Abstract
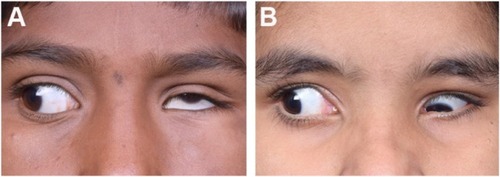
Duane retraction syndrome (DRS) is a congenital eye movement anomaly characterized by variable horizontal duction deficits, with narrowing of the palpebral fissure and globe retraction on attempted adduction, occasionally accompanied by upshoot or down-shoot. The etiopathogenesis of this condition can be explained by a spectrum of mechanical, innervational, neurologic and genetic abnormalities occurring independently or which influence each other giving rise to patterns of clinical presentations along with a complex set of ocular and systemic anomalies. Huber type I DRS is the most common form of DRS with an earlier presentation, while Huber type II is the least common presentation. Usually, patients with unilateral type I Duane syndrome have esotropia more frequently than exotropia, those with type II have exotropia and those with type III have esotropia and exotropia occurring equally common. Cases of bilateral DRS may have variable presentation depending upon the type of presentation in each eye. As regards its management, DRS classification based on primary position deviation as esotropic, exotropic or orthotropic is more relevant than Huber’s classification before planning surgery. Surgical approach to these patients is challenging and must be individualized based on the amount of ocular deviation, abnormal head position, associated globe retraction and overshoots.
Introduction
Duane retraction syndrome (DRS), also known as Stilling–Turk–Duane syndrome, is a congenital eye movement anomaly characterized by variable horizontal duction deficits, with narrowing of the palpebral fissure and globe retraction on attempted adduction, occasionally accompanied by upshoot or downshoot.Citation1 Though DRS represents <5% of all forms of strabismus,Citation2,Citation3 it was widely described in the literature as early as the 19th century. In 1879, HeuckCitation4 first reported a patient with congenitally anomalous eye movements and globe retraction in adduction. Later, SinclairCitation5 (1895), BahrCitation6 (1896), Stilling and BergmannCitation7 (1887), TurkCitation8 (1899) and several other authorsCitation9 went on to describe this entity in greater detail. It was in 1905 that Alexander DuaneCitation1 reported a series of 54 cases with DRS, giving a detailed description of its clinical features and summarizing the possible etiopathogenesis and management of this syndrome.
Decades later, with the advent of neuroimaging, muscle electrophysiology and genetic analysis, there has been greater understanding of this form of strabismus, now considered a congenital cranial dysinnervation disorder (CCDD), giving better insights into the management of this challenging syndrome.
Etiology of DRS
Various theories were proposed to explain the etiology of DRS.
Historical aspect
Mechanical anomalies
TurkCitation8 first attempted to explain the cause of globe retraction in DRS, attributing it to an abnormally tight lateral rectus (LR), which was found to behave as an “inelastic band”. WolffCitation9 similarly described a congenitally anomalous LR, which was inelastic and tight to be the cause of abnormal motility in DRS. On the other hand, a developmental anomaly leading to a tight LR due to nuclear aplasia or birth trauma was also proposed later.Citation10
Another theory put forth was the presence of dual insertionCitation6,Citation11 of the medial rectus (MR) muscle, one which rotated the globe and the other one anomalous insertion located posteriorly which retracted the globe. This was well accepted for a long time until lack of this finding intraoperatively in most of the cases made it questionable.
Innervational anomalies
Possibility of an underlying innervational etiology came into light when BreininCitation12 studied the electric potentials generated by muscles in various positions of gaze in DRS. He found that there was no LR activity recordable in attempted abduction, while maximum LR action potentials were generated in adduction, suggesting that the LR was receiving innervation in adduction. This was thought to be the cause of co-contraction and globe retraction. Few othersCitation13,Citation14 also studied these muscle potentials and found variable degrees of LR potentials from abduction to adduction along with abnormal responses in vertical and oblique gazes. The MR muscle in all these was found to be normal. Strachan and BrownCitation15 quantified this paradoxical innervations using electromyography. They found variable LR activity depending on the position of gaze, while MR spikes were almost always consistent. Occasionally, synergistic innervation between MR and the vertical rectus muscles and obliques was noted. These findings were further confirmed by MetzCitation16 who studied the saccadic velocities of eye movements in DRS. The author noted slowing of abduction saccades due to lack of normal LR innervations, along with slowing of adduction saccades, possibly due to paradoxical innervation.
Central nervous system anomalies
Understanding muscle electrophysiology in DRS made it evident that the causative etiology was absent normal sixth nerve innervation to the LR, either nuclear (eg, anomalous innervation by the oculomotor nerve) or supranuclear (eg, brainstem abnormality) in origin.
While some authors have found abducens nucleus hypoplasiaCitation17 or complete absenceCitation18 in the brain stem to be the cause of DRS, othersCitation19 have proposed that infranuclear abnormalities at the level of cavernous sinus, where the oculomotor and abducens nerves are in vicinity of each other, lead to features of DRS. These early attempts to understand the neurologic basis of DRS were only based on autopsy studies.
Further evidence to support the role of neurologic anomalies in DRS is the presence of synkinesis,Citation20 which is occasionally seen with DRS, for example, Marcus Gunn Jaw Winking phenomenon, crocodile tears etc. Also, it has been suggested that unilateral abducting nystagmus in the normal eye of DRS patients is a marker of brainstem abnormality indicative of a central neurologic anomaly.Citation21
Newer concepts
It was in 1998 that Parsa et alCitation22 demonstrated the absence of left sixth nerve in a case of unilateral DRS using high-resolution T1-weighted images on magnetic resonance imaging (MRI). This congenital absence of innervation to the muscles has been found to cause fibrotic changes in the extraocular muscles leading to abnormal motility in DRS, a concept which has evolved over the last decade to be known as CCDD.Citation23 In this case, developmental abnormalities of one or more cranial nerves cause congenital dysinnervation of the cranial muscles. This may be primary due to absence of normal innervation or secondary following aberrant innervations from other cranial nerves. CCDD is a nonprogressive entity and may also have associated bony abnormalities.Citation24
The role of genetics in the development of DRS
Congenital strabismus, in general, may be a sporadic condition or the result of mutations in genes such as PHOX2A,Citation25 HOXA1Citation26 and ROB03Citation27, which are necessary for the growth and maturation of brainstem motor neurons and axons.
DRS has been commonly found to be a sporadic disorder, but in 10% of cases, it may be familial, which includes an autosomal dominant mode of inheritance in associated syndromes.Citation28 Genetic analysis of the autosomal dominant variant has been found to be associated with mutations in the CHN1 gene. This gene mutation is said to affect the abducens (and less commonly, the oculomotor) nerve.Citation29 In cases with isolated DRS without any other systemic associations, the DURS1 locus on chromosome 2q31 has been identified and mapped. This was studied by the analysis of a dominant DRS pedigree.Citation30 In DRS with associated malformation syndromes, the Duane radial ray syndrome has been found to have a dominant inheritance with incomplete penetrance. This syndrome has been mapped to heterozygous nonsense frameshift and deletion mutations in SALL4 gene on chromosome 20.Citation31,Citation32
Other syndromes associated with this gene include Holt–Oram and acro-renal-ocular syndromes.Citation33 Additional association of DRS includes the Bosley–Salih–Alorainy syndrome (bilateral DRS with inner ear malformations, cerebrovascular and cognitive abnormalities) in which there is an autosomal recessive inheritance and has been mapped to chromosome 7p15.2 of the HOXA gene.Citation26
Studying the genetics of DRS in monozygotic twins has revealed both the following presentations – some reporting concordance for DRS, while others were found to be discordant for DRS. In monozygotic twins who had unilateral DRS, commonly, a pattern was also noted wherein each twin had the opposite eye affected known as “mirror images”.Citation34 This could be explained by the hypothesis that there were certain genes with incomplete penetrance and variable expression influenced by various stimuli during embryogenesis. These finally determined the laterality and presentation of DRS.
Patients with sporadic DRS have also been found to have associated congenital malformations. Pfaffenbach and CrossCitation35 studied these anomalies which were skeletal, auricular, ocular or neural in presentation. Generally, the crucial time for development of these congenital malformations was found to be around the fourth to eighth week of gestation. Similarly, patients with thalidomide embryopathy having DRS were also found to be associated with maternal exposure around a similar time period. These findings suggested that there could be a critical period during embryogenesis, in which an insult could result in various congenital malformations including DRS.Citation36
Pathogenesis
The preceding discussion shows that DRS is a group of entities linked by dysinnervation leading to limited horizontal gaze and globe retraction in attempted adduction. Understanding these mechanisms can help understand the pathogenesis of the presenting features of this syndrome.
Globe retraction in adduction, which is a characteristic feature of DRS, is found to be due to tight fibrotic muscles, sometimes with anomalous insertions. Usually, these changes have been observed in the horizontal rectus muscles, as is often noted while operating such patients.Citation37 Sometimes, abnormal muscle slips or foot plates attaching to the sclera are noted after disinsertion of muscles intraoperatively. These have been noted commonly in the LR and very rarely in the MR. Unless these muscle slips are released, the tightness on forced ductions remains. Second, paradoxical innervation leading to co-contraction of MR along with LR, as described by Scott, was found to contribute to globe retraction in attempted adduction.Citation18,Citation38
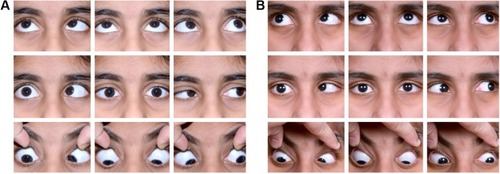
The pathogenesis of upshoots and downshoots () in DRS can be explained by these mechanical factors as well as certain innervational anomalies. The mechanical cause of these vertical movements is described as due to a “bridle effect”Citation39 of the tight LR, also known as the “leash effect”.Citation40 As the globe adducts and moves above or below the horizontal plane, there is sudden slippage of the tight LR, causing an upshoot or downshoot. In severe cases, this has been described to manifest even with the slightest of movement in adduction, known as “knife edge effect”.Citation40 These tight muscles can even give rise to an alphabet pattern of strabismus. These mechanical upshoots and downshoots in DRS are characterized by a sudden abrupt movement following a small vertical movement in adduction and usually do not have primary gaze vertical tropia.
In contrast to this, another type of vertical movement is noted in DRS, wherein there is a gradual vertical movement (elevation or depression) of the eye as it turns from abduction to adduction. This type of eye movement has been found to be due to anomalous paradoxical innervations of the LR by the oculomotor nerve. Such cases may even have vertical tropia in the primary position, and these are termed as innervational upshoots and downshoots.Citation18,Citation36
Thus, DRS is a spectrum of mechanical, innervational, neurologic and genetic abnormalities that influence each other giving rise to patterns of clinical presentations which can be categorized as described below.
Classification of DRS
Various systems of classification were proposed to understand the mechanism and presentation of DRS, as described in .
Table 1 Classification of DRS
Ahluwalia et alCitation47 modified Huber’s classification to include further subgroups in each category described by Huber. Depending upon the alignment in primary gaze, each Huber type was divided into A, B and C, indicating esotropia, exotropia and orthophoria, respectively, thus making it even more relevant clinically and surgically.
Demography
As discussed earlier, DRS represents about 1%–5% of all forms of strabismus.Citation2,Citation3
It is usually a sporadic entity (80% cases) commonly affecting females, though familial cases have also been noted in 5%–10% of patients.Citation48 DRS is also more commonly unilateral than bilateral, affecting the left eye more often.Citation49–Citation51 Bilateral cases of DRS have been reported to range from 10% to 24% of all DRS cases presenting to the clinic.Citation2,Citation52,Citation53 In both unilateral and bilateral DRS, type I is the most common presentation, followed by types III and II, respectively. Some bilateral cases have been reported to have type I in one eye and type II in the other.Citation47 A female preponderance is noted (up to 60%), especially in unilateral type I DRS. Except for one study by Tredici and von NoordenCitation54 in which only 40% of cases were females, most others reported a higher incidence in females. Some studiesCitation48,Citation55 have found DRS types II and III also to be more prevalent in females compared to males. Bilateral DRS, on the other hand, may be seen more often in males, but no predilection is noted.Citation49,Citation50
It is postulated that females are more susceptible to the expression of genes responsible for manifestation of DRS, as these are partly sex limited with incomplete penetrance.Citation45,Citation51
Another explanation put forth by Parsa and RobertCitation56 states that during embryogenesis, higher estrogen levels along with greater risk of inflammation predispose females to venous thromboembolic events. This along with right to left shunt causes more embolic phenomena that affect the left carotid artery, causing dysregulated apoptosis followed by misinnervation and ocular malformations. This predilection for the left eye is noted most often in types I and III DRS and is uncommon in type II.Citation47,Citation48
Also, some authorsCitation45 have noted evidence for a subclinical or “forme fruste” variant of DRS in the family members of the affected patient. These individuals may show extraocular muscle paresis (other than LR) or minimal palpebral fissure size changes during horizontal ductions. Miyake et al,Citation57 in their study, analyzed a family with c.443A>T CHN1 heterozygous missense mutation in a dominant pattern among five members with congenital ocular dysmotility.
Apart from DRS in two members, other phenotypes included vertical strabismus with elevation deficits. Thus, CHN1 mutations may have varied presentation and should be considered in the differential diagnosis of patients with elevation deficits, even in the absence of DRS.
Clinical presentation
Common reasons for seeking medical attention in DRS include an abnormal head position of the child, one of the eyes appearing smaller than the other due to globe retraction, pseudoptosis in adduction or abnormal eye movements. Though DRS is present at birth, in the early stages, it may appear only as an abduction deficit. As LR inelasticity and tightness develops with time, globe retraction and limitation of motility may be more pronounced.Citation58
Often, parents mistake abduction deficiency of the affected eye to be esotropia of the contralateral eye when the child looks toward the affected side. This occurs due to overadduction of the uninvolved eye due to Hering’s law.Citation59
Thus, it is important to explain to the parents and, if necessary, the pediatrician regarding the involved eye and discuss the presentation of DRS, so that is not mistaken for abducens nerve palsy, which may have serious neurologic implications.
Clinical features
Age – Despite being a congenital anomaly, patients may not always report to the clinics early. Age at presentation is variable and has been found to be younger in type I DRS, possibly due to earlier presentation to the pediatrician or pediatric ophthalmologist. In a series of 441 cases of DRS by Kekunnaya et al,Citation50 the mean age at presentation in unilateral types I, II and III, and bilateral DRS was found to be 13.5±13.46, 23.00±15.62, 21.93±13.04 and 12.40±10.30 years, respectively. No significant age difference has been found in unilateral or bilateral cases and types II and III DRS. The variations in literature are probably due to variable age of presentation in the clinics.
Refractive error and amblyopia – Considering refractive status, about 30%–80% of DRS patients have been shown to have hypermetropia or hypermetropic astigmatism greater than +1.50 Diopters (D) sphere, some even more than +4.00 DS. These cases, especially with high hyperopia, may have an associated accommodative component, which must be taken into account despite not being directly linked to the underlying etiology of DRS.Citation60 Hence, there is a need for cycloplegic refraction in all cases of DRS.
Myopia, simple myopic astigmatism and emmetropia are less common and are found to be almost equally distributed.Citation44,Citation47,Citation51,Citation61 Variable incidence of anisometropia has been noted in literature. KirkhamCitation61 found anisometropia >1 D in about 40% of cases, while Tredici and von NoordenCitation54 reported an average incidence of only 3%. Anisometropic and ametropic amblyopia has been reported to be the commonest cause of amblyopia in DRS, especially unilateral type I,Citation2,Citation47,Citation48 though O’Malley et alCitation55 found strabismic amblyopia to be the commonest cause. Less frequently, stimulus deprivation and mixed mechanisms of amblyopia have been seen.
Strabismus evaluation – A review of literatureCitation1,Citation44,Citation62–Citation64 shows that Huber type I DRS is the most common form of DRS, while type II is the least common presentation.
Also, esotropia is found to be the most common primary position deviation, followed by orthotropia, though some studies report otherwise. Usually, patients with unilateral type I Duane syndrome have esotropia more frequently than exotropia, those with type II have exotropia and those with type III have esotropia, exotropia and orthotropia occurring equally common.Citation63 Primary position deviation in cases of bilateral DRS is found to be variable depending upon the type of presentation in each eye.
Motor evaluation of strabismus
Motility – One of the most notable features of DRS is the abduction limitation. The abduction deficit in DRS accompanies a disproportionately smaller primary position deviation. This helps to distinguish it from a sixth nerve palsy, where the primary deviation and the abduction limitation are in proportion to each other.Citation59 This is most likely due to an underlying adduction deficit, which is present in varying amounts in DRS. This adduction deficit is evident from the fact that DRS patients have a remote near point of convergence. Also, these patients can be shown to have exotropia in the gaze opposite to the affected eye due to this adduction deficit.
Deviations – As discussed earlier, esotropia is the most common deviation in primary position in DRS. Measuring the deviation accurately requires the head to be in forced primary position while performing the cover test, as these patients have a strong tendency for abnormal head postures which may be very small as well. In this position, the secondary deviation is greater than the primary deviation in DRS. Also, one must remember to measure the deviations in the lateral and vertical gazes due to varying amounts of esotropia and exotropia in the lateral gazes caused by the motility deficits. In case the abduction and adduction limitations are almost equal to each other, there may be orthotropia in the primary gaze; otherwise, the predominant deficiency will determine the type of DRS and primary gaze measurement. By performing the cover test in up and down gazes, one may note any pattern strabismus. Bilateral DRS may be more likely to have pattern strabismus, compared to unilateral cases.Citation65 V pattern strabismus may be commonly seen in DRS, and sometimes an A pattern may also be noted, especially in cases of bilateral DRS. Hence, an A pattern must alert the clinician to look for bilateral DRS. Sometimes, with the presence of upshoot and downshoot, an X pattern may be seen.
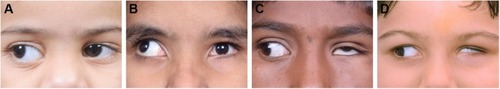
Anomalous vertical movements and globe retraction – Upshoot and downshoot are abnormal vertical eye movements frequently seen in cases of DRS. As discussed earlier, these may be due to mechanical or innervational anomalies. A patient may have both upshoot and downshoot, depending on whether the eye is moving slightly up or down during adduction and, in some cases, may have hyper- or hypotropia in the primary position itself. Kekunnaya et alCitation50 have reported an incidence of upshoot or downshoot in 43% of their cases. They found these movements to be more common in unilateral DRS types I and III, unlike Mohan et alCitation51 who found the incidence to be significantly greater in type III. In this study, Mohan et al have found the mechanical type to be more common than the innervational type (26% vs 12%). They also found a statistically significant association between exotropic DRS and mechanical upshoots and primary position vertical tropia with innervational upshoots.Citation66 Globe retraction occurs due to the co-contraction of the extraocular muscles, which is more pronounced in attempted adduction. This is accompanied by changes in the palpebral fissure, which are not merely passive changes. In the study by Isenberg and Urist,Citation63 they found that there is drooping of upper lid along with elevation of lower lid in about 52% of cases of DRS. In 18%, only upper lid changes were noted, while 21% had only lower lid elevation. A small proportion of cases (7%) did not show any lid changes with motility in DRS. In 2016, Kekunnaya et alCitation67 published a novel clinical grading system for globe retraction and overshoots to objectively quantify these. They excluded bilateral cases, cases with atypical features and vertical deviations.
Grading for globe retraction: With the involved eye in the maximum adducted position, a scale is used at the center of the palpebral fissure width to measure the palpebral aperture height and compared with that of the fellow eye in abduction.
0 No narrowing
1 <25%
2 25%–<50%
3 50%–<75%
4 ≥75%
Grading for overshoots: With the involved eye in adducted position, a straight line parallel to the intermedial canthal line is drawn from the pupillary center of the fellow eye.
0 Line bisects the pupil of involved eye.
1 Line lies between the pupillary center and the pupillary margin.
2 Line lies between the pupillary margin and the limbus.
3 Line lies at the limbus or over the sclera.
4 Cornea disappears below the lid (pumpkin seed sign).
shows various grades of retraction and overshoot.
Head posture – Head turns are very commonly seen in DRS, especially unilateral cases in both esotropic and exotropic types. This can be objectively assessed in the clinic using a goniometer. The aim of the head turn is to maintain binocular single vision and make up for the duction deficit, depending on which duction is maximally affected. Some authorsCitation68 report head turn in orthotropic DRS also, which may be to avoid the discomfort of sudden anomalous vertical eye movements. Kekunnaya et al,Citation50 in their study, were the first to report a significantly lower incidence of head turn in type II DRS, compared to types I and III.
Bilateral vs unilateral DRS – As described previously in the literature, bilateral DRS is less frequently seen than unilateral ones, with a reported incidence between 10% and 24%. Also, bilateral type I DRS has been found to be the most common type of presentation.Citation50–Citation52,Citation69 Zanin et alCitation70 have classified bilateral DRS into three types in agreement with Jampolsky,Citation71 based on the functional prognosis:
Bilateral DRS with fusion – usually bilateral type I with small angle of deviation or orthotropia and very minimal head posture, if any;
Bilateral DRS without fusion – prominent eso- or exodeviation and
Bilateral DRS with an alphabet pattern.
In this study,Citation70 the bilateral form of Duane syndrome was more likely to be associated with low visual acuity, lesser tendencies for abnormal head posture, high incidence of A and V patterns and associated congenital anomalies. The low visual acuity in bilateral DRS, also noted by Isenberg and Urist,Citation63 could be due to ametropia, anisometropia and a higher prevalence of vertical strabismus in these patients. A low incidence of abnormal head posture in bilateral Duane syndrome has been mentioned in the literature only by Raab.Citation68 This may possibly be due to symmetrical limitation of motility not requiring any head turn, or there may be very severe retraction and complex anomalous movements, which preclude normal fusion with any abnormal head posture.
Sensory evaluation of strabismus
As described previously, DRS patients try to maintain fusion with an appropriate head turn whenever possible and rarely complain of diplopia. If present, these patients have been found to ignore the second image rather than suppressing it. Hence, many cases of DRS have been found to have good stereopsis when measured in clinics.Citation2
Other variants of DRS
Vertical retraction syndrome: This is a very rare clinical entity first reported in Chinese literature. It consists of variable amounts of limitation of elevation or depression with globe retraction and palpebral fissure narrowing. The affected eye may be orthotropic, hypertropic or hypotropic in the primary position and may coexist with horizontal retraction. This vertical retraction may be due to innervational anomalies, fibrosis of vertical rectus muscles or a “tethering” effect of the horizontal rectus.Citation72
Congenital adduction deficit with synergistic divergence: This is another rare variant of DRS presenting with unilateral adduction deficit and simultaneous abduction of the eye on attempted adduction.Citation73 It may be associated with a head turn to uninvolved side, large angle exotropia, along with an abducting nystagmus on attempted adduction. This is sometimes considered to be a variant of Huber type II DRS. In some cases, it may be iatrogenically induced, called as iatrogenic simultaneous abduction or “ocular splits”Citation74 in cases where large LR resections have been performed.
Associations of DRS – DRS is found to be associated with numerous congenital anomalies, both ocular and nonocular as listed in .
Table 2 Ocular and systemic associations of DRS
Acquired retraction syndrome – Duane et alCitation82 went on to describe an entity called acquired retraction syndrome or pseudoduane syndrome. These patients present with limitation of abduction along with globe retraction in abduction. They usually have history of some kind if trauma, systemic illness and diplopia, differentiating it from true DRS. The causes of acquired retraction syndrome are as enlisted in .
Table 3 Acquired retraction syndrome mechanical causes
Other functions
Although intelligence is usually normal in DRS, intellectual disability has been reported in a few cases of DRS with borderline intelligence and cases of autism spectrum disorder associated with fetal thalidomide exposure or HOXA1-related syndromes.Citation92,Citation93
Management of DRS
Management of DRS is a challenge, and surgeons worldwide have their own preferred practices. In general, the surgical plan and aim of strabismus surgery in DRS is usually based on the following:
Primary position deviation
Degree of abnormal head posture
Severity of globe retraction and overshoots
Degree of limitation of ductions
Forced duction testing (FDT)
Extent of field of binocular single vision
DRS classification based on primary position deviation as esotropic, exotropic or orthotropic is said to be more relevant than Huber’s classification before planning surgery.
Esotropic DRS
In cases of esotropic DRS, various surgical procedures may be considered, but any coexisting accommodative component must be first corrected by prescribing spectacles preoperatively to avoid consecutive exotropia. Sometimes, even the abnormal head posture may improve upon treating with spectacles.Citation60
MR recession should be considered in situations with tight MR on intraoperative FDT. Unilateral MR recession is very commonly practiced in such cases and can correct up to 20 prism diopters (PD) of esotropia.Citation94,Citation95 However, in cases with accommodative or partially accommodative esotropia, the MR recession should be planned after measuring the deviation with hyperopic glasses to prevent overcorrection. Also, one needs to look for anomalous muscle bands in cases of very tight FDT.
Apart from improving primary position deviation, MR recession may also improve abduction of the affected eye if there was some prior lateral movement beyond the midline, thereby expanding binocular field of single vision. Various authors believe in performing MR recession no more than 6 mm or sometimes 5 mm, lest there be an induced adduction deficit creating an exotropia in contralateral gaze.
Sometimes, a large contralateral MR recession may be performed to match the abduction deficit of the affected eye and improve the binocular field of vision toward the affected side. This is a useful technique in cases where the abduction limitation is −2 or better.Citation96
However, surgeons must be careful with recessions of the contralateral MR muscle in patients with severe anomalous co-contraction. This may lead by Hering’s law to a severe co-contraction of the anomalous LR muscle in attempted rotation toward the affected gaze. Another risk here is the possibility of inducing a vertical deviation in patients with vertical dysinnervation.
On certain occasions, MR recession can also be performed as an initial procedure, followed by vertical rectus transposition as a second-stage procedure if necessary.
Bilateral MR recessions may also be required in some situations. First, in cases of esotropia with primary position deviation >20 PD, single MR recession of ≤6 mm is unlikely to be sufficient. Hence, here, bilateral MR recessions not exceeding 5–6 mm may be done.
Second, in cases with severe globe retraction, along with MR, LR may have to be recessed, thereby actually increasing the esotropia. Recessing the contralateral MR in such cases may also help to correct the total esotropia.
Lastly, bilateral MR recession may also help prevent contracture of the MR on the affected side by creating “fixation duress” in the contralateral eye. After surgery, MR of the fixing eye receives increased innervation to maintain fixation, which reduces innervation to its LR. This, in turn, reduces the innervational tone of the MR of the affected eye, lowering the risk of its contracture. This effect is said to be different from the aforementioned mechanism of matching duction deficit, and at least 7–8 mm of contralateral MR recession is necessary for it to act.Citation73
In certain cases, when esotropia is at least 25 PD with normal adduction and significantly limited abduction, MR recession can be combined with LR resection. This procedure is suitable in the absence of severe globe retraction and upshoots or downshoots. Kraft,Citation97 in his study, has recommended resecting the LR by not more than 3–3.5 mm and recessing MR not more than 5 mm in these patients. He found that with this approach, the adduction deficit induced in the DRS eye was less than that with bi-MR recessions, though both achieved a significant improvement in esotropia and abduction.
Cases with bilateral esotropic DRS, which is the commonest presentation,Citation47 are most often corrected by bilateral MR recessions, depending on the primary position deviation, muscle tightness and limitation of ductions. Sachdeva et alCitation98 studied 14 patients with bilateral esotropic DRS and found 86% success rate with a mean surgical dose of 5.6 mm (range being 5–7 mm) for bi-MR recessions. The mean preoperative esotropia in this series was 38 PD, with none of the patients developing significant postoperative adduction limitation.
Another technique to correct the eso-deviation and improve abduction in these eyes is by transposition of vertical rectus muscles to the LR along the spiral of Tillaux.Citation99 This may or may not be combined with MR recession, as there is risk of inducing anterior segment ischemia. There is also a risk of inducing new vertical or torsional deviations in these eyes, the most common being a hypotropia.Citation100 To prevent this, intraoperative FDTCitation96,Citation97 meticulous dissection of the vertical rectus to release them from the lid retractors and intraoperative monitoring of torsion is recommended after FDT.Citation101
In 2006, Johnston et alCitation102 described the role of superior rectus transposition (SRT) to the LR with or without MR recession. In their analyses of 52 patients with type I esotropic DRS, the preoperative deviation (range: 10–30 PD) improved to within 10 PD of esotropia in 95% of patients. Head turn improved in all cases, with complete elimination in 27% of the patients. Preoperative abduction limitation was noted to be from −3 to −4 in all eyes, which increased by 15°–45° after SRT. They also noted that there were no induced vertical deviations after SRT, unlike those seen after transposing both the vertical rectus muscles.
Mehendale et alCitation103 further modified this procedure by enhancing SRT with an augmentation suture to the LR (8–12 mm from insertion) with or without adjustable MR recession. They found good postoperative alignment and improvement in abduction.
Thus, in esotropic DRS, SRT may be considered as an initial procedure when the primary position deviation is 12–14 PD or lesser. On intraoperative FDT, the point when abduction restriction can be felt is the amount of abduction that may recover from a transposition. For deviations larger than 15 PD, SRT may be combined with MR recession () as described here. After performing MR recession which may be adjustable, a fornix conjunctival incision is made between the lateral border of superior rectus and the superior border of LR. Alternatively, a limbal peritomy extending up to 2 clock hours can be made in this quadrant. The superior rectus muscle is carefully dissected. The entire muscle belly and tendon is then transposed temporally to the LR, such that the temporal border of the superior rectus (SR) is 2 mm adjacent to the superior border of the LR, and the nasal border of the SR is reattached to the scleral following the spiral of Tillaux. The transposition is then augmented using a 5-0 nonabsorbable polyester suture through the sclera 8 mm posterior to the insertion of the LR, including the lateral thirds of each muscle. While augmented transpositions are practiced by the author, some surgeons describe the criteria for nonaugmented transpositions such as primary position deviation of less than or up to 18 PD and head turn of ≤18°.Citation104 Combining SRT with MR recession has been shown to be more effective than unilateral or bilateral MR recession in terms of improving abduction. Also, a combined procedure allows a smaller recession to improve both the deviation in the primary gaze and compensatory head posture.Citation105 Thus, when esotropia is >15 PD, SRT along with unilateral or bilateral adjustable MR recession up to 5 mm can be planned based on the size of deviation and muscle tightness.Citation106
Figure 3 Outcome of medial rectus recession with superior rectus transposition in type I esotropic DRS.
Abbreviation: DRS, Duane retraction syndrome.
In patients with residual esotropia after MR recession and SRT, another alternative is to add inferior rectus transposition. In those cases of residual esotropia who have undergone vertical rectus transposition and have a negative FDT, one needs to explore the transposed muscles to look for their migration, slippage or dragging of the augmentation suture. Patients may also develop consecutive exotropia following vertical rectus transposition (VRT) due to excess tightness by the VRT or a weak or excessively recessed MR. Late-onset exotropia may be explained by slipped MR, stretched scar or secondary LR tightness. If during resurgery, FDT is negative, then exotropia may be corrected with MR advancement and clearing any scar tissue. Moreover, in cases with negative FDT, the transposed muscles may need to be repositioned after releasing the augmentation suture.Citation106
Exotropic DRS
Patients with exotropic DRS usually have type III DRS followed by type II, both of which may be commonly associated with globe retraction and overshoots. Cases with isolated unilateral exotropic DRS can be managed with unilateral LR recession if the deviation is within 20 PD, increasing the surgical dosage compared to the standard surgical dose. If larger deviations are noted, bilateral LR recession may be needed, which can be performed asymmetrically with larger recession in the contralateral eye. This may help prevent worsening of abduction limitation if present.Citation101
Patients with type II exotropic DRS may have associated moderate to severe globe retraction, which can be managed with differential recessions of MR and LR in the affected eye.Citation107
In DRS types II and III, supramaximal LR recession and LR periosteal fixationCitation108 with or without vertical rectus transposition may also be done. Sharma et al,Citation109 in their study, have explained that the paradoxical innervation of LR during adduction improves with recession or periosteal fixation, while the abduction can be improved by vertical rectus transposition. They concluded that LR periosteal fixation, with and without partial VRT is effective in exotropic DRS. In addition, they found that while both procedures equally corrected the exodeviation and anomalous head posture, the abduction limitation changed from −3.8 to −3.6 in the former vs from −3.6 to −2.8 in the latter.
Management of bilateral exotropic DRS needs to be planned depending upon the presenting type in either eye. Theodorou and BurkeCitation110 treated 11 patients with bilateral exotropic DRS. Unilateral LR recession (3–18 mm recessions for deviations ranging from 12 to 60 PD) with conjunctival recession was the commonest surgery done, and cases with significant globe retraction also underwent additional small MR recession.
In the experience of the senior author, symmetrical or asymmetrical bilateral LR recessions with or without Y splitting are more effectual for treating bilateral exotropic DRS. Synergistic divergence, which is an extreme form of exotropic DRS, is very challenging to treat, and no definite surgical procedure has been found with satisfactory outcomes.Citation111
Globe retraction
As discussed in the previous section, globe retraction can be corrected with recessions of the co-contracting muscles. MR recession ranging from 5 to 6.5 mm and LR ranging from 7 to 9 mm or sometimes even periosteal fixation may be required.Citation103 When primary position esotropia is present, MR should be recessed more than LR; if not, then LR can be recessed 1 mm more than MR. It has been suggested that adults may need larger recessions than pediatric patients due to long-standing globe retraction causing orbital tissue changes, and retraction may recur due to continued co-contraction of the muscles.Citation112
Overshoots
Upshoots and downshoots in DRS may be due to the mechanical effect caused by tight muscles or due to innervational anomalies. Rogers and BremmerCitation113 have described Y splitting of the LR with or without its recession to treat overshoots caused by this mechanical leash effect. With Y split, each muscle half balances the other; thus, when the eye elevates in adduction, the lower arm contracts and prevents the globe from suddenly slipping up and vice versa in depression.
AwadeinCitation114 studied the role of inferior oblique (IO) weakening in all types of DRS which presented with a slow upshoot in which the movement was similar to IO overaction. He found that IO myectomy with or without horizontal rectus recession improved upshoot in this set of patients without inducing any vertical deviations or IO underaction.
Approach to the patient: an overview
The above review emphasizes the need to extend patient evaluation in cases of DRS beyond the routine clinical examination by a strabismologist to genetic analysis, systemic workup and neuroimaging. The understanding of DRS as a part of CCDD spectrum has evolved over time with identification of various genetic mutations as the underlying cause of these disorders. Also, phenotypic variability has been established among individuals with heterozygous CHN1 mutations, even with vertical deviations in the absence of DRS. Hence, patients with bilateral DRS with associated vertical anomalies and those with familial vertical deviations should undergo a detailed pedigree assessment and genetic screening for CHN1 mutations. Members of families with DRS as an autosomal dominant trait should be screened for mutations in the CHN1 gene, enhancing genetic counseling and permitting earlier diagnosis. Mutations such as SALL4, SALL1 and those involving chromosomes 8 and 22 are often associated with syndromic DRS; hence, a complete systemic evaluation including X-ray spine, renal function tests and audiometry is mandated, if these mutations are tested positive.Citation80 Common among these is the presence of unilateral or bilateral sensorineural deafness, as found by KirkhamCitation81 in 11% of 112 patients with DRS in his case series, and he has recommended a detailed family history and audiologic examination for all cases of DRS. Especially, the presence of neck anomalies should warn the clinician of underlying hearing loss to be likely due to Wildervanck syndrome, and an auditory evaluation should be done. MRI would be ideal in all cases of DRS, as a variable endophenotype has been noted by Demer et al. Though they found abnormal abducens nerve or LR muscle most commonly, superior oblique hypoplasia was noted in half of the tested individuals, suggesting involvement of the fourth cranial nerve in these cases. The only muscles that were not affected were those supplied by the inferior division of the oculomotor nerve: the inferior rectus, MR and IO muscles.Citation115 Thus, a combination of ocular and, when necessary, systemic evaluation, pedigree analysis, MRI and gene product characterization will help in better understanding the clinical presentation and to provide better care to DRS patients and their families.
Conclusion
DRS consists of a complex set of ocular and systemic anomalies, which may have myriad presentations. It is caused by mechanical, innervational, genetic, embryologic and central nervous system anomalies occurring together or as an independent etiology.
Management of DRS is a challenge, and the surgical approach to such a patient must be individualized based on the amount of ocular deviation, abnormal head position, associated globe retraction and overshoots.
Author contributions
Both authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Acknowledgments
The photography team of LVPEI is gratefully acknowledged. The parents or guardians of the patients gave consent for their images to be used in this paper.
Disclosure
The authors report no conflicts of interest in this work.
References
- DuaneACongenital deficiency of abduction associated with impairment of adduction, retraction movements, contraction of the palpebral fissure and oblique movements of the eyeArch Ophthalmol199611410125512568859088
- DeRespinisPCaputoAWagnerRGuoSDuane’s retraction syndromeSurv Ophthalmol19933832572888310396
- GurwoodsASTerrignoCADuane’s retraction syndrome: literature reviewOptometry2000711172277611101130
- HeuckGUber angeborenene vererbten Beweglichkeitsdefect der AugenKlin Monatsbl Augenkeilkd187917253
- SinclairWWAbnormal associated movements of the eyelidsOphthalmol Rev189514307
- BahrKVorstellung eines Falles von eigenartiger Muskelanomalie eines Auges Ber DtschGes Opthalmol189625334
- StillingJBergmannJFUntersuchungen uber die Entstehung der KurzsichtigkeitWiesbaden, Germany188713
- TurkSBemerkungen zu einem Falle von Retraction des AugesCbl Pract Augenheilk18992314
- WolffJThe occurrences of retraction movements of the eyeball together with congenital defects in the external ocular musclesArch Ophthalmol190029297
- GallusESinddiesog angeborener: Retraktionsberegungen des Auges die Folge einer GebutsverletzurgArch Augenheilkd1921873545
- AxenfeldTHSchurenbergEAngeborene RetractionsbewegungenKlin Monats190139851
- BreininGMIn discussion of: de Gindersen T, Zeavin B. Observations on the retraction syndrome of DuaneArch Ophthalmol195655576
- ZaubermanHMagoraAChacoJAn electromyographic evaluation of the retraction syndromeAm J Ophthalmol1967646110311086072985
- SatoSElectromyographic study on retraction syndromeJpn J Ophthalmol196045766
- StrachanIMBrownBHElectromyography of extraocular muscles in Duane’s syndromeBr J Ophthalmol19725685945995079406
- MetzHSThe diagnosis and treatment of abduction deficienciesAnn Ophthalmol197686683693937951
- MillerNRKielSMGreenWRClarkAWUnilateral Duane’s retraction syndrome (type 1)Arch Ophthalmol19821009146814727115176
- HotchkissMGMillerNRClarkAWGreenWRBilateral Duane’s syndromeArch Ophthalmol19809858708747378011
- HoytWFNachtigallerHAnomalies of ocular motor nervesAm J Ophthalmol19656034434485828681
- RamsayJTaylorDCongenital crocodile tears: a key to the etiology of Duane’s syndromeBr J Ophthalmol19806475185227426566
- SchmidtDCongenitale AugenmuskelparesenAlbrech v Graefes Arch Ophthalmol19741924285
- ParsaCFGrantEDillonWPdu LacSHoytWFAbsence of the abducens nerve in Duane syndrome verified by magnetic resonance imagingAm J Ophthalmol199812533994019512165
- AssafAACongenital innervation dysgenesis syndrome (CID)/congenital cranial dysinnervation disorders (CCDDs)Eye201125101251126121720410
- GutowskiNJEllardSThe congenital cranial dysinnervation disorders (CCDDs)Adv Clin Neurosci Rehabil20055810
- NakanoMYamadaKFainJHomozygous mutations in ARIX(PHOX2A) result in congenital fibrosis of the extraocular muscles type 2Nat Genet200129331532011600883
- TischfieldMABosleyTMSalihMAHomozygous HOXA1 mutations disrupt human brainstem, inner ear, cardiovascular and cognitive developmentNat Genet200537101035103716155570
- JenICChanWMBosleyTMMutations in a human ROBO gene disrupt hindbrain axon pathway crossing and morphogenesisScience200430456761509151315105459
- OystreckDTEngleECBosleyTMRecent progress in understanding congenital cranial dysinnervation disordersJ Neuroophthal20113116977
- MiyakeNChiltonJPsathaMHuman CHN1 mutations hyperactivate a2-chimaerin and cause Duane’s retraction syndromeScience2008321589083984318653847
- AppukuttanBGillandersEJuoSHLocalization of a gene for Duane retraction syndrome to chromosome 2q31Am J Hum Genet19996561639164610577917
- Al-BaradieRYamadaKHilaireCStDuane radial ray syndrome (Okihiro syndrome) maps to 20q13 and results from mutations in SALL4, a new member of the SAL familyAm J Hum Genet20027151195119912395297
- KohlhaseJHeinrichMSchubertLOkihiro syndrome is caused by SALL4 mutationsHum Mol Genet200211232979298712393809
- KohlhaseJSchubertLLiebersMMutations at the SALL4 locus on chromosome 20 result in a range of clinically overlapping phenotypes, including Okihiro syndrome, Holt-Oram syndrome, acro-renalocular syndrome, and patients previously reported to represent thalidomide embryopathyJ Med Genet200340747347812843316
- MehdornEKommerellGInherited Duane’s syndrome. Mirror-like localization of oculomotor disturbances in monozygotic twinsJ Pediatr Ophthalmol Strabismus197916152572416
- PfaffenbachDDCrossHEKearnsTPCongenital anomalies in Duane’s retraction syndromeArch Ophthalmol1972886356394628563
- MillerMTStrömlandKKWhat can we learn from the thalidomide experience: an ophthalmologic perspectiveCurr Opin Ophthalmol2011225356366421825994
- GobinMHSurgical management of Duane’s syndromeBr J Ophthalmol19745833013064834603
- MillerNRKielSMGreenWRUnilateral Duane’s retraction syndrome (Type 1)Arch Ophthalmol19821009146814727115176
- von NoordenGKMurrayEUp- and downshoots in Duane’s retraction syndromeJ Pediatr Ophthalmol Strabismus1986232122153772687
- JampolskyASurgical leashes and reverse leashes in strabismus surgical managementSymposium on Strabismus. Transactions of the New Orleans Academy of OphthalmologySt Louis, MOCV Mosby1978244268
- PapstWKlin Monbl Augenheilkd Augenarztl Fortbild. [Motility disorders of the eye due to paradoxical innervation]Klin Monatsbl Augenheilkd1962141199209 German
- PapstWEsslenESymptomatology and therapy of ocular motility disturbancesAm J Ophthalmol19645827529114202515
- PapstWParalytischer Strabismus infolge paradoxer innervationBer Dstch Ophthalmol Ges19666784
- MalbranJEstrabismos y paralysisClinica y terapeutua Buenos Anes, Editorial Oftalmologia1949625
- LyleTKBridgemanGJOWorth and Chavasse’s Squint The Binocular Reflexes and the Treatment of Strabismus9th edLondonBailliere Tindall and Cox1959251255
- HuberAElectrophysiology of the retraction syndromesBr J Ophthalmol19745832933004834602
- AhluwaliaBKGuptaNCGoelSRKhuranaAKStudy of Duane’s retraction syndromeActa Ophthalmologica19886667287303232518
- KirkhamTHInheritance of Duane’s syndromeBr J Ophthalmol19705453233295428664
- MosterMParesis of Isolated and Multiple Cranial Nerves and Painful OphthalmoplegiaYanoffMDukerJSOphthalmologyPhiladelphia, PAMosby1999
- KekunnayaRGuptaASachdevaVDuane retraction syndrome: series of 441 casesJ Pediatr Ophthalmol Strabismus201249316416922074356
- MohanKSharmaAPandavSSDifferences in epidemiological and clinical characteristics between various types of Duane retraction syndrome in 331 patientsJ AAPOS200812657658018723379
- KhanAOOystreckDTClinical characteristics of bilateral Duane syndromeJ AAPOS200610319820116814169
- KhanAOOystreckDTWilkenKAkbarFDuane retraction syndrome on the Arabian peninsulaStrabismus200715420520818058357
- TrediciTDvon NoordenGKAre anisometropia and amblyopia common in Duane’s syndrome?J Pediatr Ophthalmol Strabismus19852223253981378
- O’MalleyERHelvestonEMEllisFDDuane’s retraction syndrome-plusJ Pediatr Ophthalmol Strabismus19821931611657108702
- ParsaCFRobertMPThromboembolism and congenital malformations: from Duane syndrome to thalidomide embryopathyJAMA Ophthalmol2013131443944723710497
- MiyakeNDemerJLShaabanSExpansion of the CHN1 strabismus phenotypeInvest Ophthalmol Vis Sci20115296321632821715346
- Souza-DiazCCongenital VI nerve palsy is Duane’s syndrome until disproven (letter)Binocular Vision Ocular Motility1992770
- HeringEDie Lehre vom Binoculren SehenLeipzig, GermanyWilhelm Englemann1868
- KekunnayaRVelezFGPinelesSLOutcomes in patients with esotropic duane retraction syndrome and a partially accommodative componentIndian J Ophthalmol2013611270170424413823
- KirkhamTHAnisometropia and amblyopia in Duane’s syndromeAm J Ophthalmol1970697747775441354
- HugonnierRClayette-HuggonierSStrabismus, heterophoria, ocular motor paralysisVeronnean-TroutmanSClinical Ocular Muscle ImbalanceSt Louis, MOCV Mosby1969322327
- IsenbergSUristMJClinical observations in 101 consecutive patients with Duane’s retraction syndromeAm J Ophthalmol1977843419425900234
- RoAGummesonBOrtonRBCaderaWDuane’s retraction syndrome: southwestern ontario experienceCan J Ophthalmol19892452002032766084
- ChungMStoutJTBorchertMSClinical diversity of hereditary Duane’s retraction syndromeOphthalmology2000107350050310711888
- MohanKSarohaVSharmaAFactors predicting upshoots and downshoots in Duane’s retraction syndromeJ Pediatr Ophthalmol Strabismus200340314715112795433
- KekunnayaRMoharanaRTibrewalSPatilPCSachdevaVA simple and novel grading method for retraction and overshoot in Duane retraction syndromeBr J Ophthalmol20161001451145427574176
- RaabELClinical features of Duane’s syndromeJ Pediatr Ophthalmol Strabismus19862364683958872
- MaruoTKArimotoNKikuchiRDuane’s syndromeJpn J Ophthalmol197923453468
- ZaninEGambarelliNDenisDDistinctive clinical features of bilateral Duane retraction syndromeJ AAPOS201014429329720736120
- JampolskyADuane SyndromeRosenbaumACSantiagoAPCSM, Principles and Surgical TechniquesPhiladelphia, PAW.B. Saunders1999325346
- WildervanckLSEen geval van aandoening van Klippel-Feil Gecombineerd met Abducensparalyse, retractio bulbi en DoofstomheidNed T Geneesk1952962752275513025583
- WilcoxLMGittingerJWBreininGMCongenital adduction palsy and synergistic divergenceAm J Ophthalmol198191177234921
- MetzHSDuane’s retraction syndrome and severe adduction deficiency detteryArch Ophthalmol1986104158615873778269
- MittalVGuptaASachdevaVKekunnayaRDuane retraction syndrome with posterior microphthalmos: a rare associationJ Pediatr Ophthalmol Strabismus201249E48E5122881831
- WeissISUristMJDuane’s syndrome associated with tendon sheath syndrome and microcorneaJ Pediatr Ophthalmol Strabismus1972911415
- CibisGWCongenital familial external ophthalmoplegia with co-contractionOphthalmic Paediatr Genetics19844163168
- CollinsETEnophthalmosBr Med J18992846851
- YangMMHoMLauHHDiversified clinical presentations associated with a novel sal-like 4 gene mutation in a Chinese pedigree with Duane retraction syndromeMol Vis20131998699423687435
- GraeberCPHunterDGEngleECThe genetic basis of incomitant strabismus: consolidation of the current knowledge of the genetic foundations of diseaseSemin Ophthalmol2013285–642743724138051
- KirkhamTHDuane’s syndrome and familial perceptive deafnessBr J Ophthalmol1969533353395772619
- DuaneTDSchatzNJCaputoARPseudo-Duane’s retraction syndromeTrans Am Ophthalmol Soc197674122129867622
- GittingerJWJrHughesJPSuranELMedial orbital wall blow-out fracture producing an acquired retraction syndromeJ Clin Neuroophthalmol1986631531562946721
- KivlinJDLundermanMKAcquired Retraction syndrome associated with orbital metastasisJ Pediatr Ophthalmol Strabismus19852231091123998972
- KargiSHAtasoyHTSayarliogluHKoksalMUgurbasHSAlpayAAcquired retraction of the eye as the first sign of myositisStrabismus2005132858816020363
- SoodGCSrinathBSKrishnamurthyGAcquired Duane’s retraction syndrome following Kronlein’s operationEye Ear Nose Throat Mon19755483083101157894
- KhanAOInverse globe retraction syndrome complicating recurrent pterygiumBr J Ophthalmol2005895640641
- SmithJLDamastMAcquired retraction syndrome after sixth nerve palsyBr J Ophthalmol19735721101144694391
- AkikoYMasatoWTetsuroTKeichiGBilateral Duane’s retraction syndrome due to large pontine gliomaNeuro Ophthalmol Japan20032016670
- YükselDOrban de XivryJLefèvrePReview of the major findings about Duane retraction syndrome (DRS) leading to an updated form of classificationVision Res201050232334234720801148
- SilverbergMDemerJDuane’s syndrome with compressive denervation of the lateral rectus muscleAm J Ophthalmol2001131114614811162999
- VarmaCAroorSMundkurSCAnnamalaiKBilateral type-I duane syndrome with multiple anamolies: a case reportJ Clin Diagn Res2012681435143623205369
- AmaralDDawsonGGeschwindDAutism Spectrum DisordersOxfordOxford University Press2011866
- KraftSPSurgery for Duane’s syndromeAm Orthoptic J1993431826
- KabanTJSmithKDayCSingle medial rectus recession in unilateral Duane syndrome type IAm Orthoptic J199545108114
- KraftSPA surgical approach for Duane syndromeJ Pediatr Ophthalmol Strabismus19882531191303397857
- KraftSPResecting the lateral rectus in unilateral Duane syndrome with esotropia and limited abductionBinoc Vis Strab Q2010253149157
- SachdevaVKekunnayaRGuptaABhoompallyVRSurgical management of bilateral esotropic Duane syndromeJ AAPOS201216544544823084382
- RosenbaumALCostenbader Lecture. The efficacy of rectus muscle transposition surgery in esotropic Duane syndrome and VI nerve palsyJ AAPOS20048540941915492732
- RuthAVelezFGRosenbaumALManagement of vertical deviations after vertical rectus transposition surgeryJ AAPOS2009131161919117778
- HolmesJMHattSRLeskeDAIntraoperative monitoring of torsion to prevent vertical deviations during augmented vertical rectus transposition surgeryJ AAPOS201216213614022525168
- JohnstonSCCrouchERJrCrouchERAn innovative approach to transposition surgery is effective in treatment of Duane’s syndrome with esotropiaInvest Ophthalmol Vis Sci2006472475
- MehendaleRADagiLRWuCLedouxDJohnstonSHunterDGSuperior rectus transposition and medial rectus recession for Duane syndrome and sixth nerve palsyArch Ophthalmol2012130219520122332212
- VelezFFosterRRosenbaumAVertical rectus muscle augmented transposition in Duane syndromeJ AAPOS20015210511311304819
- YangSMacKinnonSDagiLRHunterDGSuperior rectus transposition vs medial rectus recession for treatment of esotropic Duane syndromeJAMA Ophthalmol2014132666967524763870
- KekunnayaRKraftRRaoVBVelezFGSachdevaVHunterDGSurgical management of strabismus in Duane retraction syndromeJ AAPOS2015191636925727590
- SprungerDTRecession of both horizontal rectus muscles in Duane syndrome with globe retraction in primary positionJ AAPOS199711313310530982
- VelezFGThackerNBrittMTAlcornDFosterRSRosenbaumALRectus muscle orbital wall fixation: a reversible profound weakening procedureJ AAPOS20048547348015492742
- SharmaPTomerRMenonVSaxenaRSharmaAEvaluation of periosteal fixation of lateral rectus and partial VRT for cases of exotropic Duane retraction syndromeIndian J Ophthalmol201462220420824618490
- TheodorouNBurkeJSurgical and functional outcomes in bilateral exotropic Duane’s retraction syndromeBr J Ophthalmol20139791134113723793851
- KhawamETerroAHamadehISurgical correction of synergistic divergence strabismus. A report of three casesBinocul Vis Strabismus Q200722422723418163899
- ShaulyYWeissmanAMeyerEOcular and systemic characteristics of Duane syndromeJ Pediatr Ophthalmol Strabismus1993301781838350229
- RogersGLBremmerDLSurgical treatment of the up-shoot and down-shoot in Duane’s retraction syndromeOphthalmology19849111138013826514307
- AwadeinAInferior oblique myectomy for upshoots mimicking inferior oblique overaction in Duane retraction syndromeJ AAPOS201317325325823672883
- DemerJLClarkRALimKHEngleECMagnetic resonance imaging evidence for widespread orbital dysinnervation in dominant Duane’s retraction syndrome linked to the DURS2 locusInvest Ophthalmol Vis Sci200748119420217197533