Abstract
Age-related macular degeneration (AMD) is a leading cause of vision loss in patients >50 years old. The hallmark of the disease is represented by the accumulation of extracellular material between retinal pigment epithelium and the inner collagenous layer of Bruch’s membrane, called drusen. Although identified almost 30 years ago, reticular pseudodrusen (RPD) have been recently recognized as a distinctive phenotype. Unlike drusen, they are located in the subretinal space. RPD are strongly associated with late AMD, especially geographic atrophy, type 2 and 3 choroidal neovascularization, which, in turn, are less common in typical AMD. RPD identification is not straightforward at fundus examination, and their identification should employ at least 2 different imaging modalities. In this narrative review, we embrace all aspects of RPD, including history, epidemiology, histology, imaging, functional test, natural history and therapy.
Introduction
Age-related macular degeneration (AMD) is a chronic, progressive degenerative retinal disease characterized by loss of central vision and represents one of the leading causes of blindness in subjects >50 years old.Citation1 Being a distinctive feature of AMD, drusen appear on fundus examination as focal yellowish dots clustered at the posterior pole and are constituted by extracellular material accumulation between retinal pigment epithelium (RPE) and the inner collagenous layer of Bruch’s membrane (BM).Citation1 In recent times, a distinctive phenotype, called reticular pseudodrusen (RPD), has been in the spotlight.
Here, we aim to provide the reader a comprehensive narrative review embracing all aspects of RPD.
Literature search
A PubMed engine search was carried out using the terms “reticular pseudodrusen”, “reticular drusen”, “subretinal drusenoid deposits”, and “reticular macular disease”. All studies published in English up to July 2017, irrespective of their publication status, were reviewed and relevant publications were included in this review.
History
Mimoun et alCitation2 described for the first time “pseudodrusen visible en lumière bleue” in 1990 to define retinal lesions with a variable diameter of about 100 μm that did not appear hyperfluorescent on fluorescein angiography (FA), but with enhanced visibility in blue light. In 1991, RPD were incorporated into the Wisconsin AMD grading system as “ill-defined networks of broad interlacing ribbons” on color fundus photography and were named reticular soft drusen.Citation3 In 1995, Arnold et alCitation4 further characterized RPD as “yellow interlacing network 125–250 μm wide appearing first in the superior outer macula and then extending circumferentially and beyond” and coined the terminology RPD. Since then, the nomenclature RPD spread in the current literature, although other terms (ie, subretinal drusenoid deposits, reticular macular disease, reticular drusen) have been used.
Epidemiology
Prevalence
Prevalence of RPD varies in relation to the modality of evaluation and the cohort of patients enrolled. Prevalence of RPD in general elder population was assessed by large, long-term follow-up prospective studies, namely, Beaver Dam Eye, Blue Mountains Eye and Melbourne Collaborative Cohort studies.Citation5–Citation7 In those studies, the prevalence of RPD turned to be 0.41%–1.95% and 3.0%–4.0% at baseline and 15 years, respectively.Citation5–Citation7 However, the sensitivity of color fundus photography is very low. Conversely, novel imaging modalities (ie, spectral-domain optical coherence tomography [SD-OCT], infrared [IR] reflectivity and multicolor) exhibited excellent sensitivity/specificity, and, therefore, studies employing these modalities disclosed higher prevalence of RPD.Citation8 In the Rotterdam Eye Study, RPD were detected in about 5% of participants using color fundus photograph (CFP) and near-infrared reflectance (NIR).Citation9 Prevalence of RPD was even higher ranging from 13.4% to 32% when multimodal imaging was employed.Citation10,Citation11 Interestingly, RPD seem to be less frequent in Asiatic compared with White populations.Citation12 RPD are bilateral in 50%–84% of cases.Citation6,Citation13–Citation15
Independently from the macular status, the presence of RPD in older population increased 3.4-fold the chance to suffer from AMD in general elder population.Citation11 Prevalence of RPD in patients with AMD is obviously higher than that in general population, ranging from 16.8% to 79%, depending on the imaging modality used, stage and type of AMD.Citation8,Citation11,Citation16–Citation20 Surprisingly, RPD prevalence was even higher (85%) in postmortem histological samples of patients with AMD.Citation21 Although their overall presence in neovascular AMD ranges between 22% and 36%, RPD are more easily found in eyes with type 3 choroidal neovascularization (CNV) (68.4%–83%). Conversely, they are less commonly seen in eyes with typical neovascular AMD (type 1 and 2) or polypoidal choroidal vasculopathy with a prevalence of 9%–13.9% and 2%–3.4%, respectively.Citation17,Citation19,Citation22,Citation23 In eyes with geographic atrophy, the prevalence of RPD ranges between 29% and 50%.Citation14,Citation17 An association between RPD and acquired vitelliform detachment has been reported, suggesting RPE dysfunction and impairment to outer segments turnover.Citation24,Citation25
Although RPD are commonly found in AMD, they have also been associated with other retinal affections, including early-onset drusen,Citation26 pseudoxanthoma elasticum,Citation27,Citation28 Sorsby macular dystrophyCitation29 and adult-onset foveomacular vitelliform dystrophy.Citation30 RPD have been anecdotally reported in late-onset macular degeneration,Citation31 vitamin A deficiencyCitation32 and IgA nephropathy.Citation33
Risk factors
RPD occur in the setting of AMD, and, therefore, phenotypic features of AMD are associated with higher risk of RPD presence. Patients with late AMD have the highest odds of having RPD, and the risk is greater in the geographic atrophy (GA) over the CNV phenotype, followed by intermediate and early stages.Citation4,Citation5,Citation34,Citation35 Large drusen and focal pigmentary changes are additional risk factors for RPD presence.Citation5 AMD and RPD share several non-ocular risk factors, including older age,Citation5–Citation7,Citation36 female sex,Citation5,Citation7,Citation19,Citation36 current smokingCitation6,Citation7 and high body mass index.Citation6 Other reported risk factors for RPD include less education,Citation7 B-vitamin complex use,Citation7 history of steroid eye drop useCitation7 and glaucoma.Citation7 As for AMD, the prevalence of RPD is lower in patients with diabetes.Citation7,Citation37 In the Beaver Dam Eye Study,Citation7 RPD were an independent risk factor for mortality, and Cymerman et alCitation38 have recently reported higher prevalence of RPD in patients with coronary artery disease. Compared with AMD without RPD, patients with RPD demonstrated higher rates of systemic hypertension, angina and mortality.Citation39 However, this association is still under debate, and other studies did not confirm association between RPD and cardiovascular risk factors.Citation40,Citation41 Decreased renal function represents another vascular association; interestingly, both choroid and kidney share a lobular, end-arterial vascular system, and this could account for this connection.Citation42
With the advent of the enhanced depth imaging (EDI)-and swept-source (SS)-OCT, several studies focused on the choroidal imaging. A reduction of the subfoveal choroidal thickness has been extensively demonstrated in patients with RPD when compared not only with healthy subjects but also with AMD without RPD.Citation43–Citation46 Also, choroidal thinning involves all the macula area and even areas outside the macula, such as in the peripapillary region, suggesting a widespread choroidal involvement.Citation47–Citation50 Employing choroidal binarization, Corvi et alCitation51 showed that stromal area is more represented in patients suffering from AMD with RPD compared with those without RPD, suggesting a choroidal vascular depletion and fibrotic replacement. Zheng et alCitation52 illustrated choroidal vessel density reduction in eyes with RPD, suggesting that RPD may be a sign of choroidal vasculopathy. Interestingly, areas of lowest vessel density did not correspond to the location of thinnest choroid, and, therefore, RPD may be related to a global choroidal dysfunction leading to choriocapillaris hypoperfusion, possibly indicating an ocular sign of systemic vascular deficiency.Citation52,Citation53
Little is known regarding genetic susceptibility and RPD. Two major AMD risks alleles, namely, complement factor H (CHF, rs1061170) 402H on chromosome 1q32 and the age-related maculopathy susceptibility 2 (ARMS2, rs10490924) 69S on chromosome 10q26, have been pointed out by large studies.Citation54–Citation57 Data regarding RPD are controversial. The Beaver Eye StudyCitation7 and the Blue Mountain Eye StudyCitation6 revealed a linkage between RPD and both ARMS2 and CFH Y402H. Smith et alCitation58 found ARMS2 and CHF 402H variant as a risk and protective factor, respectively. In the Melbourne Collaborative Cohort Study,Citation5 the ARMS2 single-nucleotide polymorphism (SNP) rs10490924, HTRA1 SNPs rs11200638 and rs3793917, and CFH SNPs rs393955, rs1061170 and rs2274700 were linked to higher prevalence of RPD. Association between RPD and ARMS2 allele was also confirmed in Japanese population.Citation17 Buitendijk et alCitation9 found CFH, C2/FB and ARMS2 to be associated with both RPD and soft drusen, whereas C3 was linked only to RPD.
Conversely, Puche et alCitation40 did not find any genotypic difference between AMD patients with and without RPD. Similarly, Boddu et alCitation36 did not find any association between RPD and both ARMS2 and CHF alleles.
Histology
Different from drusen, which are made of lipids gathering in the BM (basal deposits), RPD are histologically characterized by the accumulation of material in the subretinal space extending up to the outer segment and even in the outer nuclear layers.Citation59,Citation60 Neighboring structures are affected by the presence of RPD, which is associated with RPE polymegathism, photoreceptor disruption and reactive gliosis.Citation60
Although RPD have some similarities with drusen in their composition (ie, neutral lipid, cholesterol, amyloid, complement factor, and membranous debris), they have significant differences in their components, and, therefore, RPD are not just drusen material located above the RPE.Citation60–Citation63 RPD have higher concentration of unesterified cholesterol, vitronectin, include opsins, peanut agglutinin, and photoreceptor pigments (including precursors of A2E/lipofuscin).Citation60–Citation63 Since RPD and drusen lipids did not stain with the same dye, differences in lipid composition have been postulated.Citation60 Moreover, RPD are rich in immune cells, predominantly immune-reactive microglia and macrophages, confirming the role of inflammation.Citation60 However, precise composition of RPD is yet to be determined.
Imaging
Different imaging modalities alone or in combination have been used to investigate RPD, including CFP, IR, short wave-length (SW)- and NIR-fundus autofluorescence (FAF), multicolor, OCT, en face OCT, OCT angiography (OCT-A), FA, indocyanine green angiography (ICGA) and adaptive optics.Citation63 shows multimodal imaging of an eye with RPD. Written informed consent was received from all patients for publication of images in this review.
Figure 1 Multicolor imaging, FAF, IR and SD-OCT of a patient affected by RPD.
Abbreviations: FAF, fundus autofluorescence; IR, infrared reflectivity; SD-OCT, spectral-domain optical coherence tomography; RPD, reticular pseudodrusen; RPE, retinal pigment epithelium; EZ, ellipsoid zone.
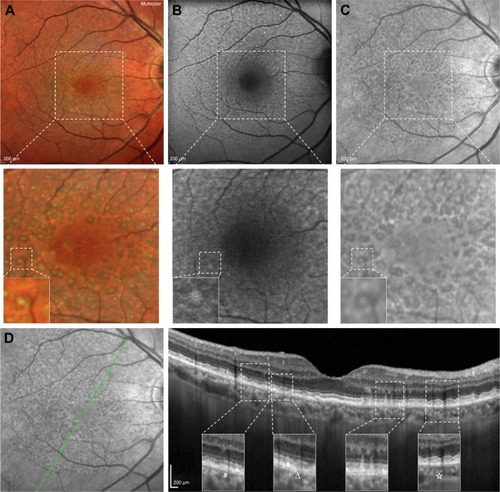
Multimodal imaging studies revealed that IR, FAF, SD-OCT and widefield en face SS-OCT have the highest sensitivity in detecting RPD, whereas late phases of ICGA, blue channel of CFP and confocal blue reflectance (CBR) have almost perfect specificity.Citation14,Citation18,Citation64 However, there is no real gold standard for PRD detection, and use of at least 2 imaging modalities has been advocated.Citation65 It has been suggested to use a high sensible modality (ie, SD-OCT, IR) as a screening test and a high specific one (ie, ICGA, CFP, CBR) as confirmation.Citation18
Color fundus photograph
CFP reveals RPD as yellowish, ill-defined network of broad, interlacing ribbons preferentially located in the superior aspect of the macular area, which tends to become more punctate as they become closer to the fovea.Citation59,Citation66 The blue channel of CFP allows better contrast of RPD since the SW of blue light is highly reflected by the RPD, but preferentially absorbed by melanin contained in the RPE surrounding the RPD, creating contrast.Citation18 Although some milestone studiesCitation5–Citation7 employed CFP to individuate RPD, the diagnostic ability of this technique is unsatisfactory, due to the low sensitivity.Citation18 On the contrary, its specificity is extremely high, virtually reaching 100%, and, therefore, it could be a powerful confirmatory test.Citation18
Fundus autofluorescence
SW-FAF (excitation λ=488 nm; emission λ>500 nm) reveals RPD as hypofluorescent.Citation23,Citation35,Citation67–Citation69 The reason why RPD appear as hypofluorescent is still uncertain.Citation18,Citation70 One possible explanation is that the reflection and blockage of the blue excitation light prevent it from reaching the RPE.
On the other hand, most RPD deposits/subretinal materials may have an isofluorescent core conferring to these lesions a “target aspect”.Citation24 This peculiar aspect could derive from the accumulation in the RPD core of lipofuscin-like material, probably representing unphagocytized photoreceptor outer segments; alternatively, it could be related to the interruption of ellipsoid zone (EZ).Citation24 The absence of the target aspect in some RPD could be ascribed to heterogeneity in RPD composition or confocal selective laser ophthalmoscopy properties.Citation24 Interestingly, a small subset of patients may have intense hyperfluorescent RPD, which have been initially reported as less associated with late AMD,Citation71 even though it was not confirmed by later analysis.Citation71,Citation72 It has been speculated that hyperfluorescent RPD could be a transient form of pseudodrusen or small foci of acquired vitelliform material.Citation71 RPD extent increases over time, and it has been estimated to have a mean growth rate of 4.4 mm2/year.Citation73 Using quantitative FAF (qFAF), it has been observed that eyes with RPD have lower qFAF values compared with patients with soft drusen, cuticular drusen and controls even in unaffected retina areas.Citation74 Reduced qFAF values could reflect reduction in (intracellular but not extracellular) lipofuscin accumulation due to dysfunction in choroid-BM-RPE complex, slowing in visual cycle, reduced cone and rode densities and different composition of lipofuscin.Citation74 Since it is rapid, noninvasive, highly reliable, accurate (sensitivity: 86%, specificity: 92%–95%), SW-FAF is a valuable imaging modality to identify RPD.Citation18,Citation68,Citation75 Conversely, NIR-FAF (excitation λ=787 nm; emission λ>800 nm) has not good diagnostic abilities.Citation18
IR and multicolor
RPD appearance at IR imaging parallels FAF showing a hyporeflective reticular pattern and a variable target aspect, with an isoreflective core surrounded by hyporeflective halo.Citation24,Citation35,Citation73,Citation76 Using multimodal imaging (including CFP, IR and OCT), Suzuki et alCitation66 subclassified RPD in 3 different subtypes: dot, ribbon and midperipheral. In the first type, IR reveals perifoveal hyporeflective dots often showing a target aspect; the second type is characterized by perifoveal faint hyporeflective ribbons; and the third subtype is featured by midperipheral hyperreflective spots.Citation66 Different RPD subtypes may have distinct components and may confer differing risk of disease progression.Citation66 Since it is noninvasive, fast, high sensible and reliable, IR imaging has been advocated as a screening test to identify RPD.Citation18
MultiColor (Spectralis SD-OCT; Heidelberg Engineering, Heidelberg, Germany) merges 3 different laser channels (ie, IR: λ=820 nm; green reflectance: λ=515 nm; blue reflectance at λ=488 nm) into a single pseudocolor image. RPD have a peculiar yellowish-green reticular pattern and are more evident at green and IR than at blue reflectance.Citation77 Similar to IR, RPD may have a target appearance with a more intense yellowish/greenish core surrounded by a decreased intensity.Citation75,Citation77 MultiColor demonstrated equal identification rates of FAF and IR and higher individuation of target aspect.Citation75,Citation78 The green–blue enhanced mode, which stresses blue and green channels, reveals RPD as green/gray lesions, but has lower detection rates than standard MultiColor, confirming the value of the IR component.Citation75
OCT, en face OCT and OCT-A
SD-OCT shows RPD as discrete accumulation of hyperreflective material above the RPE in the subretinal space.Citation79,Citation80 Although contradicted by some studies, it is widely accepted that that hyperreflective subretinal drusenoid material seen at SD-OCT corresponds to RPD seen at other imaging modalities.Citation15,Citation76,Citation80,Citation81 Zweifel et alCitation80 cataloged RPD in 3 different stages: 1) diffuse accumulation of granular hyperreflective material between RPE and EZ, 2) mounds of material bowing and distorting EZ profile and 3) conical amassing with focal interruption of EZ. Subsequently, Querques et alCitation79 elucidated that RPD are dynamic structures characterized by agglomeration of subretinal material; moreover, they illustrated that the drusenoid material eventually reabsorbs and migrates into the inner retinal layers, and this was referred to as stage 4. Auge et alCitation82 confirmed the dynamism of RPD and emphasized the importance of dense scan protocols and exact registration of B-scans in the follow-up. Advanced stages of pseudodrusen life cycle are associated with sublesional RPE degeneration and shortening of photoreceptors.Citation83 Small series reported that RPD can completely disappear during the time, and this phenomenon may be unilateral and asymmetric.Citation84 RPD disappearance may leave outer retinal atrophy and focal reduction in choroidal thickness, and this could represent a novel phenotype of late AMD not included in the current classification systems.Citation85 It has been speculated that outer retinal atrophy following RPD regression may eventually involve RPE and choriocapillaris, leading to GA.Citation86
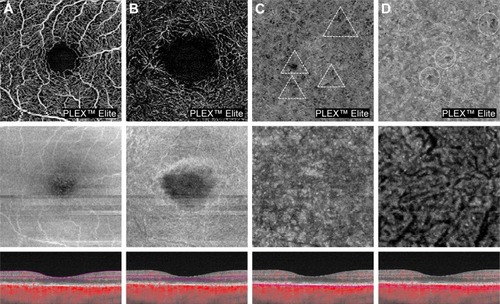
As previously discussed, several studies using EDI- and SS-OCT demonstrated that eyes with RPD are characterized by choroidal vascular depletion and fibrotic replacement, resulting in a widespread reduction in choroidal thickness even in comparison with other AMD phenotypes.Citation43–Citation52 Mrejen and SpaideCitation87 observed that RPD have low prevalence in eyes with very thin choroid (ie, pathologic myopia), and, therefore, they argued that RPD accumulation is unlikely to be related to a primary dysfunction at the choroidal level, but rather explained by other factors (eg, RPE dysfunction). However, this argument fails to consider that the thin choroid in high myopia can be functionally and structurally healthy, compatible with normal visual function. Interestingly, high prevalence of RPD has been reported in eyes suffering from age-related choroidal atrophy, which is an entity recently described characterized by choroidal thickness reduction, pigmentary changes and scarcity of visible choroidal vessels, reflecting a disease of small vessels involving the choroid.Citation88 Using OCT-A (), eyes with RPD exhibited a reduction in choriocapillary vessel density more extensive than in those with drusen and no RPD and which has been associated with poor visual acuity.Citation89–Citation91
Figure 2 OCT-A of a patient affected by RPD.
Abbreviations: OCT-A, optical coherence tomography angiography; RPD, reticular pseudodrusen; OCT, optical coherence tomography.
SD-OCT has excellent diagnostic properties and reliability in identification of RPD.Citation18 Schaal et alCitation64,Citation92 demonstrated that en face OCT can image RPD, and this methodology alone has similar diagnostic abilities of conventional multimodal imaging (ie, CFP, IR and FAF). Polarization-sensitive (PS)-OCT is able to provide more contrast for structures, such as RPE, that are able to alter the polarization state of the light.Citation93 PS-OCT can identify RPD, which preserve polarization, and distinguish them from drusen, which, conversely, alter polarization.Citation94 However, PS-OCT has not broken into the clinical practice, and its diagnostic properties are still unknown.
In addition to RPD identification, some authors have tried to determine whether OCT could provide information on the natural history of the disease. RPD thickness was a robust biomarker of future areas of GA growth.Citation95–Citation97 Moreover, EZ disruption pattern has been related to GA progression over time.Citation98 Identification of biomarkers and anatomic surrogates predicting future vision loss could be beneficial for clinical trials concerning early or intermediate AMD.Citation99
FA and ICGA
FA may reveal RPD as a filling defect in the choriocapillaris in early frames; however, due to its low sensitivity and invasive nature, it is seldom performed.Citation63
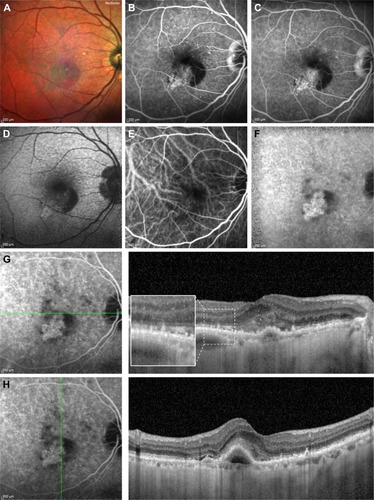
RPD are seen as hypofluorescent dots at mid and late phases of ICGA, which co-localize with dots seen at IR and FAF, suggesting a pathology internal to RPE.Citation14,Citation100 Using IR and ICGA, Querques et alCitation44 showed that RPD co-localize with choroidal intervascular stroma with sparing of areas above large choroidal vessels, suggesting impaired choroidal filling. Similarly, Alten et alCitation101 observed that RPD are located in areas of choroidal watershed, reinforcing the pathogenic role of choroidal hypoxia. However, further study did not find concordance between RPD and large choroidal blood vessels.Citation102 Although it owns good diagnostic abilities (sensitivity: 73%, specificity: 100%), ICGA is not the first choice test because of its invasive nature.Citation18 As shown in , FA and ICGA have a pivotal role in diagnosing CNV associated with RPD.
Figure 3 Multicolor imaging, FA, fundus autofluorescence, ICGA and structural SD-OCT of a patient affected by RPD and a type 1 CNV.
Abbreviations: FA, fluorescein angiography; ICGA, indocyanine green angiography; SD-OCT, spectral-domain optical coherence tomography; RPD, reticular pseudodrusen; CNV, choroidal neovascularization.
Adaptive optics
By improving the transverse resolution to 2 μm, adaptive optics visualize cone photoreceptors in vivo. Adaptive optics illustrate RPD as isoreflective lesions surrounded by a continuous/discontinuous hyporeflective halo, with the hyperreflective core ascribed to the drusenoid material itself and the hyporeflective halo related to absent or degenerated photoreceptors correspondent to EZ disruption.Citation103 Photo receptors mosaic is perturbed by RPD according to lesion stage; specifically, stages 1 and 2 are characterized by a reduction in photoreceptor density, whereas only stage 3 has an annular configuration.Citation104,Citation105 As shown by OCT studies, dynamism of RPD was further confirmed using adaptive optics.Citation106
Mrejen et alCitation107 showed that cone density over and around RPD is lower than drusen and healthy controls, suggesting that RPD could affect retinal function even in the absence of CNV or GA. On the contrary, Querques et alCitation103 found that cone density similarly decreased in drusen and RPD.
Functional tests
AMD is characterized by abnormal retinal function even in early or intermediate stage. Impairment in dark adaption and low-luminance visual acuity have been demonstrated in AMD, and these parameters are more severely affected by the presence of RPD potentially reflecting impairment in rod function.Citation68,Citation108,Citation109 In agreement with this hypothesis, Steinberg et alCitation110 illustrated a more pronounced deterioration of mean threshold sensitivity at scotopic than at photopic microperimetry. A recent study comparing eyes in normal macular health with and without RPD illustrated no significant difference regarding photopic acuity, mesopic acuity, low-luminance acuity, photopic contrast sensitivity and photopic light sensitivity.Citation111 In the same study, eyes with RPD exhibited delayed dark adaptation, although significant differences in age between the 2 groups could be imputable for such a difference.Citation111
Eyes with RPD have reduction in microperimetric retinal sensitivity despite preserved visual acuity.Citation112 Compared with drusen, RPD are associated with a more pronounced impairment in macular sensitivity.Citation113 Whether the extent of RPD is related to macular sensitivity is still unclear and contrasting results have been published.Citation114,Citation115
By recording local electroretinographic responses from the central 40°–50° of the macula, multifocal electroretinography (mfERG) allows assessing retinal function in a topographic way. Studies involving mfERG reported contrasting results.Citation115–Citation117 Alten et alCitation116,Citation117 did not find any significant difference between patients with RPD and healthy controls. Comparing areas with and without pseudodrusen, they observed no significant differences at baseline; conversely, reduction in amplitude was seen in affected areas during the follow-up, suggesting a progressive decline in retinal function over time, which did not correlate with structural data.Citation116,Citation117 Wu et alCitation115 reported conflicting results, showing that RPD presence and extent in intermediate AMD are independently linked to worse mfERG implicit time, but not to amplitude.
Natural history
As illustrated in the aforementioned paragraphs, RPD are dynamic structures evolving and even reabsorbing with time. RPD confer a higher risk of developing AMD during follow-up. In elder population with an otherwise healthy macula, RPD were found in the 25% of subjects conferring a 2-fold risk to develop early AMD.Citation118 In addition, the presence of RPD increase is significantly associated with higher odds to progress to late AMD.Citation6,Citation119 In fellow eyes of patients with unilateral CNV, the presence of AMD conferred extra odds to progress to late AMD.Citation34 RPD are linked to the development of GA, especially with multilobular type, and higher progression rates.Citation96,Citation97,Citation120 RPD are a strong risk factor for developing type 3 CNV, and, moreover, they are associated with higher earlier onset and bilateral disease.Citation121,Citation122 Eyes affected by type 3 CNV have higher odds to have also RPD, and, for this reason, RPD have been advocated as a diagnostic sign to distinguish type 3 CNV from other forms.Citation123,Citation124 Eyes with exclusively RPD have been associated with type 2 CNV.Citation125 In case of CNV development, RPD fade nearby the CNV itself, but they may still be observed more peripherally.Citation14,Citation69
RPD are a risk factor to develop atrophy following intravitreal anti-vascular endothelial growth factor (VEGF).Citation126–Citation128 However, presence of RPD did not interfere with the response to intravitreal anti-VEGF, at least in the short term.Citation129
Therapy
No specific therapy is available for RPD. Since RPD are in the spectrum of AMD, they can benefit from vitamin supplementation (ie, AREDS and AREDS2 formula) in case of early and intermediate diseases.Citation130 Beyond reducing the risk of late AMD development, supplementation with macular pigment component (ie, lutein, zeaxanthin and meso-zeaxanthin) may increase the macular pigment optical density primarily, if not only, in the RPD phenotype.Citation131 Presence of RPD in the setting of late AMD has no impact on the therapeutic strategy.
Conclusions
RPD represent a specific component of AMD, although they may be found even in other ocular diseases. They differ from drusen not only for their location (ie, subretinal space) but also for their components. Their presence should always be sought in patients suffering from AMD, since they are strongly associated with late stages of disease. RPD identification should employ at least 2 different imaging modalities, since their recognition may not be straightforward with one technique only. Future clinical trials and epidemiological studies should take into account RPD phenotype employing imaging modalities suitable for their identification.
Disclosure
GQ is a consultant for Alimera Sciences (Alpharetta, Georgia, USA), Allergan Inc. (Irvine, California, USA), Bayer Schering-Pharma (Berlin, Germany), Heidelberg (Germany), Novartis (Basel, Switzerland), Sandoz (Berlin, Germany), Zeiss (Dublin, OH, USA). FB has the following disclosures: Allergan (s), Alimera (s), Bayer (s), Farmila-Thea (s), Schering Pharma (s), Sanofi-Aventis (s), Novagali (s), Pharma (s), Hoffmann-La Roche (s), Genentech (s) and Novartis (s). The other authors report no conflicts of interest in this work.
References
- LimLSMitchellPSeddonJMHolzFGWongTYAge-related macular degenerationLancet201237998271728173822559899
- MimounGSoubraneGCoscasGLes drusen maculaires. [Macular drusen]J Fr Ophtalmol19901310511530 French2081842
- KleinRDavisMDMagliYLSegalPKleinBEHubbardLThe Wisconsin age-related maculopathy grading systemOphthalmology1991987112811341843453
- ArnoldJJSarksSHKillingsworthMCSarksJPReticular pseudodrusen. A risk factor in age-related maculopathyRetina19951531831917569344
- FingerRPChongEMcGuinnessMBReticular Pseudodrusen and Their Association with Age-Related Macular Degeneration: The Melbourne Collaborative Cohort StudyOphthalmology2016123359960826681391
- JoachimNMitchellPRochtchinaETanAGWangJJIncidence and progression of reticular drusen in age-related macular degeneration: findings from an older Australian cohortOphthalmology2014121491792524332537
- KleinRMeuerSMKnudtsonMDIyengarSKKleinBEThe epidemiology of retinal reticular drusenAm J Ophthalmol2008145231732618045568
- De BatsFMathisTMauget-FaysseMJoubertFDenisPKodjikianLPrevalence of reticular pseudodrusen in age-related macular degeneration using multimodal imagingRetina2016361465226090899
- BuitendijkGHHooghartAJBrusseeCEpidemiology of reticular pseudodrusen in age-related macular degeneration: the Rotterdam studyInvest Ophthalmol Vis Sci201657135593560127768796
- ChanHCougnard-GregoireADelyferMNMultimodal imaging of reticular pseudodrusen in a population-based setting: the alienor studyInvest Ophthalmol Vis Sci20165773058306527367498
- ZarubinaAVNeelyDCClarkMEPrevalence of subretinal drusenoid deposits in older persons with and without age-related macular degeneration, by multimodal imagingOphthalmology201612351090110026875000
- JoachimNMitchellPYounanCEthnic variation in early age-related macular degeneration lesions between white Australians and Singaporean AsiansInvest Ophthalmol Vis Sci20145574421442924970259
- AltenFClemensCRMilojcicCEterNSubretinal drusenoid deposits associated with pigment epithelium detachment in age-related macular degenerationRetina20123291727173222466490
- SmithRTSohrabMABusuiocMBarileGReticular macular diseaseAm J Ophthalmol20091485733743.e219878758
- SohrabMASmithRTSalehi-HadHSaddaSRFawziAAImage registration and multimodal imaging of reticular pseudodrusenInvest Ophthalmol Vis Sci20115285743574821693600
- CohenSYDuboisLTadayoniRDelahaye-MazzaCDebibieCQuentelGPrevalence of reticular pseudodrusen in age-related macular degeneration with newly diagnosed choroidal neovascularisationBr J Ophthalmol200791335435916973663
- Ueda-ArakawaNOotoSNakataIPrevalence and genomic association of reticular pseudodrusen in age-related macular degenerationAm J Ophthalmol20131552260269.e26223111182
- Ueda-ArakawaNOotoSTsujikawaAYamashiroKOishiAYoshimuraNSensitivity and specificity of detecting reticular pseudodrusen in multimodal imaging in Japanese patientsRetina201333349049723403515
- WildeCPatelMLakshmananAMoralesMADhar-MunshiSAmoakuWMPrevalence of reticular pseudodrusen in eyes with newly presenting neovascular age-related macular degenerationEur J Ophthalmol201626212813426350997
- WuZAytonLNLuuCDBairdPNGuymerRHReticular pseudodrusen in intermediate age-related macular degeneration: prevalence, detection, clinical, environmental, and genetic associationsInvest Ophthalmol Vis Sci20165731310131626998717
- CurcioCAMessingerJDSloanKRMcGwinGMedeirosNESpaideRFSubretinal drusenoid deposits in non-neovascular age-related macular degeneration: morphology, prevalence, topography, and biogenesis modelRetina201333226527623266879
- KimJHChangYSKimJWLeeTGKimCGPrevalence of subtypes of reticular pseudodrusen in newly diagnosed exudative age-related macular degeneration and polypoidal choroidal vasculopathy in Korean patientsRetina201535122604261226049615
- SmithRTChanJKBusuoicMSivagnanavelVBirdACChongNVAutofluorescence characteristics of early, atrophic, and high-risk fellow eyes in age-related macular degenerationInvest Ophthalmol Vis Sci200647125495550417122141
- QuerquesGQuerquesLMartinelliDPathologic insights from integrated imaging of reticular pseudodrusen in age-related macular degenerationRetina201131351852621150696
- ZweifelSASpaideRFYannuzziLAAcquired vitelliform detachment in patients with subretinal drusenoid deposits (reticular pseudodrusen)Retina201131222923420966822
- De BatsFWolffBMauget-FaysseMMeunierIDenisPKodjikianLAssociation of reticular pseudodrusen and early onset drusenISRN Ophthalmol2013201327308524563787
- GliemMMullerPLBirtelJHendigDHolzFGCharbel IssaPFrequency, phenotypic characteristics and progression of atrophy associated with a diseased bruch’s membrane in pseudoxanthoma elasticumInvest Ophthalmol Vis Sci20165773323333027367499
- ZweifelSAImamuraYFreundKBSpaideRFMultimodal fundus imaging of pseudoxanthoma elasticumRetina201131348249120966826
- GliemMMullerPLMangoldEReticular pseudodrusen in sorsby fundus dystrophyOphthalmology201512281555156226077580
- WildeCLakshmananAPatelMMoralesMUDhar-MunshiSAmoakuWMPrevalence of reticular pseudodrusen in newly presenting adult onset foveomacular vitelliform dystrophyEye (Lond)201630681782427034200
- CukrasCFlamendorfJWongWTAyyagariRCunninghamDSievingPALongitudinal structural changes in late-onset retinal degenerationRetina201636122348235627388725
- AlemanTSGarritySTBruckerAJRetinal structure in vitamin A deficiency as explored with multimodal imagingDoc Ophthalmol2013127323924323900584
- LallyDRBaumalCSubretinal drusenoid deposits associated with complement-mediated IgA nephropathyJAMA Ophthalmol2014132677577724921170
- PumariegaNMSmithRTSohrabMALetienVSouiedEHA prospective study of reticular macular diseaseOphthalmology201111881619162521550118
- Schmitz-ValckenbergSAltenFSteinbergJSGeographic Atrophy Progression (GAP) Study GroupReticular drusen associated with geographic atrophy in age-related macular degenerationInvest Ophthalmol Vis Sci20115295009501521498612
- BodduSLeeMDMarsigliaMMarmorMFreundKBSmithRTRisk factors associated with reticular pseudodrusen versus large soft drusenAm J Ophthalmol20141575985993.e224491417
- ChoBJHeoJWShinJPAhnJKimTWChungHEpidemiological association between systemic diseases and age-related macular degeneration: the Korea National Health and Nutrition Examination Survey 2008–2011Invest Ophthalmol Vis Sci20145574430443724970257
- CymermanRMSkolnickAHColeWJNabatiCCurcioCASmithRTCoronary artery disease and reticular macular disease, a subphenotype of early age-related macular degenerationCurr Eye Res201641111482148827159771
- RastogiNSmithRTAssociation of age-related macular degeneration and reticular macular disease with cardiovascular diseaseSurv Ophthalmol201661442243326518628
- PucheNBlanco-GaravitoRRichardFGenetic and environmental factors associated with reticular pseudodrusen in age-related macular degenerationRetina2013335998100423549092
- McCarterRVMcKayGJQuinnNBEvaluation of coronary artery disease as a risk factor for reticular pseudodrusenBr J Ophthalmol Epub2017819
- LeisyHBAhmadMMarmorMSmithRTAssociation between decreased renal function and reticular macular disease in age-related macular degenerationOphthalmology Retina2017114248
- GargAOllMYzerSReticular pseudodrusen in early age-related macular degeneration are associated with choroidal thinningInvest Ophthalmol Vis Sci201354107075708124071958
- QuerquesGQuerquesLForteRMassambaNCoscasFSouiedEHChoroidal changes associated with reticular pseudodrusenInvest Ophthalmol Vis Sci20125331258126322222508
- ThorellMRGoldhardtRNunesRPAssociation between subfoveal choroidal thickness, reticular pseudodrusen, and geographic atrophy in age-related macular degenerationOphthalmic Surg Lasers Imaging Retina201546551352126057754
- SwitzerDWJrMendoncaLSSaitoMZweifelSASpaideRFSegregation of ophthalmoscopic characteristics according to choroidal thickness in patients with early age-related macular degenerationRetina20123271265127122222760
- YunCOhJAhnSEHwangSYKimSWHuhKPeripapillary choroidal thickness in patients with early age-related macular degeneration and reticular pseudodrusenGraefes Arch Clin Exp Ophthalmol2016254342743525971212
- HaasPEsmaeelpourMAnsari-ShahrezaeiSDrexlerWBinderSChoroidal thickness in patients with reticular pseudodrusen using 3D 1060-nm OCT mapsInvest Ophthalmol Vis Sci20145542674268124651554
- Ueda-ArakawaNOotoSEllabbanAAMacular choroidal thickness and volume of eyes with reticular pseudodrusen using swept-source optical coherence tomographyAm J Ophthalmol20141575994100424491418
- CapuanoVSouiedEHMiereAJungCCostanzoEQuerquesGChoroidal maps in non-exudative age-related macular degenerationBr J Ophthalmol2016100567768226347526
- CorviFSouiedEHCapuanoVChoroidal structure in eyes with drusen and reticular pseudodrusen determined by binarisation of optical coherence tomographic imagesBr J Ophthalmol2017101348352
- ZhengFGregoriGSchaalKBChoroidal thickness and choroidal vessel density in nonexudative age-related macular degeneration using swept-source optical coherence tomography imagingInvest Ophthalmol Vis Sci201657146256626427849311
- A MartilloMMarsigliaMD LeeMPumariegaNBearellySSmithRTIs reticular macular disease a choriocapillaris perfusion problem?Med Hypothesis Discov Innov Ophthalmol201212374124600618
- EdwardsAORitterR3rdAbelKJManningAPanhuysenCFarrerLAComplement factor H polymorphism and age-related macular degenerationScience2005308572042142415761121
- JakobsdottirJConleyYPWeeksDEMahTSFerrellREGorinMBSusceptibility genes for age-related maculopathy on chromosome 10q26Am J Hum Genet200577338940716080115
- KleinRJZeissCChewEYComplement factor H polymorphism in age-related macular degenerationScience2005308572038538915761122
- RiveraAFisherSAFritscheLGHypothetical LOC387715 is a second major susceptibility gene for age-related macular degeneration, contributing independently of complement factor H to disease riskHum Mol Genet200514213227323616174643
- SmithRTMerriamJESohrabMAComplement factor H 402H variant and reticular macular diseaseArch Ophthalmol201112981061106621825189
- KhanKNMahrooOAKhanRSDifferentiating drusen: Drusen and drusen-like appearances associated with ageing, age-related macular degeneration, inherited eye disease and other pathological processesProg Retin Eye Res2016537010627173377
- GreferathUGuymerRHVesseyKABrassingtonKFletcherELCorrelation of histologic features with in vivo imaging of reticular pseudodrusenOphthalmology201612361320133127039021
- CurcioCAPresleyJBMillicanCLMedeirosNEBasal deposits and drusen in eyes with age-related maculopathy: evidence for solid lipid particlesExp Eye Res200580676177515939032
- JohnsonLVLeitnerWPStaplesMKAndersonDHComplement activation and inflammatory processes in Drusen formation and age related macular degenerationExp Eye Res200173688789611846519
- SivaprasadSBirdANitiahpapandRPerspectives on reticular pseudodrusen in age-related macular degenerationSurv Ophthalmol201661552153726994868
- SchaalKBLegarretaADFeuerWJComparison between widefield en face swept-source OCT and conventional multimodal imaging for the detection of reticular pseudodrusenOphthalmology2017124220521427856030
- AltenFEterNCurrent knowledge on reticular pseudodrusen in age-related macular degenerationBr J Ophthalmol201599671772225232026
- SuzukiMSatoTSpaideRFPseudodrusen subtypes as delineated by multimodal imaging of the fundusAm J Ophthalmol201415751005101224503406
- BindewaldABirdACDandekarSSClassification of fundus autofluorescence patterns in early age-related macular diseaseInvest Ophthalmol Vis Sci20054693309331416123434
- HoggRESilvaRStaurenghiGClinical characteristics of reticular pseudodrusen in the fellow eye of patients with unilateral neovascular age-related macular degenerationOphthalmology201412191748175524856310
- SarksJArnoldJHoIVSarksSKillingsworthMEvolution of reticular pseudodrusenBr J Ophthalmol201195797998521109695
- LoisNOwensSLCocoRHopkinsJFitzkeFWBirdACFundus autofluorescence in patients with age-related macular degeneration and high risk of visual lossAm J Ophthalmol2002133334134911860971
- LeeMYHamDISubretinal drusenoid deposits with increased autofluorescence in eyes with reticular pseudodrusenRetina2014341697623743636
- QuerquesGSouiedEHCorrespondenceRetina2015351e4e625473787
- SteinbergJSAugeJJaffeGJFleckensteinMHolzFGSchmitz-ValckenbergSGAP Study GroupLongitudinal analysis of reticular drusen associated with geographic atrophy in age-related macular degenerationInvest Ophthalmol Vis Sci20135464054406023633663
- GliemMMullerPLFingerRPMcGuinnessMBHolzFGCharbel IssaPQuantitative fundus autofluorescence in early and intermediate age-related macular degenerationJAMA Ophthalmol2016134781782427253610
- AltenFClemensCRHeiduschkaPEterNCharacterisation of reticular pseudodrusen and their central target aspect in multi-spectral, confocal scanning laser ophthalmoscopyGraefes Arch Clin Exp Ophthalmol2014252571572124276561
- SpaideRFColocalization of pseudodrusen and subretinal drusenoid deposits using high-density en face spectral domain optical coherence tomographyRetina201434122336234525380066
- QuerquesGSrourMMassambaNPucheNSouiedEHReticular pseudodrusenOphthalmology2013120487223552084
- BadalJBiarnesMMonesJPerformance characteristics of multicolor versus blue light and infrared imaging in the identification of reticular pseudodrusenInt Ophthalmol Epub2017121
- QuerquesGCanoui-PoitrineFCoscasFAnalysis of progression of reticular pseudodrusen by spectral domain-optical coherence tomographyInvest Ophthalmol Vis Sci20125331264127022266524
- ZweifelSASpaideRFCurcioCAMalekGImamuraYReticular pseudodrusen are subretinal drusenoid depositsOphthalmology20101172303312.e119815280
- HeifermanMJFernandesJKMunkMMirzaRGJampolLMFawziAAReticular pseudodrusen on infrared imaging are topographically distinct from subretinal drusenoid deposits on en face optical coherence tomographyRetina201535122593260326131588
- AugeJSteinbergJSFleckensteinMHolzFGSchmitz-ValckenbergSRetikuläre Drusen über die Zeit mittels SD-OCT. [Reticular drusen over time with SD-OCT]Ophthalmologe20141118765771 German24114561
- XuXLiuXWangXRetinal pigment epithelium degeneration associated with subretinal drusenoid deposits in age-related macular degenerationAm J Ophthalmol2017175879827986424
- SchickTErsoyLKirchhofBLiakopoulosSAsymmetrical behaviour of disappearance of reticular pseudodrusen in both eyes during long-term follow-up with spectral domain optical coherence tomographyGMS Ophthalmol Cases20144Doc0627625941
- SpaideRFOuter retinal atrophy after regression of subretinal drusenoid deposits as a newly recognized form of late age-related macular degenerationRetina20133391800180823764969
- SpaideRFImproving the age-related macular degeneration construct: a new classification systemRetina Epub2017526
- MrejenSSpaideRFThe relationship between pseudodrusen and choroidal thicknessRetina20143481560156624732697
- SpaideRFAge-related choroidal atrophyAm J Ophthalmol2009147580181019232561
- AltenFHeiduschkaPClemensCREterNExploring choriocapillaris under reticular pseudodrusen using OCT-angiographyGraefes Arch Clin Exp Ophthalmol2016254112165217327193430
- CicinelliMVRabioloAMarcheseAChoroid morphometric analysis in non-neovascular age-related macular degeneration by means of optical coherence tomography angiographyBr J Ophthalmol201710191193120028057649
- NesperPLSoetiknoBTFawziAAChoriocapillaris nonperfusion is associated with poor visual acuity in eyes with reticular pseudodrusenAm J Ophthalmol2017174425527794427
- SchaalKBLegarretaADGregoriGWidefield en face optical coherence tomography imaging of subretinal drusenoid depositsOphthalmic Surg Lasers Imaging Retina201546555055926057758
- PircherMHitzenbergerCKSchmidt-ErfurthUPolarization sensitive optical coherence tomography in the human eyeProg Retin Eye Res201130643145121729763
- RobertsPKBaumannBSchlanitzFGRetinal pigment epithelial features indicative of neovascular progression in age-related macular degenerationBr J Ophthalmol Epub201737
- NiuSde SisternesLChenQRubinDLLengTFully automated prediction of geographic atrophy growth using quantitative spectral-domain optical coherence tomography biomarkersOphthalmology201612381737175027262765
- MarsigliaMBodduSBearellySAssociation between geographic atrophy progression and reticular pseudodrusen in eyes with dry age-related macular degenerationInvest Ophthalmol Vis Sci201354127362736924114542
- XuLBlonskaAMPumariegaNMReticular macular disease is associated with multilobular geographic atrophy in age-related macular degenerationRetina20133391850186223632954
- Giocanti-AureganATadayoniRFajnkuchenFDourmadPMagazzeniSCohenSYPredictive value of outer retina en face OCT imaging for geographic atrophy progressionInvest Ophthalmol Vis Sci201556138325833026747761
- SchaalKBRosenfeldPJGregoriGYehoshuaZFeuerWJAnatomic clinical trial endpoints for nonexudative age-related macular degenerationOphthalmology201612351060107926952592
- ArnoldJJQuarantaMSoubraneGSarksSHCoscasGIndocyanine green angiography of drusenAm J Ophthalmol199712433443569439360
- AltenFClemensCRHeiduschkaPEterNLocalized reticular pseudodrusen and their topographic relation to choroidal watershed zones and changes in choroidal volumesInvest Ophthalmol Vis Sci20135453250325723599330
- VongkulsiriSOotoSMrejenSSuzukiMSpaideRFThe lack of concordance between subretinal drusenoid deposits and large choroidal blood vesselsAm J Ophthalmol2014158471071525034112
- QuerquesGKamami-LevyCBlanco-GaravitoRAppearance of medium-large drusen and reticular pseudodrusen on adaptive optics in age-related macular degenerationBr J Ophthalmol201498111522152724985725
- ZhangYWangXRiveroEBPhotoreceptor perturbation around subretinal drusenoid deposits as revealed by adaptive optics scanning laser ophthalmoscopyAm J Ophthalmol20141583584596.e124907433
- MeadwayAWangXCurcioCAZhangYMicrostructure of subretinal drusenoid deposits revealed by adaptive optics imagingBiomed Opt Express20145371372724688808
- ZhangYWangXGodaraPDynamism of dot subretinal drusenoid deposits in age-related macular degeneration demonstrated with adaptive optics imagingRetina Epub2017210
- MrejenSSatoTCurcioCASpaideRFAssessing the cone photoreceptor mosaic in eyes with pseudodrusen and soft Drusen in vivo using adaptive optics imagingOphthalmology2014121254555124183341
- FlamendorfJAgronEWongWTImpairments in dark adaptation are associated with age-related macular degeneration severity and reticular pseudodrusenOphthalmology2015122102053206226253372
- SevillaMBMcGwinGJrLadEMRelating retinal morphology and function in aging and early to intermediate age-related macular degeneration subjectsAm J Ophthalmol2016165657726940163
- SteinbergJSFitzkeFWFimmersRFleckensteinMHolzFGSchmitz-ValckenbergSScotopic and photopic microperimetry in patients with reticular drusen and age-related macular degenerationJAMA Ophthalmol2015133669069725811917
- NeelyDZarubinaAVClarkMEAssociation between visual function and subretinal drusenoid deposits in normal and early age-related macular degeneration eyesRetina20173771329133628633153
- ForteRCennamoGde CrecchioGCennamoGMicroperimetry of subretinal drusenoid depositsOphthalmic Res2014511323624158037
- QuerquesGMassambaNSrourMBoulangerEGeorgesASouiedEHImpact of reticular pseudodrusen on macular functionRetina201434232132923842105
- OotoSEllabbanAAUeda-ArakawaNReduction of retinal sensitivity in eyes with reticular pseudodrusenAm J Ophthalmol2013156611841191.e223972310
- WuZAytonLNMakeyevaGGuymerRHLuuCDImpact of reticular pseudodrusen on microperimetry and multifocal electroretinography in intermediate age-related macular degenerationInvest Ophthalmol Vis Sci20155632100210625736790
- AltenFHeiduschkaPClemensCREterNMultifocal electroretinography in eyes with reticular pseudodrusenInvest Ophthalmol Vis Sci201253106263627022918638
- AltenFHeiduschkaPClemensCREterNLongitudinal structure/function analysis in reticular pseudodrusenInvest Ophthalmol Vis Sci20145596073608125146989
- HuisinghCMcGwinGJrNeelyDThe association between subretinal drusenoid deposits in older adults in normal macular health and incident age-related macular degenerationInvest Ophthalmol Vis Sci201657273974526906160
- GilJQMarquesJPHoggRClinical features and long-term progression of reticular pseudodrusen in age-related macular degeneration: findings from a multicenter cohortEye (Lond)201731336437127768118
- FingerRPWuZLuuCDReticular pseudodrusen: a risk factor for geographic atrophy in fellow eyes of individuals with unilateral choroidal neovascularizationOphthalmology201412161252125624518615
- ChangYSKimJHYooSJLewYJKimJFellow-eye neovascularization in unilateral retinal angiomatous proliferation in a Korean populationActa Ophthalmol2016941e49e5325981599
- SawaMUenoCGomiFNishidaKIncidence and characteristics of neovascularization in fellow eyes of Japanese patients with unilateral retinal angiomatous proliferationRetina201434476176724100709
- RaveraVBottoniFGianiACigadaMStaurenghiGRetinal angiomatous proliferation diagnosis: a multiimaging approachRetina201636122274228127870798
- MarsigliaMBodduSChenCYCorrelation between neovascular lesion type and clinical characteristics of nonneovascular fellow eyes in patients with unilateral, neovascular age-related macular degenerationRetina201535596697425627089
- NaysanJJungJJDansinganiKKBalaratnasingamCFreundKBType 2 (Subretinal) neovascularization in age-related macular degeneration associated with pure reticular pseudodrusen phenotypeRetina201636344945726383711
- ChoHJYooSGKimHSRisk factors for geographic atrophy after intravitreal ranibizumab injections for retinal angiomatous proliferationAm J Ophthalmol20151592285292.e125447115
- MunkMRCeklicLEbneterAHufWWolfSZinkernagelMSMacular atrophy in patients with long-term anti-VEGF treatment for neovascular age-related macular degenerationActa Ophthalmol2016948e757e76427417506
- SaitoMIidaTKanoMItagakiKTwo-year results of combined intravitreal ranibizumab and photodynamic therapy for retinal angiomatous proliferationJpn J Ophthalmol2016601425026498642
- Nghiem-BuffetSGiocanti-AureganAJungCReticular pseudodrusen are not a predictive factor for the 1-year response to intravitreal ranibizumab in neovascular age-related macular degenerationRetina2017371535927380430
- BandelloFSacconiRQuerquesLCorbelliECicinelliMVQuerquesGRecent advances in the management of dry age-related macular degeneration: a reviewF1000Res2017624528529701
- CorviFSouiedEHFalfoulYPilot evaluation of short-term changes in macular pigment and retinal sensitivity in different phenotypes of early age-related macular degeneration after carotenoid supplementationBr J Ophthalmol2017101677077327587715
