous ocular pain/discomfort characterized by different terms (eg, dryness, grittiness, burning, stinging, etc), while other patients may report evoked pain, in particular by wind and light.Citation1 Yet others report visual phenomena in the form of blurry or fluctuating vision.Citation24 Furthermore, some individuals describe transient symptoms, while other have a chronic disease course.Citation1 Several standardized questionnaires have been developed to assess DE symptoms and their effect on QOL. These include the Dry Eye Questionnaire 5,Citation25 the Ocular Surface Disease Index (OSDI),Citation26 the Impact of Dry Eye on Everyday Life,Citation27 and Visual Function Questionnaire-25.Citation28 Most arrive at a DE severity score by lumping together various DE symptoms and there is no consensus on which questionnaire provides the best assessment tool.Citation26,Citation27,Citation29
DE signs
A careful slit-lamp evaluation with a few additional tests can reveal many signs of DE. These signs include decreased tear production measured by Schirmer tear test, tear film instability measured by tear-film breakup time, and corneal or conjunctival epithelial damage measured by the degree of punctate epithelial erosions, best seen after administration of fluorescein, rose bengal, or lissamine green dyes.Citation30 Signs of MGD such as plugging of the meibomian glands, eyelid telangiectasias, and abnormal meibum quality may also be present.Citation30
Point-of-care tests can be used to evaluate subclinical markers of DE, namely, ocular surface inflammation and tear osmolarity. Ocular surface inflammation is assessed by measuring the levels of matrix metallopeptidase (MMP)-9, a protease produced by the epithelial cells in response to inflammatory stress (Inflammadry; Quantel, San Diego, CA, USA).Citation31 Tear film osmolarity is measured with a portable osmometer (TearLAB, San Diego, CA, USA).Citation32,Citation33
Lacrimal functional unit and DE pathophysiology
The lacrimal functional unit (LFU) is composed of the eyelids, lacrimal glands (main and accessory), meibomian glands, cornea, conjunctiva, and their accompanying sensory, autonomic, and motor innervation.Citation34,Citation35 The LFU has three major functions as follows:
to maintain a pure optical surface for light refraction;Citation36,Citation37
to maintain ocular surface homeostasis;Citation35 and
to upregulate and downregulate immune response as needed.Citation38
The LFU accomplishes these goals by regulation of neuronal and hormonal pathways that ultimately control tear flow and quality.Citation35 In a commonly presented schematic, the tear film is statically illustrated as having three layers – an outer lipid layer, a middle aqueous layer, and an inner mucinous layer. The reality, however, is likely more complex as dynamic interactions have been demonstrated between lipids (phospholipids, fatty acids, and cholesterol) in meibum and proteins (lipocalin) in the aqueous layer.Citation39,Citation40
Despite the complexity, it is generally agreed that lipids are produced by meibomian glands located in the tarsal plate.Citation41 They drain a clear oil called meibum that helps reduce the rate of evaporation of the aqueous layer.Citation42
The aqueous layer is the largest component of the tear film and is produced by the lacrimal glands (main and accessory) with smaller contributions from the conjunctival epithelium.Citation43 It is mostly composed of salts that maintain an adequate osmolar gradient and proteins with a wide array of functions. These include growth factors (eg, epidermal growth factor, hepatocyte growth factor, platelet-derived growth factor)Citation44 and defense proteins (eg, lactoferrin, lysozyme, immunoglobulin A)Citation45 that are important for ocular surface repair and immunologic defenses. Anti-inflammatory proteins are also found, including transforming growth factor (TGF)-β, which suppresses antigen-presenting cells (APCs), and tissue inhibitor metalloproteinases (TIMP-1 and TIMP-2), which suppress corneal neovascularization and limit inflammatory cell trafficking into the ocular surface.Citation46–Citation48 Anti-inflammatory omega-3–derived lipids (lipoxins, resolvins, and neuroprotectin) are released into the tear film by resident regulatory polymorphonuclear neutrophils, corneal epithelial cells, and the lacrimal gland.Citation49–Citation54
The mucin layer is produced by conjunctival goblet cells (GCs) which secrete a large gel-forming mucin (MUC5AC) with contributions by the lacrimal glands producing a smaller and soluble mucin (MUC7).Citation55 The mucin layer interacts with the glycocalyx of ocular surface epithelial cells, forming a dense barrier particularly against pathogens, thereby playing an important role in mucosal immunotolerance.Citation55,Citation56 In addition, the mucin layer serves to trap debris and sloughed off epithelial cells as well as facilitates sliding of the eyelid against the corneal and conjunctival epithelium during blinking.Citation57,Citation58
A unifying mechanism in DE is the disruption of one or more components of the LFU, leading to tear film instability and impairment of one or more of its three main functions. The loss of a pure optical surface can lead to aberrant light refraction which can create various visual phenomena.Citation24,Citation37 Tear film instability can give rise to a hyperosmolar tear film either by rapid evaporation of the aqueous component or by normal evaporation of a reduced aqueous component.Citation59 This creates a hostile environment for ocular surface epithelial cells which respond by undergoing apoptosis and releasing proinflammatory mediators as they are injured.Citation60,Citation61 Inflammation is amplified by the loss of anti-inflammatory mechanisms of the LFU.Citation38 Persistent inflammation further damages the ocular surface and can impact sensory nerves, thus dampening reflex tear secretion and causing further tear film instability.Citation1 A feedback cycle can ensue with chronic inflammation leading to more ocular surface damage and, thus, more inflammation.
Inflammation in animal models of DE
Ocular surface inflammation begins with a rapid, but nonspecific innate immune response to stress. In mice, hyperosmolar stress on the ocular surface triggered the release of inflammatory mediators such as interleukins (IL)-1β, tumor necrosis factor (TNF)-α, and MMP-9, which initiate the mitogen-activated protein kinase (MAPK) signaling pathways c-jun n-terminal kinases, extracellular-regulated kinases, and p38.Citation62 In a similar manner, desiccating stress (DS), created by scopolamine plus low humidity, leads to the induction of a wide variety of inflammatory mediators on the ocular surface, including IL-1α, IL-1β, CC chemokine ligand (CCL)2, CCL3, CCL5, C-X-C motif chemokine ligand (CXCL)10, TNF-α, interferon (IFN)-γ, IL-2, IL-6, IL-10, and MMP-9. Again, this inflammatory milieu was found to activate the MAPK signaling pathways.Citation63,Citation64 Interestingly, aging mice were found to spontaneously develop ocular surface inflammation, with elevated IFN-γ and IL-7α on the ocular surface.Citation65
This innate immune response gives way to a slower, but more specific adaptive immune response requiring a complex interaction between APCs, namely, macrophages and dendritic cells (DCs), and CD4+ T cells. In a study by Schaumburg et al,Citation66 acute cytokine production from the initial innate immune response phase of DS-induced DE mice was associated with an increased number of CD11c+ DCs and increased expression of DC maturation markers (major histocompatibility complex II, CD83, CD86, C-C motif receptor 7) which preceded activation of CD4+ T cells.
Once activated by APCs, CD4+ T cells infiltrate and damage the ocular surface with corneal irregularity, corneal barrier disruption, and decreased conjunctival GCs noted.Citation67 The specific antigen(s) targeted by these pathogenic CD4+ T cells remains unknown.
One possibility is that T cell response is aimed at self-antigens of the cornea and conjunctiva. In fact, adoptive transfer of CD4+ T cells from DS-induced DE mice to nude mice was enough to reproduce disease with CD4+ T cells localizing to the lacrimal gland, cornea, and conjunctiva in the nude mice.Citation67 Adoptive transfer of CD4+ T cells from aging mice with signs of DE was similarly effective in replicating disease.Citation65 The important interplay between the APCs and T cells became evident as APC depletion prior to DS induction mitigated the ability of T cells to recreate the disease in nude mice recipients.Citation66
T regulatory cells (Tregs) also play an important role as inflammatory modulators in DE mice. Adoptive transfer of pathogenic CD4+ T cells from DS-induced DE mice to nude mice is effectively attenuated by reconstitution of Treg cells in nude recipient mice.Citation67–Citation69 In DE mice, Treg dysfunction contributes to pathogenesis as Tregs from DS-induced DE mice were less capable of suppressing proliferation of T helper (Th)17 cells, leading to higher expression of IL-17 and higher number of Th17 cells in regional lymph nodes.Citation70
Another possibility is that T cell response is aimed at harmless exogenous antigens as part of a derailed immunologic response in the setting of dysfunctional mucosal immunotolerance.Citation56 Guzman et al studied the role of mucosal tolerance in DS-induced DE mice.Citation71 Ocular exposure to ovalbumin (OVA) antigen in wild-type (WT) mice led to immunotolerance demonstrated by reduced in vitro antigen-specific T cell proliferation and in vivo delayed-type hypersensitivity (DTH) response to OVA immunization. Immunotolerance was also retained early on in DS-induced DE mice when they were exposed to ocular OVA on day 1 of DS. However, when OVA instillation was applied on day 4 of DS, DS-induced DE mice exhibited loss of immunotolerance with elevated in vitro antigen-specific T cell proliferation and in vivo DTH response to OVA immunization, suggesting time-dependent deterioration of ocular mucosal tolerance. Inhibition of NF-kB, a key regulator of mucosal tolerance, restored mucosal tolerance and decreased corneal staining and inflammatory markers (IL-1B and IL-6) in DS-induced DE mice. Similarly, time-dependent mucosal tolerance loss and mitigation of corneal damage were demonstrated in a DE mice model created by resection of extraorbital lacrimal glands.Citation72
Loss of GCs is often noted in DE models and may contribute to mucosal immunotolerance loss, given their critical role in modulating antigen distribution and exposure to adjacent APCs.Citation56,Citation67,Citation73,Citation74 Barbosa et alCitation73 studied the role of GCs in immune tolerance using SAM pointed domain ETS transcription factor knockout (Spdef−/−) mice, a DE mice model that lacks GCs and exhibits signs of DE. In WT mice, topically applied antigen OVA was effectively delivered to the stroma through GC-associated passages for uptake by adjacent APCs (CD11b+ F4/80+ macrophages), while Spdef−/− mice retained OVA within the epithelium. APCs isolated from conjunctival draining cervical lymph nodes of Spdef−/− mice showed stronger induction of antigen-specific lymphoproliferation, greater IFN-γ production, and lesser Treg proliferation, compared to WT. These findings were consistent with the loss of immune tolerance observed in Spdef−/− mice compared to WT, as assessed by cutaneous DTH to OVA following immunization with complete Freund’s adjuvant mixed with OVA.
In addition to regulating antigen exposure to DCs, GCs play a role in modulation of DCs phenotype. Studies by Contreras-Ruiz and Masli revealed that GCs TSP-1–dependent expression of TGF-β, particularly the TGF-β2 isoform, plays a role in modulation of DC phenotype.Citation75 When DCs were co-cultured with WT globet cells from mice, they were found to have reduced major histocompatibility complex II and costimulatory molecules (CD80 and CD86) expression, compared to cultures of DCs alone. This effect was dependent on TSP-1 expression by GCs.
Irrespective of whether T cell activation is triggered by self-antigens or exogenous antigens, T cell migration to the ocular surface is a key event in DE and is driven by a variety of inflammatory cytokines and chemokines as well as other proteins. MRL/lpr mice are homozygous for the recessive lpr (lymphoproliferative) gene and used as Sjögren’s syndrome DE models.Citation76 These mice have high expression of intercellular adhesion molecule (ICAM)-1, an adhesion molecule important for homing and activation of infiltrating lymphocytes, in the conjunctival epithelium and vascular endothelium along with infiltrating lymphocytes within the lacrimal gland tissue.Citation77 In fact, ICAM-1 expression is positively correlated with disease progression and severity.Citation77
The importance of these inflammatory mediators is further highlighted by mice studies showing improvement of DE with the use of anti-inflammatory therapy targeting IL-1,Citation78 IL-17,Citation70,Citation79 and C-C motif receptor 2.Citation80 Furthermore, T cell infiltration decreased with the use of monoclonal antibodies against ICAM-1 and its receptor lymphocyte functional associated antigen-1 (LFA-1).Citation77
Inflammation in humans with DE
Hyperosmolarity is a known trigger of inflammation. Cell culture experiments demonstrated increased proinflammatory mediators (eg, IL-1, IL-6, TNF-α, and MMP-9) after human corneal epithelium was subjected to hyperosmolar stress.Citation81,Citation82 Similar results were noted in limbal epithelial cells with elevated levels of IL-1β, IL-8, and TNF-α found through the c-jun n-terminal kinase and extracellular-regulated kinase MAPK signaling pathway.Citation61
Similar to mice, patients with DE (defined in a number of different ways) have increased tear levels of proinflammatory cytokines, chemokines, and chemokine receptors including IL-2, IL-4, IL-5, IL-6, IL-8, IL-10, IL-12, IL-13, IL-17, IL-21, IFN-γ, TNF-α, CXCL9, CXCL10, CXCL11, CXCR3, IL-1Ra, IP-10/CXCL10, fractalkine/CX3CL1, CCL-5, and vascular endothelial growth factor.Citation83–Citation90 In fact, levels of IL-6, IL-17, IL-21, TNF-α, CXCL-9, CXCL-10, and CXCL-11 were found to be positively correlated with DE signs and symptoms (eg, Schirmer test, tear-film breakup time, punctate epithelial erosions, GC density, OSDI).Citation88,Citation90–Citation92
Patients with DE have other features in common with mouse models, including T cell infiltration of the ocular surface and elevated ICAM expression. Stern et alCitation93 studied patients with moderate to severe ADDE and found CD4+ T cell infiltration in the conjunctiva, along with increased markers of immune activation (HLA-DR and HLA-DQ). ADDE patients were also found to have increased ICAM-1 expression in the conjunctival epithelium and lacrimal gland venule endothelium, near CD4+ T cells.Citation77,Citation93–Citation95
Inflammation in DE can also affect the corneal nerves, disrupting normal tearing reflex and blink rate.Citation96 Many studies using in vivo confocal microscope, a minimally invasive tool for imaging ocular surface, have shown decreased sub-basal nerve density of the corneaCitation97–Citation103 and morphologic changes of the sub-basal nerves (increased tortuosity, nerve sprouting, increased bead-like formation, and decreased reflectivity) in DE patients.Citation97–Citation99,Citation101,Citation104,Citation105 Additionally, DE patients generally show higher DC density and morphologic changes including larger DC size and additional dendrites at the sub-basal nerve plexus.Citation98,Citation100,Citation104,Citation106,Citation107 A few studies have attempted to correlate in vivo confocal microscope parameters to DE signs and symptoms. Decreased sub-basal nerve density is correlated to decreased corneal sensitivity,Citation99,Citation102 increased DC density at the sub-basal nerve plexus,Citation98 and increased DE symptoms and signs.Citation97,Citation98,Citation108 On the other hand, studies correlating DC density to DE signs and symptoms have yielded conflicting results.Citation100,Citation107,Citation109
Treatment modalities: targeting inflammation
Corticosteroids
Mechanism of action
Topical corticosteroids decrease inflammation primarily by binding to glucocorticoid receptors and regulating the expression of anti-inflammatory and proinflammatory genes.Citation110 Specifically, corticosteroids suppress NF-kB, a key transcription factor in inflammation, leading to suppression of proinflammatory mediators and promoting lymphocyte apoptosis. There is a wide range of proinflammatory mediators that are suppressed by corticosteroids, including ICAM-1, MMPs, prostaglandins, cytokines, chemokines, and phospholipase A2. Non-genomic effects of corticosteroids also aid in suppressing leukocyte infiltration into areas of inflammation.Citation111
Clinical data in DE
Topical corticosteroids have proven effective in the treatment of DE in several studies.Citation112–Citation116 One of these studies included a multicenter, randomized, double-masked, placebo-controlled clinical trial with 64 moderate to severe ADDE patients receiving either 0.5% loteprednol or placebo four times a day for 4 weeks.Citation115 This study evaluated symptoms on a visual analog scale and signs by corneal staining, conjunctival injection, and lid margin injection. At 2 weeks, treatment with 0.5% loteprednol improved the signs of DE, including lid margin injection and conjunctival injection. Furthermore, in a subset analysis of patients with moderate clinical inflammatory component, treatment with 0.5% loteprednol improved corneal staining, conjunctival injection, and DE symptoms (eye redness).
Side effects
The use of corticosteroids is limited due to a wide range of side effects including glaucoma, ocular infection, corneal thinning, and formation of cataracts.Citation117 Given the chronic nature of DE, topical corticosteroid use tends to be limited to short-term treatment of DE exacerbations.
Cyclosporine
Mechanism of action
Cyclosporine is FDA approved for the treatment of DE signs. It suppresses inflammation by binding to cyclophilins and inhibiting calcineurin, a calcium-dependent phosphatase, thereby preventing nuclear factor of activated T-cells, cytoplasmic 1 (NFATc1) dephosphorylation which reduces IL-2 levels suppressing T cell activation.Citation118
Formulation
Cyclosporine requires suspension and emulsion forms due to its poor solubility in water. Restasis is formulated with 0.05% cyclosporine in an emulsion of glycerin (2.2%), castor oil (1.25%), polysorbate 80 (1.00%), carbomer copolymer type A (0.05%), purified water (to 100%), and sodium hydroxide for pH adjustment.Citation119
Clinical data in DE
Studies with topical cyclosporine treatment in patients with moderate to severe DE generally showed significant improvement of DE signs and variable improvement of DE symptoms.Citation120 Large multicenter, randomized, double-blind, controlled studies with moderate to severe DE patients have shown improvement in DE signs (corneal staining, tear production) over emulsion placebo.Citation121–Citation123 Symptomatic improvement in these studies was more variable, with the most consistent improvement found in reports of “dryness” symptoms.Citation121–Citation124 Similarly, smaller prospective randomized studies of moderate to severe DE patients treated with cyclosporine or combinations of cyclosporine (cyclosporine plus artificial tears [AT] or cyclosporine plus vitamin A) consistently showed improvement in DE signs (corneal staining, conjunctival staining, GC density, tear production, tear film stability)Citation125–Citation131 and, in some cases, DE symptoms (OSDI, blurry vision, burning, and photophobia).Citation125,Citation127–Citation129
Side effects
Cyclosporine use is limited by its side effects of mild to moderate burning and irritation of the eye.Citation123 Tolerance is improved with the use of concomitant topical corticosteroids.Citation132,Citation133
Lifitegrast
Mechanism of action
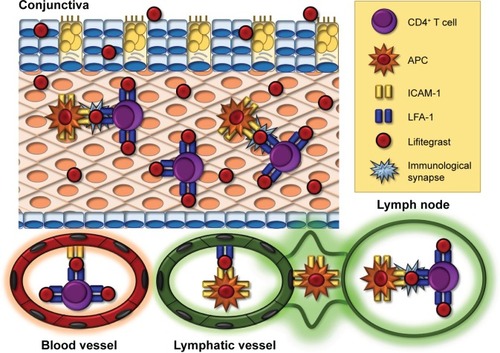
Lifitegrast (formerly SAR1118) is the only FDA-approved treatment for both signs and symptoms of DE. Lifitegrast suppresses inflammation by mimicking ICAM-1, thus blocking the interaction between ICAM-1 and LFA-1, a cell surface protein belonging to the β2 family of integrins and found on leukocytes ().Citation134,Citation135 LFA-1/ICAM-1 interaction has been implicated in various aspects of lymphocyte activation and migration.Citation136 It promotes migration of DC to lymph nodes where they can activate naïve T cells.Citation137,Citation138 It also plays a direct role in T cell activation by maintaining initial contact between naïve T cells and APCs in lymph nodesCitation139 and enhancing T cell sensitivity to antigens by stabilization of the immunologic synapse between naïve T cells and APCs in the lymph nodes.Citation140–Citation142 Beyond T cell activation, LFA-1/ICAM-1 interaction also plays a role in T cell migration by allowing firm adhesion to the endothelium with subsequent transmigration into inflamed tissue.Citation143,Citation144 By blocking the interaction between LFA-1 and ICAM-1, lifitegrast is able to disrupt various key steps in T cell–mediated inflammation.
Figure 1 Mechanism of action of lifitegrast.
Abbreviations: APCs, antigen-presenting cells; DC, dendritic cell; ICAM-1, intercellular adhesion molecule; LFA-1, lymphocyte functional associated antigen-1.
Formulation
Lifitegrast is prepared in PBS with a pH, tonicity, and osmolarity range consistent with other currently approved topical ophthalmic solutions.Citation145 It is preservative-free and comes in single-unit dose ampules.Citation145
Animal studies with lifitegrast in non-DE conditions
A few animal studies have shown the anti-inflammatory effect of lifitegrast in various inflammatory-based ocular diseases. A study by Sun et al tested the efficacy of lifitegrast in inhibiting inflammation in mice with corneal inflammation induced by antibiotic-killed Pseudomonas aeruginosa and Staphylococcus aureus in the presence of contact lenses. Lifitegrast reduced neutrophilic infiltration into the cornea, reduced clinical signs of corneal inflammation, and prevented P. aeruginosa- and S. aureus- induced inflammation compared to controls.Citation146 In rat streptozotocin model of diabetic retinopathy, lifitegrast was able to significantly reduce the number of adherent leukocytes and level of myeloperoxidase, a leukocyte-derived proinflammatory protein, to levels comparable to those of normal retina.Citation147 Lifitegrast also significantly reduced blood–retinal barrier breakdown, compared to vehicle-control.Citation147
Animal studies with lifitegrast in DE
A study of 10 idiopathic keratoconjunctivitis sicca dogs receiving 1% lifitegrast three times a day for 12 weeks showed a significant increase in tear production from baseline for 11 of 18 study eyes, which corresponded with a decreased inflammatory cell infiltrate of the conjunctiva from baseline.
Human studies with lifitegrast
In vitro studies demonstrate lifitegrast’s ability to inhibit human T cell binding to ICAM-1 and inhibit the release of proinflammatory cytokines including IFN-γ, TNF-α, macrophage inflammatory protein-1α, IL-1α, IL-1β, IL-2, IL-4, and IL-6 from activated lymphocytes.Citation148 Additionally, imaging studies reveal inhibition of immunologic synapse formation in the presence of lifitegrast.Citation149
The clinical efficacy of lifitegrast in the treatment of signs and symptoms of DE has been demonstrated across four prospective, multicenter, randomized, double-masked, vehicle-controlled trials (). The first of these trials was a phase II clinical trial of 230 patients with moderate to severe DE receiving either 0.1%, 1%, or 5% lifitegrast vs vehicle twice a day for 84 days.Citation150 Key eligibility criteria included adult DE patients with corneal staining score ≥2, un-anesthetized Schirmer >1 and <10, no active lid margin disease, and worsening of signs (inferior corneal staining score [ICSS] increase >1 point) and symptoms (ocular discomfort score [ODS] increase >1 point) in response to controlled adverse environment (CAE), an environment with specified humidity, temperature, airflow, and lighting designed to worsen DE. The use of topical cyclosporine and AT was prohibited 6 weeks and 3 days from visit 1, respectively. Three visits occurred during the treatment period at days 14, 42, and 84 of treatment during which patients were exposed to additional 90-minute sessions of CAE. Lifitegrast (1% and 5%) showed significant improvement in ICSS (primary sign endpoint) at 84 days from baseline, compared to vehicle. This study also showed improvement in tear production with 5% lifitegrast at 14 days from baseline, compared to vehicle. In terms of symptoms, all lifitegrast groups showed significant improvement in the OSDI at 14 days and 1% and 5% lifitegrast showed significant improvement in the vision-related subscale score of OSDI at 14 and 84 days from baseline, compared to vehicle. Baseline ODS showed trends toward improvement, but was not significantly different from vehicle.
Table 1 Summary of studies using LIF for the treatment of dry eye
This was followed by the first Phase III trial, OPUS-1, consisting of 588 patients with moderate to severe DE receiving 5% lifitegrast vs vehicle twice a day for 84 days.Citation151 Unlike the previously described Phase II study, CAE was only used during screening visits and not during the in-treatment visits. Once again, lifitegrast significantly improved ICSS (primary end point) at 84 days from baseline, compared to vehicle. Additional improvements in DE signs over vehicle included superior corneal staining, total corneal staining, nasal conjunctival staining, and conjunctival staining, with some of these changes starting as early as at 14 days of treatment. Lifitegrast significantly improved DE symptoms over vehicle, including decreased ODS and visual analog eye dryness score (VAS-EDS); however, this study failed to show significant improvement in vision-related subscale score of OSDI (primary symptom end point) over vehicle.
The next Phase III trial was OPUS-2 consisting of 718 patients with moderate to severe DE receiving 5% lifitegrast vs vehicle twice a day for 84 days.Citation152 Study methods were similar to the previous two studies; however, no CAE was used in this study and AT use within 30 days and VASEDS ≥40 were added to the inclusion criteria based on post hoc analysis from OPUS-1, suggesting that the drug effect is increased in patients with recent AT use and VAS-EDS ≥40. In this study, primary symptom end point was met as lifitegrast showed significant improvement from baseline in VAS-EDS as early as 14 days and continuing to 84 days, over vehicle. Additionally, lifitegrast was superior in decreasing ODS at 84 days from baseline, compared to vehicle. Primary sign end point of ICSS showed improvement from baseline, but did not reach significant difference compared to vehicle. The authors hypothesize that the lack of ICSS improvement was related to patients’ recent AT use which hindered detection of drug effect, or the enrollment of more severe DE patients with more advanced ocular surface damage.
The final phase III trial was OPUS-3 consisting of 711 patients with moderate to severe DE receiving 5% lifitegrast vs vehicle twice a day for 84 days.Citation153 Study methods were similar to those of OPUS-2. This study also met its primary symptom end point of VAS-EDS improvement from baseline compared to vehicle starting at 14 days and continuing to 84 days. Other symptoms significantly improved with the use of lifitegrast over vehicle, including visual analog scale for itching, foreign body sensation, and eye discomfort at 42 days of treatment.
Safety studies with lifitegrast
The safety profile of lifitegrast has been explored in several dose-escalation tolerance studies (). Dose-escalation tolerance studies using healthy dogs with a maximum dosage of 10% lifitegrast administered three times per day for 1 month did not demonstrate any adverse effect on the ocular surface.Citation148 Similarly, dose-escalation tolerance assessment with a maximum dosage of 3% lifitegrast administered three times per day for 13 weeks did not show any adverse effect on the ocular surface, but was associated with a transient period of blinking and squinting upon drop administration, which appeared to improve after the first few days of instillation.Citation148
Table 2 Summary of safety studies using LIF for the treatment of dry eye
Short-term dose-escalation tolerance studies of healthy individuals and patients undergoing pars plana vitrectomies have tested lifitegrast in dosages ranging from 0.1% to 5% at frequencies as high as three times per day.Citation154,Citation155 In these studies, adverse events from lifitegrast were mild to moderate in severity and consisted of transient ocular irritation and ocular hyperemia. Non-ocular adverse events included dysgeusia, headaches, erythema, and musculoskeletal pain. No effects were seen in intraocular pressure, visual acuity, ocular surface exams, healing time, and hematologic and chemistry panels. SONATA was a multicenter, randomized, double-blind, vehicle-controlled trial that was conducted to assess the long-term safety profile of lifitegrast 5% vs vehicle twice daily for 1 year in 331 DE patients.Citation156 Eligibility criteria included Schirmer tear test result ≥1 and ≤10 mm in 5 minutes, no active lid margin disease, corneal staining score ≥2.0, EDS ≥40, and use and/or desire to use AT in the past 6 months. Patients receiving lifitegrast had a higher percentage of treatment-emergent adverse effects (TEAEs; 53.6% lifitegrast vs 34.2% vehicle), most of which were mostly transient and mild to moderate in severity. TEAEs included instillation site irritation (15.0% lifitegrast vs 4.5% vehicle), instillation site reaction (13.2% lifitegrast vs 1.8% vehicle), reduction in visual acuity (11.4% lifitegrast vs 6.3% vehicle), DE (1.8% lifitegrast vs 5.4% vehicle), and dysgeusia (16.4% lifitegrast vs 1.8% vehicle). Drop comfort improved at each visit. Dysgeusia was likely the result of tear drainage into the oropharynx, while decreased visual acuity may have been due to transient alterations in the tear film leading to altered refractive properties. Despite the decreased visual acuity, changes in best-corrected visual acuity from baseline to 1 year were minimal in both groups. No serious ocular adverse events occurred in this study. All non-ocular TEAEs, except for dysgeusia, were considered to be unrelated to lifitegrast. There was no evidence of immunosuppression as per CD3, CD4 and CD8 serum levels and no alterations in hematologic, renal and liver panels. Concomitant AT use appeared to increase the rates of ocular TEAEs in both groups, but was associated with lower discontinuation rates due to TEAEs in both groups.
Conclusion and remaining questions
Evidence suggests that ocular surface inflammation is an important component of DE. Stress to the ocular surface stimulates production of proinflammatory mediators inducing maturation of resident APCs that migrate to the lymph nodes to activate autoreactive CD4+ T cells which migrate to the ocular surface causing more inflammation and damage. Lifitegrast blocks the interaction between ICAM-1 and LFA-1, which has been shown to be critical for APC migration to the lymph nodes as well as CD4+ T cell activation and migration to the ocular surface. In four large multicenter, randomized controlled trials, lifitegrast has proven to be effective at controlling both the signs and symptoms of DE with minimal side effects.
Despite its success, many questions remain. It is known that not all individuals with DE symptoms have detectable levels of inflammation as measured by ocular surface MMP-9 levels.Citation157 DE patients with ADDE are more likely to have inflammation, especially in the setting of systemic inflammation such as Sjögren’s syndrome and graft vs host disease.Citation157 Could these patients also be more likely to respond to anti-inflammatory therapy such as lifitegrast?
Another question is whether anti-inflammatory therapies for DE could work better in combination. While studies of topical cyclosporine and methylprednisolone combinations showed faster symptomatic relief, at this time, no combination studies have been done with lifitegrast. Additionally, no comparative studies have been done with lifitegrast to determine if one anti-inflammatory agent could be better than the others. These are all important avenues of future investigation.
Of note, we focused our review on the inflammatory component of DE, given our focus on lifitegrast as a new therapeutic modality in DE. However, it is known that not all patients with DE symptoms have ocular surface inflammation (as measured by Inflammadry).Citation157 Other contributors to DE include MGD and resultant EDE.Citation3 The role of inflammation in the initiation and propagation of MGD is less well clarified.Citation158 Furthermore, many other exposures contribute to DE, which do not clearly fit into the autoreactive and/or loss of mucosal tolerance story. These include environmental exposures such as air pollution and low humidity,Citation159 dietary patterns such as a high consumption of free fatty acids,Citation160 and psychosocial considerations such as depression and chronic widespread pain, to name a few.Citation161 The role of inflammation, along with the other contributors, needs to be considered when evaluating a patient with DE.
Acknowledgments
This study was supported by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Clinical Sciences Research EPID-006-15S (Dr Galor), R01EY026174 (Dr Galor), NIH Center Core Grant P30EY014801, and Research to Prevent Blindness Unrestricted Grant.
Disclosure
The authors report no conflicts of interest in this work.
References
- The epidemiology of dry eye disease: report of the Epidemiology Subcommittee of the International Dry Eye WorkShop (2007)Ocul Surf2007529310717508117
- PouyehBViteriEFeuerWImpact of ocular surface symptoms on quality of life in a United States veterans affairs populationAm J Ophthalmol201215361061.e31066.e322330309
- CraigJPNicholsKKAkpekEKTFOS DEWS II definition and classification reportOcul Surf201715327628328736335
- SternMESchaumburgCSPflugfelderSCDry eye as a mucosal autoimmune diseaseInt Rev Immunol2013321194123360156
- GodinMRGuptaPKLifitegrast ophthalmic solution in the treatment of signs and symptoms of dry eye disease: design, development, and place in therapyClin Ophthalmol20171195195728579745
- LempMACrewsLABronAJFoulksGNSullivanBDDistribution of aqueous-deficient and evaporative dry eye in a clinic-based patient cohort: a retrospective studyCornea201231547247822378109
- SchaumbergDASullivanDABuringJEDanaMRPrevalence of dry eye syndrome among US womenAm J Ophthalmol2003136231832612888056
- SullivanDATearful relationships? Sex, hormones, the lacrimal gland, and aqueous-deficient dry eyeOcul Surf2004229212317216082
- MossSEKleinRKleinBEPrevalence of and risk factors for dry eye syndromeArch Ophthalmol200011891264126810980773
- GalorAFeuerWLeeDJDepression, post-traumatic stress disorder, and dry eye syndrome: a study utilizing the national United States Veterans Affairs administrative databaseAm J Ophthalmol20121542340.e2346.e222541654
- YuEYLeungARaoSLamDSEffect of laser in situ keratomileusis on tear stabilityOphthalmology2000107122131213511097583
- KasetsuwanNSatitpitakulVChangulTJariyakosolSIncidence and pattern of dry eye after cataract surgeryPLoS One2013811e7865724265705
- BuchholzPSteedsCSSternLSUtility assessment to measure the impact of dry eye diseaseOcul Surf20064315516116900272
- SchiffmanRMWaltJGJacobsenGDoyleJJLebovicsGSumnerWUtility assessment among patients with dry eye diseaseOphthalmology200311071412141912867401
- DeschampsNRicaudXRabutGLabbeABaudouinCDenoyerAThe impact of dry eye disease on visual performance while drivingAm J Ophthalmol20131561184.e3189.e323706501
- RidderWH3rdZhangYHuangJFEvaluation of reading speed and contrast sensitivity in dry eye diseaseOptom Vis Sci2013901374423222922
- MiljanovićBDanaRSullivanDASchaumbergDAImpact of dry eye syndrome on vision-related quality of lifeAm J Ophthalmol20071433409.e2415.e217317388
- LiMGongLSunXChapinWJAnxiety and depression in patients with dry eye syndromeCurr Eye Res20113611721174591
- KimKWHanSBHanERAssociation between depression and dry eye disease in an elderly populationInvest Ophthalmol Vis Sci201152117954795821896858
- LeQZhouXGeLWuLHongJXuJImpact of dry eye syndrome on vision-related quality of life in a non-clinic-based general populationBMC Ophthalmol2012122222799274
- McDonaldMPatelDAKeithMSSnedecorSJEconomic and humanistic burden of dry eye disease in Europe, North America, and Asia: a systematic literature reviewOcul Surf201614214416726733111
- YuJAscheCVFairchildCJThe economic burden of dry eye disease in the United States: a decision tree analysisCornea201130437938721045640
- NicholsKKNicholsJJMitchellGLThe lack of association between signs and symptoms in patients with dry eye diseaseCornea200423876277015502475
- GotoEYagiYMatsumotoYTsubotaKImpaired functional visual acuity of dry eye patientsAm J Ophthalmol2002133218118611812420
- ChalmersRLBegleyCGCafferyBValidation of the 5-Item Dry Eye Questionnaire (DEQ-5): Discrimination across self-assessed severity and aqueous tear deficient dry eye diagnosesCont Lens Anterior Eye2010332556020093066
- SchiffmanRMChristiansonMDJacobsenGHirschJDReisBLReliability and validity of the ocular surface disease indexArch Ophthalmol2000118561562110815152
- AbetzLRajagopalanKMertzanisPBegleyCBarnesRChalmersRDevelopment and validation of the impact of dry eye on everyday life (IDEEL) questionnaire, a patient-reported outcomes (PRO) measure for the assessment of the burden of dry eye on patientsHealth Qual Life Outcomes2011911122152125
- MangioneCMLeePPGutierrezPRDevelopment of the 25-item National Eye Institute Visual Function QuestionnaireArch Ophthalmol200111971050105811448327
- NicholsKKMitchellGLZadnikKPerformance and repeatability of the NEI-VFQ-25 in patients with dry eyeCornea200221657858312131034
- MessmerEMThe pathophysiology, diagnosis, and treatment of dry eye diseaseDtsch Arztebl Int201511257181 quiz 225686388
- LanzaNLValenzuelaFPerezVLGalorAThe matrix metalloproteinase 9 point-of-care test in dry eyeOcul Surf201614218919526850527
- MessmerEMBulgenMKampikAHyperosmolarity of the tear film in dry eye syndromeDev Ophthalmol20104512913820502033
- LempMABronAJBaudouinCTear osmolarity in the diagnosis and management of dry eye diseaseAm J Ophthalmol20111515792.e1798.e121310379
- SternMEBeuermanRWFoxRIGaoJMircheffAKPflugfelderSCThe pathology of dry eye: the interaction between the ocular surface and lacrimal glandsCornea19981765845899820935
- SternMEGaoJSiemaskoKFBeuermanRWPflugfelderSCThe role of the lacrimal functional unit in the pathophysiology of dry eyeExp Eye Res200478340941615106920
- DursunDMonroyDKnightonRThe effects of experimental tear film removal on corneal surface regularity and barrier functionOphthalmology200010791754176010964840
- LiuZPflugfelderSCCorneal surface regularity and the effect of artificial tears in aqueous tear deficiencyOphthalmology1999106593994310328393
- BarabinoSChenYChauhanSDanaROcular surface immunity: homeostatic mechanisms and their disruption in dry eye diseaseProg Retin Eye Res201231327128522426080
- DeanAWGlasgowBJMass spectrometric identification of phospholipids in human tears and tear lipocalinInvest Ophthalmol Vis Sci20125341773178222395887
- YehPTCaseyRGlasgowBJA novel fluorescent lipid probe for dry eye: retrieval by tear lipocalin in humansInvest Ophthalmol Vis Sci20135421398141023361507
- McCulleyJPShineWEThe lipid layer of tears: dependent on meibomian gland functionExp Eye Res200478336136515106913
- BronAJTiffanyJMGouveiaSMYokoiNVoonLWFunctional aspects of the tear film lipid layerExp Eye Res200478334736015106912
- HollyFJLempMATear physiology and dry eyesSurv Ophthalmol19772226987335548
- KlenklerBSheardownHJonesLGrowth factors in the tear film: role in tissue maintenance, wound healing, and ocular pathologyOcul Surf20075322823917660896
- GarreisFGottschaltMPaulsenFPAntimicrobial peptides as a major part of the innate immune defense at the ocular surfaceDev Ophthalmol201045162220502023
- SackRAConradiLKrumholzDBeatonASatheSMorrisCMembrane array characterization of 80 chemokines, cytokines, and growth factors in open- and closed-eye tears: angiogenin and other defense system constituentsInvest Ophthalmol Vis Sci20054641228123815790883
- WalterSDGronertKMcClellanALLevittRCSarantopoulosKDGalorAω-3 tear film lipids correlate with clinical measures of dry eyeInvest Ophthalmol Vis Sci20165762472247827138739
- GuptaAMonroyDJiZYoshinoKHuangAPflugfelderSCTransforming growth factor beta-1 and beta-2 in human tear fluidCurr Eye Res19961566056148670763
- BitemanBHassanIRWalkerEInterdependence of lipoxin A4 and heme-oxygenase in counter-regulating inflammation during corneal wound healingFASEB J20072192257226617384141
- GaoYMinKJZhangYBSuJGreenwoodMGronertKFemale-specific downregulation of tissue polymorphonuclear neutrophils drives impaired regulatory T cell and amplified effector T cell responses in autoimmune dry eye diseaseJ Immunol201519573086309926324767
- LeedomAJSullivanABDongBLauDGronertKEndogenous LXA4 circuits are determinants of pathological angiogenesis in response to chronic injuryAm J Pathol20101761748420008149
- WangSBHuKMSeamonKJManiVChenYGronertKEstrogen negatively regulates epithelial wound healing and protective lipid mediator circuits in the corneaFASEB J20122641506151622186873
- CortinaMSHeJLiNBazanNGBazanHENeuroprotectin D1 synthesis and corneal nerve regeneration after experimental surgery and treatment with PEDF plus DHAInvest Ophthalmol Vis Sci201051280481019797230
- KenchegowdaSBazanHESignificance of lipid mediators in corneal injury and repairJ Lipid Res201051587989119965607
- GipsonIKArguesoPRole of mucins in the function of the corneal and conjunctival epitheliaInt Rev Cytol200323114914713002
- GallettiJGGuzmanMGiordanoMNMucosal immune tolerance at the ocular surface in health and diseaseImmunology2017150439740728108991
- NornMSDead, degenerate, and living cells in conjunctival fluid and mucous threadActa ophthalmologica1969475110211154190824
- HodgesRRDarttDATear film mucins: front line defenders of the ocular surface; comparison with airway and gastrointestinal tract mucinsExp Eye Res2013117627823954166
- BaudouinCAragonaPMessmerEMRole of hyperosmolarity in the pathogenesis and management of dry eye disease: proceedings of the OCEAN group meetingOcul Surf201311424625824112228
- LuoLLiDQPflugfelderSCHyperosmolarity-induced apoptosis in human corneal epithelial cells is mediated by cytochrome c and MAPK pathwaysCornea200726445246017457195
- LiDQLuoLChenZKimHSSongXJPflugfelderSCJNK and ERK MAP kinases mediate induction of IL-1beta, TNF-alpha and IL-8 following hyperosmolar stress in human limbal epithelial cellsExp Eye Res200682458859616202406
- LuoLLiDQCorralesRMPflugfelderSCHyperosmolar saline is a proinflammatory stress on the mouse ocular surfaceEye Contact Lens200531518619316163009
- RashidSJinYEcoiffierTBarabinoSSchaumbergDADanaMRTopical omega-3 and omega-6 fatty acids for treatment of dry eyeArch Ophthalmol2008126221922518268213
- LuoLLiDQDoshiAFarleyWCorralesRMPflugfelderSCExperimental dry eye stimulates production of inflammatory cytokines and MMP-9 and activates MAPK signaling pathways on the ocular surfaceInvest Ophthalmol Vis Sci200445124293430115557435
- McClellanAJVolpeEAZhangXOcular surface disease and dacryoadenitis in aging C57BL/6 miceAm J Pathol2014184363164324389165
- SchaumburgCSSiemaskoKFDe PaivaCSOcular surface APCs are necessary for autoreactive T cell-mediated experimental autoimmune lacrimal keratoconjunctivitisJ Immunol201118773653366221880984
- NiederkornJYSternMEPflugfelderSCDesiccating stress induces T cell-mediated Sjogren’s Syndrome-like lacrimal keratoconjunctivitisJ Immunol200617673950395716547229
- SiemaskoKFGaoJCalderVLIn vitro expanded CD4+ CD25+ Foxp3+ regulatory T cells maintain a normal phenotype and suppress immune-mediated ocular surface inflammationInvest Ophthalmol Vis Sci200849125434544018658093
- RatayMLGlowackiAJBalmertSCTreg-recruiting microspheres prevent inflammation in a murine model of dry eye diseaseJ Control Release201725820821728501670
- ChauhanSKEl AnnanJEcoiffierTAutoimmunity in dry eye is due to resistance of Th17 to Treg suppressionJ Immunol200918231247125219155469
- GuzmanMKeitelmanISabbioneFTrevaniASGiordanoMNGallettiJGDesiccating stress-induced disruption of ocular surface immune tolerance drives dry eye diseaseClin Exp Immunol2016184224825626690299
- GuzmanMKeitelmanISabbioneFTrevaniASGiordanoMNGallettiJGMucosal tolerance disruption favors disease progression in an extraorbital lacrimal gland excision model of murine dry eyeExp Eye Res2016151192227443502
- BarbosaFLXiaoYBianFGoblet cells contribute to ocular surface immune tolerance-implications for dry eye diseaseInt J Mol Sci2017185113
- McDoleJRWheelerLWMcDonaldKGGoblet cells deliver luminal antigen to CD103+ dendritic cells in the small intestineNature2012483738934534922422267
- Contreras-RuizLMasliSImmunomodulatory cross-talk between conjunctival goblet cells and dendritic cellsPLoS One2015103e012028425793763
- HoffmanRWAlspaughMAWaggieKSDurhamJBWalkerSESjogren’s syndrome in MRL/l and MRL/n miceArthritis Rheum19842721571656421291
- GaoJMorganGTieuDICAM-1 expression predisposes ocular tissues to immune-based inflammation in dry eye patients and Sjogrens syndrome-like MRL/lpr miceExp Eye Res200478482383515037117
- OkanoboAChauhanSKDastjerdiMHKodatiSDanaREfficacy of topical blockade of interleukin-1 in experimental dry eye diseaseAm J Ophthalmol20121541637122541929
- De PaivaCSChotikavanichSPangelinanSBIL-17 disrupts corneal barrier following desiccating stressMucosal Immunol20092324325319242409
- GoyalSChauhanSKZhangQDanaRAmelioration of murine dry eye disease by topical antagonist to chemokine receptor 2Arch Ophthalmol2009127788288719597109
- WangCShiXChenX17-beta-estradiol inhibits hyperosmolarity-induced proinflammatory cytokine elevation via the p38 MAPK pathway in human corneal epithelial cellsMol Vis2012181115112222605923
- LiDQChenZSongXJLuoLPflugfelderSCStimulation of matrix metalloproteinases by hyperosmolarity via a JNK pathway in human corneal epithelial cellsInvest Ophthalmol Vis Sci200445124302431115557436
- LeeSYHanSJNamSMAnalysis of tear cytokines and clinical correlations in Sjogren syndrome dry eye patients and non-Sjogren syndrome dry eye patientsAm J Ophthalmol20131562247.e1253.e123752063
- MeadowsJFDionneKNicholsKKDifferential profiling of T-cell cytokines as measured by protein microarray across dry eye subgroupsCornea201635332933526751989
- LamHBleidenLde PaivaCSFarleyWSternMEPflugfelderSCTear cytokine profiles in dysfunctional tear syndromeAm J Ophthalmol20091472198.e1205.e118992869
- Enriquez-de-SalamancaACastellanosESternMETear cytokine and chemokine analysis and clinical correlations in evaporative-type dry eye diseaseMol Vis20101686287320508732
- HeCLaiPWengJToll-like receptor 2-mediated NF-kappaB inflammatory responses in dry eye associated with cGVHDMol Vis2011172605261122025895
- YoonKCParkCSYouICExpression of CXCL9, −10, −11, and CXCR3 in the tear film and ocular surface of patients with dry eye syndromeInvest Ophthalmol Vis Sci201051264365019850844
- MassingaleMLLiXVallabhajosyulaMChenDWeiYAsbellPAAnalysis of inflammatory cytokines in the tears of dry eye patientsCornea20092891023102719724208
- YoonKCJeongIYParkYGYangSYInterleukin-6 and tumor necrosis factor-alpha levels in tears of patients with dry eye syndromeCornea200726443143717457192
- MrugaczMOstrowskaLBrylASzulcAZelazowska-RutkowskaBMrugaczGPro-inflammatory cytokines associated with clinical severity of dry eye disease of patients with depressionAdv Med Sci201762233834428511072
- LimSANamDHLeeJHKwokSKParkSHChungSHAssociation of IL-21 cytokine with severity of primary Sjogren syndrome dry eyeCornea201534324825225603233
- SternMEGaoJSchwalbTAConjunctival T-cell subpopulations in Sjogren’s and non-Sjogren’s patients with dry eyeInvest Ophthalmol Vis Sci20024382609261412147592
- SaitoITerauchiKShimutaMExpression of cell adhesion molecules in the salivary and lacrimal glands of Sjogren’s syndromeJ Clin Lab Anal1993731801878509947
- JonesDTMonroyDJiZAthertonSSPflugfelderSCSjogren’s syndrome: cytokine and Epstein-Barr viral gene expression within the conjunctival epitheliumInvest Ophthalmol Vis Sci1994359349335048056525
- DarttDADysfunctional neural regulation of lacrimal gland secretion and its role in the pathogenesis of dry eye syndromesOcul Surf200422769117216081
- VillaniEGalimbertiDViolaFMapelliCRatigliaRThe cornea in Sjogren’s syndrome: an in vivo confocal studyInvest Ophthalmol Vis Sci20074852017202217460255
- TepelusTCChiuGBHuangJCorrelation between corneal innervation and inflammation evaluated with confocal microscopy and symptomatology in patients with dry eye syndromes: a preliminary studyGraefes Arch Clin Exp Ophthalmol Epub2017520
- LabbeALiangQWangZCorneal nerve structure and function in patients with non-sjogren dry eye: clinical correlationsInvest Ophthalmol Vis Sci20135485144515023833066
- ShettyRSethuSDeshmukhRCorneal dendritic cell density is associated with subbasal nerve plexus features, ocular surface disease index, and serum vitamin D in Evaporative dry eye diseaseBioMed Res Int20162016436975026904676
- ErdelyiBKraakRZhivovAGuthoffRNemethJIn vivo confocal laser scanning microscopy of the cornea in dry eyeGraefes Arch Clin Exp Ophthalmol20072451394416874525
- Benitez-Del-CastilloJMAcostaMCWassfiMARelation between corneal innervation with confocal microscopy and corneal sensitivity with noncontact esthesiometry in patients with dry eyeInvest Ophthalmol Vis Sci200748117318117197530
- Benitez del CastilloJMWasfyMAFernandezCGarcia-SanchezJAn in vivo confocal masked study on corneal epithelium and subbasal nerves in patients with dry eyeInvest Ophthalmol Vis Sci20044593030303515326117
- TuiskuISKonttinenYTKonttinenLMTervoTMAlterations in corneal sensitivity and nerve morphology in patients with primary Sjogren’s syndromeExp Eye Res200886687988518436208
- TuominenISKonttinenYTVesaluomaMHMoilanenJAHelintoMTervoTMCorneal innervation and morphology in primary Sjogren’s syndromeInvest Ophthalmol Vis Sci20034462545254912766055
- KheirkhahARahimi DarabadRCruzatACorneal epithelial immune dendritic cell alterations in subtypes of dry eye disease: a pilot in vivo confocal microscopic studyInvest Ophthalmol Vis Sci201556127179718526540656
- MachettaFFeaAMActisAGde SanctisUDalmassoPGrignoloFMIn vivo confocal microscopic evaluation of corneal langerhans cells in dry eye patientsOpen Ophthalmol J20148515925317216
- ZhangXChenQChenWCuiLMaHLuFTear dynamics and corneal confocal microscopy of subjects with mild self-reported office dry eyeOphthalmology2011118590290721146227
- VillaniEGaroliETermineVPichiFRatigliaRNucciPCorneal Confocal Microscopy in Dry Eye Treated with CorticosteroidsOptom Vis Sci2015929e290e29525909241
- StahnCButtgereitFGenomic and nongenomic effects of glucocorticoidsNat Clin Pract Rheumatol200841052553318762788
- RhenTCidlowskiJAAntiinflammatory action of glucocorticoids–new mechanisms for old drugsN Engl J Med2005353161711172316236742
- MarshPPflugfelderSCTopical nonpreserved methylprednisolone therapy for keratoconjunctivitis sicca in Sjogren syndromeOphthalmology1999106481181610201607
- AvundukAMAvundukMCVarnellEDKaufmanHEThe comparison of efficacies of topical corticosteroids and nonsteroidal anti-inflammatory drops on dry eye patients: a clinical and immunocytochemical studyAm J Ophthalmol2003136459360214516798
- JungHHJiYSSungMSKimKKYoonKCLong-term outcome of treatment with topical corticosteroids for severe dry eye associated with Sjogren’s SyndromeChonnam Med J2015511263225914877
- PflugfelderSCMaskinSLAndersonBA randomized, double-masked, placebo-controlled, multicenter comparison of loteprednol etabonate ophthalmic suspension, 0.5%, and placebo for treatment of keratoconjunctivitis sicca in patients with delayed tear clearanceAm J Ophthalmol2004138344445715364229
- Pinto-FragaJLopez-MiguelAGonzalez-GarciaMJTopical fluorometholone protects the ocular surface of dry eye patients from desiccating stress: a randomized controlled clinical trialOphthalmology2016123114115326520171
- McGheeCNDeanSDanesh-MeyerHLocally administered ocular corticosteroids: benefits and risksDrug Saf2002251335511820911
- MatsudaSKoyasuSMechanisms of action of cyclosporineImmunopharmacology2000472–311912510878286
- AllerganPrescribing information for RESTASIS® Available from: http://www.allergan.com/assets/pdf/restasis_pi.pdfAccessed June 25, 2017
- AmesPGalorACyclosporine ophthalmic emulsions for the treatment of dry eye: a review of the clinical evidenceClin Investig (Lond)201553267285
- StevensonDTauberJReisBLEfficacy and safety of cyclosporin A ophthalmic emulsion in the treatment of moderate-to-severe dry eye disease: a dose-ranging, randomized trial. The Cyclosporin A Phase 2 Study GroupOphthalmology2000107596797410811092
- ChenMGongLSunXA comparison of cyclosporine 0.05% ophthalmic emulsion vs vehicle in Chinese patients with moderate to severe dry eye disease: an eight-week, multicenter, randomized, double-blind, parallel-group trialJ Ocul Pharmacol Ther201026436136620698799
- SallKStevensonODMundorfTKReisBLTwo multicenter, randomized studies of the efficacy and safety of cyclosporine ophthalmic emulsion in moderate to severe dry eye disease. CsA Phase 3 Study GroupOphthalmology2000107463163910768324
- Baiza-DuranLMedrano-PalafoxJHernandez-QuintelaELozano-AlcazarJAlaniz-de laOJA comparative clinical trial of the efficacy of two different aqueous solutions of cyclosporine for the treatment of moderate-to-severe dry eye syndromeBr J Ophthalmol201094101312131520679084
- RaoSNTopical cyclosporine 0.05% for the prevention of dry eye disease progressionJ Ocul Pharmacol Ther201026215716420415623
- KunertKSTisdaleASGipsonIKGoblet cell numbers and epithelial proliferation in the conjunctiva of patients with dry eye syndrome treated with cyclosporineArch Ophthalmol2002120333033711879137
- LaibovitzRASolchSAndrianoKO’ConnellMSilvermanMHPilot trial of cyclosporine 1% ophthalmic ointment in the treatment of keratoconjunctivitis siccaCornea19931243153238339560
- DeveciHKobakSThe efficacy of topical 0.05% cyclosporine A in patients with dry eye disease associated with Sjogren’s syndromeInt Ophthalmol20143451043104824442759
- KimECChoiJSJooCKA comparison of vitamin a and cyclosporine a 0.05% eye drops for treatment of dry eye syndromeAm J Ophthalmol20091472206.e3213.e318848318
- PrabhasawatPTesavibulNMahawongWA randomized double-masked study of 0.05% cyclosporine ophthalmic emulsion in the treatment of meibomian gland dysfunctionCornea201231121386139323135530
- DemiryayEYaylaliVCetinENYildirimCEffects of topical cyclosporine a plus artificial tears vs artificial tears treatment on conjunctival goblet cell density in dysfunctional tear syndromeEye Contact Lens201137531231521792057
- SheppardJDScoperSVSamudreSTopical loteprednol pretreatment reduces cyclosporine stinging in chronic dry eye diseaseJ Ocul Pharmacol Ther2011271232721133792
- ByunYJKimTIKwonSMEfficacy of combined 0.05% cyclosporine and 1% methylprednisolone treatment for chronic dry eyeCornea201231550951319730097
- GadekTRBurdickDJMcDowellRSGeneration of an LFA-1 antagonist by the transfer of the ICAM-1 immunoregulatory epitope to a small moleculeScience200229555571086108911834839
- ZhongMGadekTRBuiMDiscovery and development of potent LFA-1/ICAM-1 antagonist SAR 1118 as an ophthalmic solution for treating dry eyeACS Med Chem Lett20123320320624900456
- PflugfelderSCSternMZhangSShojaeiALFA-1/ICAM-1 interaction as a therapeutic target in dry eye diseaseJ Ocul Pharmacol Ther201733151227906544
- TeijeiraAGarasaSPelaezRLymphatic endothelium forms integrin-engaging 3D structures during DC transit across inflamed lymphatic vesselsJ Invest Dermatol201313392276228523528818
- RouzautAGarasaSTeijeiraADendritic cells adhere to and transmigrate across lymphatic endothelium in response to IFN-alphaEur J Immunol201040113054306321061437
- VargaGNippeNBalkowSLFA-1 contributes to signal I of T-cell activation and to the production of T(h)1 cytokinesJ Invest Dermatol201013041005101220072134
- HartmanNCNyeJAGrovesJTCluster size regulates protein sorting in the immunological synapseProc Natl Acad Sci U S A200910631127291273419622735
- FooksmanDRVardhanaSVasiliver-ShamisGFunctional anatomy of T cell activation and synapse formationAnnu Rev Immunol2010287910519968559
- de la FuenteHMittelbrunnMSanchez-MartinLSynaptic clusters of MHC class II molecules induced on DCs by adhesion molecule-mediated initial T-cell scanningMol Biol Cell20051673314332215872088
- PorterJCBrackeMSmithADaviesDHoggNSignaling through integrin LFA-1 leads to filamentous actin polymerization and remodeling, resulting in enhanced T cell adhesionJ Immunol2002168126330633512055249
- SmithAStanleyPJonesKSvenssonLMcDowallAHoggNThe role of the integrin LFA-1 in T-lymphocyte migrationImmunol Rev200721813514617624950
- SembaCPGadekTRDevelopment of lifitegrast: a novel T-cell inhibitor for the treatment of dry eye diseaseClin Ophthalmol2016101083109427354762
- SunYZhangRGadekTRO’NeillCAPearlmanECorneal inflammation is inhibited by the LFA-1 antagonist, lifitegrast (SAR 1118)J Ocul Pharmacol Ther201329439540223215542
- RaoVRPrescottEShelkeNBDelivery of SAR 1118 to the retina via ophthalmic drops and its effectiveness in a rat streptozotocin (STZ) model of diabetic retinopathy (DR)Invest Ophthalmol Vis Sci201051105198520420445119
- MurphyCJBentleyEMillerPEThe pharmacologic assessment of a novel lymphocyte function-associated antigen-1 antagonist (SAR 1118) for the treatment of keratoconjunctivitis sicca in dogsInvest Ophthalmol Vis Sci20115263174318021330663
- DustinMLGrovesJTReceptor signaling clusters in the immune synapseAnnu Rev Biophys20124154355622404679
- SembaCPTorkildsenGLLonsdaleJDA phase 2 randomized, double-masked, placebo-controlled study of a novel integrin antagonist (SAR 1118) for the treatment of dry eyeAm J Ophthalmol201215361050.e11060.e122330307
- SheppardJDTorkildsenGLLonsdaleJDLifitegrast ophthalmic solution 5.0% for treatment of dry eye disease: results of the OPUS-1 phase 3 studyOphthalmology2014121247548324289915
- TauberJKarpeckiPLatkanyRLifitegrast ophthalmic solution 5.0% vs placebo for treatment of dry eye disease: results of the randomized Phase III OPUS-2 StudyOphthalmology2015122122423243126365210
- HollandEJLuchsJKarpeckiPMLifitegrast for the treatment of dry eye disease: results of a Phase III, Randomized, Double-Masked, Placebo-Controlled Trial (OPUS-3)Ophthalmology20171241536028079022
- SembaCPSwearingenDSmithVLSafety and pharmacokinetics of a novel lymphocyte function-associated antigen-1 antagonist ophthalmic solution (SAR 1118) in healthy adultsJ Ocul Pharmacol Ther20112719910420334535
- PaskowitzDMNguyenQDGehlbachPSafety, tolerability, and bioavailability of topical SAR 1118, a novel antagonist of lymphocyte function-associated antigen-1: a phase 1b studyEye (Lond)201226794494922538219
- DonnenfeldEDKarpeckiPMMajmudarPASafety of lifitegrast ophthalmic solution 5.0% in patients with dry eye disease: a 1-year, multicenter, randomized, placebo-controlled studyCornea201635674174827055211
- LanzaNLMcClellanALBatawiHDry eye profiles in patients with a positive elevated surface matrix metalloproteinase 9 point-of-care test versus negative patientsOcul Surf201614221622326807724
- BaudouinCMessmerEMAragonaPRevisiting the vicious circle of dry eye disease: a focus on the pathophysiology of meibomian gland dysfunctionBr J Ophthalmol2016100330030626781133
- GalorAKumarNFeuerWLeeDJEnvironmental factors affect the risk of dry eye syndrome in a United States veteran populationOphthalmology2014121497297324560568
- MiljanovicBTrivediKADanaMRGilbardJPBuringJESchaumbergDARelation between dietary n-3 and n-6 fatty acids and clinically diagnosed dry eye syndrome in womenAm J Clin Nutr200582488789316210721
- GalorAFelixERFeuerWDry eye symptoms align more closely to non-ocular conditions than to tear film parametersBr J Ophthalmol20159981126112925710726
