Abstract
Objective
To assess the efficacy and tolerability of a fixed combination of bimatoprost and timolol (BTFC) in a large patient sample in a clinical setting.
Methods
In this multicenter, observational, noncontrolled, open-label study, patients (n = 1862) with primary open-angle glaucoma or ocular hypertension were treated with BTFC. Assessments were made at baseline, six weeks, and three months.
Results
Prior to starting BTFC, 92.3% of patients were taking other ocular hypotensive medications. In the overall group at three months, mean intraocular pressure was reduced from baseline (21.7 ± 4.5 mmHg and 21.8 ± 4.9 mmHg for the right and left eye, respectively) to 16.1 ± 3.0 mmHg for each eye (P < 0.0001). The majority of patients (92%) reported no adverse events. The most commonly reported adverse events (in >1% of patients) were eye irritation, and ocular and conjunctival hyperemia. Adherence to treatment was generally better than (35.4%) or the same as (57.5%) with prior therapy. BTFC tolerability was rated as excellent or good by 92.3% of physicians and 85.8% of patients.
Conclusions
In a large group of patients with primary open-angle glaucoma or ocular hypertension, treatment with BTFC was associated with consistent reductions in IOP, improved adherence to treatment, and good tolerability.
Introduction
Glaucoma is the second most common cause of blindness in adults, both in Central Europe and worldwide.Citation1 A significant risk factor associated with glaucoma is elevated intraocular pressure (IOP).Citation2,Citation3 The importance of IOP as a modifiable risk factor for visual field progression in glaucoma is well established.Citation4–Citation8 For example, in the Canadian Glaucoma Study, mean IOP at follow-up was significantly associated with visual field progression, with every 1 mmHg increment in IOP increasing the risk of progression by 19%.Citation4 Other risk factors for glaucoma include increased age, optic disc and visual defects, corneal thickness, and myopia.Citation2,Citation3
Commonly prescribed glaucoma medicines may take the form of monotherapies, adjunctive combinations, or fixed combination treatments. In the long term, monotherapy provides insufficient IOP lowering in the majority of patients.Citation9 However, the adjunctive use of several hypotensive medications also has potential disadvantages, such as an increased dosing frequency, which has been linked with poor compliance,Citation9–Citation11 the washout effect of multiple instillations,Citation10 and the increased adverse event burden of multiple medications.Citation12
Randomized clinical trials have shown that the fixed combination of bimatoprost and timolol (BTFC) is effective in reducing IOP.Citation13,Citation14 Bimatoprost is a prostamide which is thought to lower IOP largely by increasing uveoscleral out-flow, whereas timolol blocks β-adrenergic receptors in the ciliary body, thus decreasing aqueous humor production.Citation15 In a study comparing BTFC with its individual constituents in patients with glaucoma or ocular hypertension, BTFC caused a greater reduction in IOP than timolol or bimatoprost alone.Citation13 The purpose of this observational study was to assess the IOP-lowering efficacy, tolerability, compliance, and therapeutic safety of BTFC in a large patient sample in a routine clinical setting.
Methods
Study design
This was a multicenter, observational, open-label, exploratory, noninterventional, nonblinded study conducted in 400 centers in Germany. The centers and physicians were selected by Allergan GmbH, Germany. Patients were followed over three visits, ie, a baseline visit and follow-up visits at six weeks and three months. Patients were treated with BTFC (Ganfort®; Allergan Inc., Dublin, Ireland; 0.3 mg/mL bimatoprost, 5 mg/mL timolol) at a dose determined by their physician and guided by the summary of product characteristics within the clinical setting. Participation in the study did not influence the prescription of BTFC or any other drug. Data were gathered anonymously, in accordance with German law.
Patients
The study included patients with primary open-angle glaucoma or ocular hypertension, primarily those with insufficient IOP adjustment from previous β-blocker monotherapy.
Measurements
At the first visit, demographic and risk factor information (elevated IOP, family history of glaucoma, visual and optic disc defects), previous therapy, and IOP readings were recorded. The presence of optic cup and visual field defects was rated on a three-step scale (mild, moderate, and advanced). The reasons for changing therapy in patients who were receiving previous IOP-lowering therapy prior to BTFC treatment were recorded according to several categories, ie, insufficient IOP adjustment, appearance of glaucoma-related damage, progression of glaucoma-related damage, insufficient tolerability, lack of compliance, and other reasons. More than one reason could be recorded for each patient. Individual target IOP for right and left eyes was recorded.
The primary efficacy variable was the mean change in IOP from baseline to end of study at the third visit. IOP measurements were made for each eye three times (baseline, six weeks, three months) over the study period after switching to BTFC.
Other efficacy measures included physician-reported assessment of BTFC in terms of IOP reduction using a four-point scale, ie, excellent, good, moderate, and insufficient. Tolerability was assessed by questionnaires completed by both physicians and patients at the final examination using a four-point scale, ie, excellent, good, moderate, and bad. Patient compliance with BTFC was compared with previous therapy and rated by the physician as better, equal, or worse. All adverse events were recorded at the final visit as free-text entries using a questionnaire.
Statistical analysis
The planned sample size was 2000 patients from up to 400 ophthalmology centers; this patient number allowed for the detection of uncommon adverse events with an incidence of <0.1% at least once (α = 0.05, binomial distribution). Analyses were performed on the total sample unless otherwise stated. IOP was calculated as mean and standard deviation (SD). A two-sided paired-difference t-test was performed on the null hypothesis that IOP does not change after three months of study treatment. All statistics were performed using SAS® software (version 9.1.3; SAS Institute Inc., Cary, NC).
Results
The study included 1862 patients (57.4% female, 42.6% male) with primary open-angle glaucoma or ocular hypertension (). Most (72.5%) patients were >60 years old. Elevated IOP was the most commonly reported risk factor for glaucoma (81%), with 10% reporting both high IOP and a family history. The mean time since first diagnosis of elevated IOP was 7.7 years. Most patients had at least mild visual defects and optic disc defects at the baseline visit. The mean target IOP (mean of all individually identified target IOP ± SD) for the population was 16.2 ± 2.3 mmHg in both the right and left eyes.
Table 1 Patient demographics at baseline (n = 1862)
Prior therapy
Prior to switching to BTFC, 92.3% of patients (n = 1719) were recorded as taking other medications (). The remaining 143 (7.7%) patients either had not been receiving prior IOP-lowering therapy or had no available information regarding previous therapy. Prior therapy was timolol-based in the majority of patients (57.5%, either monotherapy or in combination with other therapies). Three patients (0.2%) had previously been using bimatoprost and timolol as an adjunctive combination. Of the 1719 patients previously taking other medications, 1387 (81%) were taking monotherapies, 292 (17%) were taking two adjunctive therapies, and 40 (2%) were taking three or more adjunctive therapies. Reasons for the change in medication were insufficient IOP lowering (86.3%), progression of glaucoma-related damage (25.6%), insufficient tolerability (13.1%), lack of compliance (11.8%), appearance of glaucoma-related damage (10.4%), and other reasons (2.3%), with some patients changing for more than one reason.
Table 2 Prior medications taken by >1% of patients, among those whose prior therapy was documented (n = 1719)Table Footnote*
BTFC therapy
At each visit, the dosage of BTFC was reassessed and prescribed as either none, once, twice or more times a day for each eye. The most common dose, used by 93.8% of patients, was once a day for each eye, as would be expected, given that this agent is licensed for once-daily dosing.
At the baseline visit, 88.5% of patients were using BTFC alone, 10.3% were using BTFC with additional medication, and data were unavailable for 1.2%. At the second visit (mean duration of BTFC treatment 6.7 ± 5.3 weeks), 84.6% of patients were using BTFC as single therapy, 12.0% were using BTFC with additional medication, and data were missing for 3.4%. At the final visit (mean duration of BTFC treatment 16.4 ± 8.1 weeks), 80.2% of patients were using BTFC as single therapy, 13.4% were taking additional medication, and data were unavailable for 6.3%.
Effect on IOP
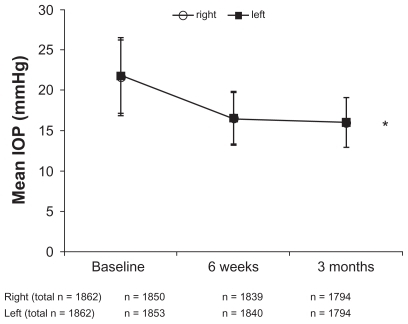
Baseline IOP (mean ± SD) in the total group was 21.7 ± 4.5 mmHg (n = 1850) and 21.8 ± 4.9 mmHg (n = 1853) for the right and left eyes, respectively (). In the total group at six weeks, mean IOP was reduced to 16.6 ± 3.3 mmHg for both right and left eyes (). At three months, mean IOP was further reduced to 16.1 ± 3.0 mmHg for both right and left eyes (, P < 0.0001 by t-test).
Figure 1 Mean IOP (± SD) at baseline, six weeks, and three months, for right and left eyes in the total population.
Abbreviation: IOP, intraocular pressure.
Mean IOP was also calculated for patients with complete data (n = 1775 patients with right eye data, n = 1778 patients with left eye data). Mean baseline IOP was 21.8 ± 4.5 mmHg in the right eye and 21.8 ± 4.8 mmHg in the left eye. After three months, mean IOP was 16.1 ± 3.0 mmHg in both the right and left eyes for this subset of patients with complete data.
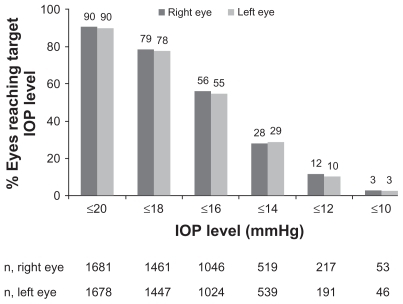
Over half of the total population achieved an IOP of ≤16 mmHg by the end of the study at three months; 56.2% of right eyes and 54.8% of left eyes achieved an IOP ≤16 mmHg (). In over a quarter of eyes studied (left and right), IOP was further reduced to ≤14 mmHg by three months (). Physicians rated overall efficacy of BTFC on IOP reduction as “excellent” or “good” in 89.2% of the overall group.
Tolerability and safety
Few adverse events were associated with the use of BTFC (7.7%), and most patients (92%) reported no adverse events. The most commonly reported adverse events (in >1% of patients) were eye irritation (2.2%), ocular hyperemia (1.6%), and conjunctival hyperemia (1.5%). The tolerability of BTFC was rated as “excellent” or “good” by 92.3% of physicians and 85.8% of patients at three months. Compliance with treatment, as rated by the physician, was rated as better than (35.4%) or the same as (57.5%) that with previous therapy. Continuation of BTFC therapy beyond the end of the study was 82.9%.
Discussion
In this German observational study reflecting clinical practice conditions, a large group of patients with primary open-angle glaucoma and ocular hypertension achieved further IOP reductions after switching therapy to BTFC. The change in IOP over the three-month observation period was highly significant (P < 0.0001 by t-test). Most patients were switched to BTFC because of insufficient IOP lowering with their previous therapy. BTFC treatment was also associated with a low incidence of conjunctival hyperemia (1.5%), good tolerability, and improved compliance compared with prior treatment.
In a double-masked, randomized study of 445 patients with glaucoma or ocular hypertension, a significantly lower incidence of conjunctival hyperemia was observed with the fixed combination of BTFC (8.5%) versus the nonfixed combination (12.5%) or single agent bimatoprost (18.9%).Citation14 A further study in 53 patients with glaucoma showed an improvement in hyperemia in 69% of patients switching from prior combination treatment to BTFC.Citation16
Prior to switching to BTFC, patients in this study were taking a variety of therapies, including monotherapies and combined treatments, with the majority receiving timolol (57.5%) or latanoprost (23.8%). There are few published data regarding switching from a prior therapy to BTFC, but some smaller studies have shown significant reductions in IOP after switching to BTFC in patients whose IOP was inadequately controlled on previous therapies.Citation16–Citation18 An observational study of 606 patients switched to BTFC from monotherapy (66.8%), nonfixed combinations (17.2%), and other fixed combinations (16.0%) showed additional IOP reductions versus baseline. Furthermore, patients who had previously received β-blocker or prostaglandin analog treatment achieved an additional 25.8% or 22.6% decrease in IOP from baseline, respectively, after switching to BTFC treatment.Citation18 After three months of BTFC treatment, 85% of all eyes achieved an IOP of ≤18 mmHg, which is similar to the findings in the current study.
Another study of 102 patients who switched to BTFC following inadequate treatment with monotherapy, or combination or fixed combination treatments also showed a decrease in mean IOP from baseline after two months.Citation17 The reasons for the greater IOP lowering achieved when patients switch from previous therapy to BTFC may relate to improved compliance resulting from once-daily administration.Citation11
This was an open-label observational study including many centers. Such a study has inherent limitations, which must be acknowledged. For instance, the noninterventional nature of the study means that there could be no washout period between the earlier prior medications and the switch to BTFC. The design is also essentially uncontrolled, making interpretation more difficult. However, the study captures important information regarding the use of fixed combinations in everyday life, and the results confirm that BTFC offers good efficacy and tolerability to patients with primary open-angle glaucoma or ocular hypertension with insufficient IOP control on previous therapy. These findings should be considered alongside results from well controlled, randomized clinical trials to guide the use of IOP-lowering therapy in clinical practice.
Conclusion
In this observational study, three months of BTFC treatment resulted in significant IOP reductions in a large group of patients in a German clinical setting, where most patients were previously insufficiently controlled on other hypotensive agents. BTFC was well tolerated and associated with a low rate of adverse events, a high level of both patient and physician reported satisfaction, and good adherence to treatment.
Figures and Tables
Disclosure
Allergan Ltd funded this study and provided the services of Darwin Healthcare Communications for editorial support.
References
- ResnikoffSPascoliniDEtya’aleDGlobal data on visual impairment in the year 2002Bull World Health Organ20048284485115640920
- GordonMOBeiserJABrandtJDThe Ocular Hypertension Treatment Study: Baseline factors that predict the onset of primary open-angle glaucomaArch Ophthalmol200212071472012049575
- BurrJMMowattGHernandezRThe clinical effectiveness and cost-effectiveness of screening for open angle glaucoma: A systematic review and economic evaluationHealth Technol Assess20071141iiiivixx1190
- ChauhanBCMikelbergFSBalasziAGLeBlancRPLeskMRTropeGECanadian Glaucoma Study: Risk factors for the progression of open-angle glaucomaArch Ophthalmol20081261030103618695095
- Collaborative Normal-Tension Glaucoma Study GroupComparison of glaucomatous progression between untreated patients with normaltension glaucoma and patients with therapeutically reduced intraocular pressuresAm J Ophthalmol19981264874979780093
- HeijlALeskeMCBengtssonBHymanLBengtssonBHusseinMReduction of intraocular pressure and glaucoma progression: Results from the Early Manifest Glaucoma TrialArch Ophthalmol20021201268127912365904
- KassMAHeuerDKHigginbothamEJThe Ocular Hypertension Treatment Study: A randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary openangle glaucomaArch Ophthalmol200212070171312049574
- LichterPRMuschDCGillespieBWInterim clinical outcomes in the Collaborative Initial Glaucoma Treatment Study comparing initial treatment randomized to medications or surgeryOphthalmology20011081943195311713061
- European Glaucoma SocietyTerminology and Guidelines for Glaucoma3rd edSavona, ItalyEditrice, Dogma2008
- ChraiSSMakoidMCEriksenSPRobinsonJRDrop size and initial dosing frequency problems of topically applied ophthalmic drugsJ Pharm Sci1974633333384820359
- PatelSCSpaethGLCompliance in patients prescribed eyedrops for glaucomaOphthalmic Surg1995262332367651690
- DunkerSSchmuckerAMaierHTolerability, quality of life, and persistency of use in patients with glaucoma who are switched to the fixed combination of latanoprost and timololAdv Ther20072437638617565929
- BrandtJDCantorLBKatzLJBatoosinghALChouCBossowskaIBimatoprost/timolol fixed combination: A 3-month double-masked, randomized parallel comparison to its individual components in patients with glaucoma or ocular hypertensionJ Glaucoma20081721121618414107
- HommerAA double-masked, randomized, parallel comparison of a fixed combination of bimatoprost 0.03%/timolol 0.5% with non-fixed combination use in patients with glaucoma or ocular hypertensionEur J Ophthalmol200717536217294383
- LimKSNauCBO’ByrneMMMechanism of action of bimatoprost, latanoprost, and travoprost in healthy subjects. A crossover studyOphthalmology200811579079518452763
- SkorkovskaKComparison of intraocular pressure lowering efficacy of bimatoprost/timolol fixed combination and other glaucoma medications in the treatment of glaucomaCesk Slov Oftalmol200864144148 Czech18780653
- ZafarAA switch to bimatoprost/timolol fixed combination resulted in improved IOP reduction in patients with POAG, regardless of previous IOP-lowering therapyPresented at: World Glaucoma CongressBoston, MAJuly 8–11, 2009
- FeuerhakeCBuchholzPKimmichFEfficacy, tolerability and safety of the fixed combination of bimatoprost 0.03% and timolol 0.5% in a broad patient population: Multicenter, open-label observational studyCurr Med Res Opin2009251037104319290780