Abstract
Objective
To assess the efficacy of nepafenac 0.1% ophthalmic suspension in improving the clinical outcomes following cataract surgery (CS) in patients with nonproliferative diabetic retinopathy.
Methods
In two similar multicenter, randomized studies, patients received either nepafenac 0.1% or vehicle, instilled three times daily starting a day prior to surgery and continuing for 90 days postoperatively. A post hoc analysis of these two studies was conducted to assess 1) the likelihood for development of postoperative macular edema (ME), based on the percentage of patients who developed ME (≥30% increase from preoperative baseline in central subfield macular thickness) within 90 days following CS and 2) best-corrected visual acuity (BCVA) endpoints, including the percentage of patients with a BCVA improvement of ≥15 letters from preoperative baseline to Day 14 and maintained through Day 90. Results for individual studies and their pooled estimates (only visual acuity endpoints) are reported. Primary inference was based on odds ratio (OR).
Results
This post hoc analysis included 411 patients (nepafenac 0.1%: 205; vehicle: 206). The incidence of postoperative ME within 90 days of CS was notably lower in the nepafenac-treated patients than in vehicle-treated patients (study 1: 3.2% vs 16.7%; OR =0.2, 95% confidence interval [CI] =0.1, 0.5, P=0.001; study 2: 5.0% vs 17.5%; OR =0.2, 95% CI =0.1, 0.8, P=0.018). A higher percentage of nepafenac-treated patients than vehicle-treated patients gained ≥15 letters from preoperative baseline to Day 14, which was maintained through Day 90 (study 1: 38.4% vs 21.4%; OR =2.4, 95% CI =1.4, 4.2, P=0.003; study 2: 35.0% vs 25.0%; OR =1.6, 95% CI =0.8, 3.2, P=0.172; pooled: 37.1% vs 22.8%; OR =2.0, 95% CI =1.3, 3.1, P=0.001). The odds of >5-letter and >10-letter loss in BCVA from postoperative Day 7 were higher in vehicle-treated than in nepafenac-treated patients.
Conclusion
These results support the clinical benefit of prophylactic use of nepafenac 0.1% for reducing the risk of postoperative ME and for improvement in BCVA outcomes following CS in patients with nonproliferative diabetic retinopathy.
Introduction
Postoperative macular edema (ME) following cataract surgery is an important cause for suboptimal visual outcomes, particularly in high-risk patients such as those with diabetes.Citation1–Citation8 A retrospective database study of 81,984 consecutive phacoemulsification cataract operations found the incidence of postoperative ME to be four times higher in patients with diabetes and the relative risk of postoperative ME to increase in proportion with the severity of diabetic retinopathy (DR).Citation9
The prostaglandin-mediated inflammatory response triggered as a result of trauma during cataract surgery is considered to be an underlying cause for the development of ME.Citation4 The effectiveness of topical anti-inflammatory agents, such as corticosteroids and nonsteroidal anti-inflammatory drugs (NSAIDs), that are commonly used for the management of postoperative ocular inflammation and pain, has also been studied for the prevention of postoperative ME.Citation1,Citation2,Citation10,Citation11
Nepafenac (Alcon Research Ltd, Fort Worth, TX, USA), a topical ocular NSAID, is available as an ophthalmic suspension in concentrations of 0.1% and 0.3%.Citation12,Citation13 Unlike other NSAIDs, nepafenac is a prodrug that is deaminated to its active metabolite (amfenac) in the ocular tissues.Citation14,Citation15 Both nepafenac and amfenac are potent inhibitors of cyclooxygenase isoforms, COX-1 and COX-2, and distribute rapidly in both the anterior and posterior segments of the eye.Citation14–Citation16 The high bioavailability of nepafenac in the posterior segment serves as a reservoir for hydrolysis to amfenac and accounts for nepafenac’s prolonged activity in the ocular tissues.Citation14–Citation16
The nepafenac 0.1% and 0.3% ophthalmic suspensions are the only NSAIDs approved in Europe for the reduction in the risk of postoperative ME associated with cataract surgery in patients with diabetes, with a recommended use for up to 60 days postoperatively.Citation7 An extended treatment with nepafenac ophthalmic suspension 0.1% for 90 days after cataract surgery was shown to result in statistically significant and clinically relevant reduction in the risk of postoperative ME, and also a better maintenance of visual acuity (VA) in patients with nonproliferative diabetic retinopathy (NPDR).Citation17,Citation18
To gain additional understanding of the efficacy of nepafenac 0.1% in improving clinical outcomes following cataract surgery in patients with diabetes and NPDR, a post hoc analysis was conducted for data from the two individual nepafenac 0.1% studies as well as the pooled data from the two studies.
Methods
Study design
This post hoc analysis included data from two, multicenter, randomized, double-masked, controlled, parallel-group trials. One was a phase II study conducted between 2008 and 2010 at study centers in the USA (NCT00782717; study 1) and the other was a phase III study conducted between 2009 and 2010 at study centers in the USA, Europe, Middle East, and Asia Pacific (NCT00939276; study 2). The detailed study designs of the two studies have been published previously.Citation17,Citation18 Both the studies were conducted in accordance with the Good Clinical Practice and the Declaration of Helsinki. Each study protocol was reviewed and approved by an Independent Ethics Committee or an Institutional Review Board for each participating center. All patients provided written informed consent before entering the respective study.
The two trials were similar in design (). Briefly, patients were administered either nepafenac 0.1% or vehicle, instilled three times daily in the study eye, on the day prior to cataract surgery, on the day of surgery, and for 90 days thereafter. An additional one drop of the study medication was administered 30–120 minutes prior to surgery. In all patients, regardless of the assigned study drug, a corticosteroid (study 1: OMNIPRED™, prednisolone acetate ophthalmic suspension; study 2: TOBRADEX® tobramycin and dexamethasone ophthalmic suspension) was instilled into the study eye four times daily for 2 weeks postsurgery. There were six postsurgical visits (days 1, 7, 14, 30, 60, and 90) in both studies. The only differences between the two studies were the eligibility requirements in central subfield macular thickness (CSMT) and the method for optical coherence tomography (OCT). In the phase II study, macular thickness was measured using time domain OCT and the ME cut-off was CSMT ≥250 µm, while in the phase III study, spectral domain OCT (SD-OCT) was used and the ME cut-off was CSMT >320 µm.Citation17,Citation18
In both studies, eligible patients were aged ≥18 years and had a cataract that required extraction by phacoemulsification with planned implantation of the posterior chamber intraocular lens, and had diabetes (Type 1 or Type 2) and were diagnosed with NPDR (mild, moderate, or severe) as defined by the International Clinical Diabetic Retinopathy Disease Severity Scale.Citation17,Citation18 At least 50% of the randomized patients in each treatment arm had to have moderate or severe NPDR. Patients with pre-existing ME in the study eye were excluded.
Post hoc analyses assessments
The efficacy and safety data from the two individual studies have been published previously.Citation17,Citation18 In this post hoc analysis, the endpoints assessed in the individual studies were the following: the percentage of patients who developed ME (defined as ≥30% increase from preoperative baseline in CSMT) within 90 days following cataract surgery; the percentage of patients with a best-corrected visual acuity (BCVA) improvement of ≥15 letters from preoperative baseline to Day 14 and maintained through Day 90; the percentage of patients with a BCVA improvement of ≥15 letters from preoperative baseline to Day 90 and Day 60; the percentage with BCVA loss of >5 letters and >10 letters from Day 7 to any visit; and mean change from baseline in BCVA. The post hoc analysis of pooled data from the two studies only included the VA endpoints, since different OCT methods were used in the two studies for assessment of ME. There were no safety assessments in this post hoc analysis.
Statistical analyses
The post hoc efficacy analysis was conducted on the full analysis set which consisted of all patients who completed the implant surgery and had at least one on-therapy postsurgical visit. For the pooled analysis, the full analysis sets of the respective studies were pooled for each treatment arm.
A logistic regression model was employed that included treatment and retinopathy severity terms to compare odds of event outcomes. The primary inference for ME and BCVA endpoints was based on the odds ratio (OR). The estimated OR, associated 95% confidence intervals (CIs), and P-values were calculated. The percentage of patients with BCVA improvement of ≥15 letters from preoperative baseline to Day 14 and maintained through Day 90 was based on a binary variable (positive outcome; negative outcome) which was derived using the change from preoperative baseline in BCVA at Day 14, Day 30, Day 60, and Day 90. A positive outcome required that BCVA improvement from preoperative baseline be ≥15 letters at all four visits (days 14, 30, 60, and 90). Any response short of BCVA improvement of ≥15 letters at all four visits was considered a negative outcome. A patient with BCVA data missing at one or more than one visit was considered as a negative outcome. For the mean change from baseline, the estimated difference in means by visit and the associated 95% CIs were provided. Descriptive statistics were used to summarize baseline and demographic characteristics, and data were presented by treatment group.
Results
The baseline and demographic characteristics of all patients included in the analysis are shown in . The pooled population included 411 patients (nepafenac 0.1% group: 205; vehicle: 206). Overall, the two treatment groups were well balanced. The mean age of patients was 67 years, ~46% were males, and >80% were white. In both treatment groups, >50% patients had moderate NPDR and 38% patients had mild NPDR ().
Table 1 Demographic and baseline characteristics of patients in each treatment group by individual study and pooled data (FAS)
ME endpoint
As previously reported, in both studies, a significantly lower percentage of patients in nepafenac 0.1%-treated group developed ME relative to those in the vehicle group (study 1: 3.2% vs 16.7%, P=0.001; study 2: 5.0% vs 17.5%, P=0.012).Citation17,Citation18 In this post hoc analysis, the odds of developing postoperative ME within 90 days of cataract surgery were observed to be notably lower in nepafenac 0.1%-treated group than in the vehicle-treated group in the two studies (study 1: OR =0.2, 95% CI =0.1, 0.5, P=0.001; study 2: OR =0.2; 95% CI =0.1, 0.8, P=0.018). No pooled analysis was performed for this endpoint due to differences in the OCT methodology used in the two studies.
BCVA endpoints
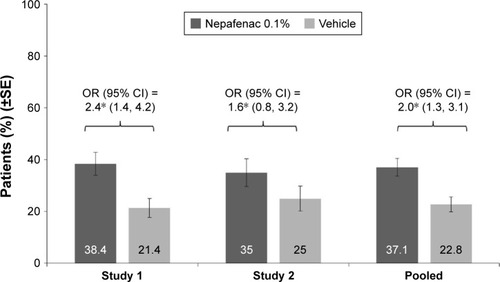
As shown in , a higher percentage of nepafenac 0.1%-treated patients had a BCVA improvement of ≥15 letters from preoperative baseline to Day 14 and maintained through Day 90, compared with vehicle (study 1: 38.4% vs 21.4%, P=0.003; study 2: 35.0% vs 25.0%, P=0.172). The analysis of pooled data from the two studies also showed that treatment with nepafenac 0.1% was associated with a higher odds of achieving a BCVA improvement of ≥15 letters from preoperative baseline to Day 14 maintained through Day 90, as compared to vehicle (37.1% vs 22.8%; OR =2.0, 95% CI =1.3, 3.1; P=0.001).
Figure 2 Percentage of patients with BCVA improvement of ≥15 letters from preoperative baseline to Day 14 and maintained through Day 90 in each treatment group by individual study and pooled data (FAS).
Abbreviations: BCVA, best-corrected visual acuity; CI, confidence interval; FAS, full analysis set; OR, odds ratio; SE, standard error.
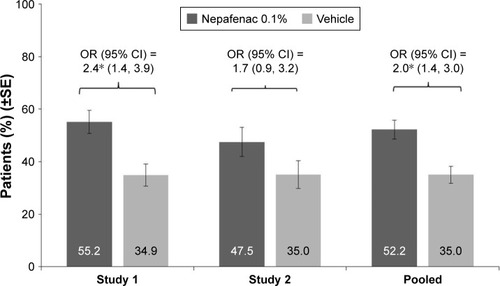
As shown in , while the percentage of patients with an improvement in VA of ≥15 letters from preoperative baseline to Day 90 was higher in the nepafenac 0.1% group than in the vehicle group, the difference between the groups was notable in study 1 (55.2% vs 34.9%, P=0.001), but not in study 2 (47.5% vs 35%, P=0.111). However, in the pooled estimate, treatment with nepafenac 0.1% was associated with an increased odds of improvement in VA of ≥15 letters from preoperative baseline to Day 90, compared with vehicle (52.2% vs 35%; OR =2.0, 95% CI =1.4, 3.0; P<0.001).
Figure 3 Percentage of patients with BCVA improvement of ≥15 letters from preoperative baseline and maintained through Day 90 in each treatment group by individual study and pooled data (FAS).
Abbreviations: BCVA, best-corrected visual acuity; CI, confidence interval; FAS, full analysis set; OR, odds ratio; SE, standard error.
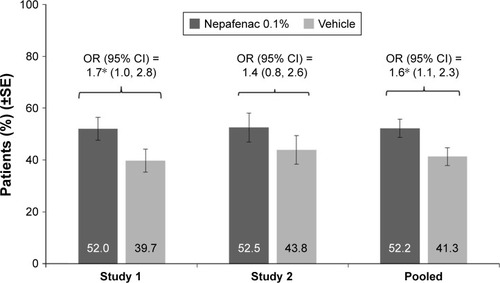
Similarly, the percentage of patients with an improvement in VA of ≥15 letters from preoperative baseline to Day 60 was higher in the nepafenac 0.1% group than in vehicle, the difference between treatment groups was notable in study 1 (P=0.046), but not in study 2 (P=0.272; ). In the pooled estimate, treatment with nepafenac 0.1% was associated with an increased odds of improvement in VA of ≥15 letters from preoperative baseline to Day 60 (OR =1.6; 95% CI =1.1, 2.3; P=0.025; ).
Figure 4 Percentage of patients with BCVA improvement of ≥15 letters from preoperative baseline and maintained through Day 60 in each treatment group by individual study and pooled data (FAS).
Abbreviations: BCVA, best-corrected visual acuity; CI, confidence interval; FAS, full analysis set; OR, odds ratio; SE, standard error.
The percentage of patients with a loss in VA of >5 letters or >10 letters from Day 7 to any visit was more in the vehicle than in the nepafenac 0.1% group. For both these BCVA endpoints, the odds for postoperative loss in VA were notably lower with nepafenac 0.1% compared with vehicle in study 1 and in the analysis of pooled data from the two studies (P<0.001), but not in study 2 (P>0.05; ).
Table 2 Summary of BCVA outcomes in each treatment group by individual study and pooled data (FAS)
The mean changes in BCVA for patients in nepafenac 0.1%- and vehicle-treated groups at all postoperative visits are shown in . The mean change from baseline in BCVA at all postoperative visits up to Day 90 was greater in the nepafenac 0.1% group than in the vehicle group, although the difference between the two treatment groups was notable only at Day 60 (in study 1 and in the analysis of pooled data from the two studies, but not in study 2; ).
Table 3 Mean BCVA at baseline and at each postoperative visit in each treatment group by individual study and pooled data (FAS)
Discussion
The results of this post hoc analyses corroborated the findings from the two individual studies, that prophylactic treatment with nepafenac 0.1%, initiated 1 day prior to and continued for 90 days after the cataract surgery, reduces the risk of postoperative ME in patients with diabetes and NPDR, compared to vehicle. Treatment with nepafenac 0.1% was found to lower the odds of postoperative ME by 80% in these patients, compared with vehicle. This finding is consistent with the primary analysis of data in the two studies that had shown the incidence of postoperative ME to be three- to five fold lower in the nepafenac 0.1% group than in the vehicle group.Citation17,Citation18 Recently, the nepafenac 0.3% ophthalmic suspension administered once daily for 90 days postoperatively has also been shown to reduce the risk of postoperative ME in patients with NPDR.Citation19
Furthermore, in study 1 and in pooled analysis, compared to vehicle-treated patients, a notably higher percentage of nepafenac 0.1%-treated patients had a faster recovery in VA, with a clinically meaningful improvement (≥15 letters gain) in BCVA from preoperative baseline to postoperative Day 14, which was sustained throughout the study period (Day 90). Higher percentage of patients in the vehicle group had a >5-letter and >10-letter loss after postoperative Day 7, compared with the nepafenac 0.1% group. Overall, for all BCVA endpoints in this study, patients treated with nepafenac 0.1% showed better VA improvements, consistent with results from previous studies.Citation20,Citation21
One of the quality measures of cataract surgery is the percentage of patients achieving a VA of 20/40 or better (distance or near) within 90 days following an uneventful cataract extraction.Citation22 While good visual prognosis is reported in diabetic patients after cataract surgery, the percentage of patients, especially those with DR, achieving better than 20/40 vision is lower than that reported for nondiabetic patients.Citation23–Citation25 Suboptimal visual function severely impacts the quality of life; therefore, early restoration of vision after cataract surgery is highly desirable to the patients.Citation26 Patients with DR are more prone to inflammatory effects, due to a compromised blood–retinal barrier function that can result in exacerbation of ME or retinopathy progression.Citation27–Citation31 Therefore, DR patients require careful management of post-operative inflammation to ensure stable vision, and thus, a preventive approach would help in reducing the risk of visual impairment due to ME.
Contrasting reports exist regarding the beneficial role of NSAIDs in reducing the risk of postoperative ME after cataract surgery.Citation1,Citation2,Citation10,Citation11,Citation28–Citation41 However, a comparison of results across studies is limited due to factors such as differences in the definition of ME used, the method used for detecting ME (fluorescein angiography vs OCT), duration of treatment, concurrent medication used, and the patient population included. Very few of these studies have assessed both postoperative ME and BCVA outcomes.
NSAIDs have been reported to be more effective than topical corticosteroids in reducing/preventing postoperative ME after an uneventful cataract surgery.Citation1,Citation11 It was observed in a systematic review of randomized clinical trials that in patients with diabetes, a combination of topical NSAIDs and corticosteroids significantly reduced the odds of developing postoperative ME after cataract surgery than corticosteroids alone.Citation1 Kim et al have suggested that this is likely due to the additive effect of the two anti-inflammatory drugs.Citation2 However, results from the two nepafenac 0.1% studies reported here suggest that the reduction in risk of postoperative ME observed in the two studies is primarily due to nepafenac 0.1% treatment. Although all patients had received additional corticosteroid treatment, the ME and VA findings were in favor of the group receiving nepafenac 0.1% than vehicle at all postoperative time points in both the studies. This is supported by another study that directly compared nepafenac with a topical corticosteroid in low-risk patients; after cataract extraction, nepafenac was found to be more effective in preventing cystoid ME and leading to more rapid visual recovery.Citation20
A limitation of the results presented in this study was that all analyses were post hoc. Thus, claims to statistical significance were not made. Furthermore, study 2 was terminated early, with only 67% of the planned sample size enrolled due to patient recruitment difficulties. The reduced sample size in study 2 could have contributed to the relatively weaker results for VA endpoints, compared to study 1 results. However, VA outcomes in study 2 were numerically in favor of nepafenac 0.1% suspension than vehicle and, thus, directionally consistent with study 1. Though study 1 and study 2 had similar inclusion criteria, they differed in the OCT technique used: time domain OCT vs SD-OCT. Due to greater sensitivity of SD-OCT to detect retinal anatomy, a high percentage of screened patients, such as even those with small cystoid abnormalities/more severe retinopathy, were excluded, which severely limited the enrollment in study 2.
Safety was not assessed as part of this post hoc analysis. However, the safety profile of nepafenac has been established across several studies. The adverse events reported in the two nepafenac 0.1% studies included in this post hoc analyses were consistent with those reported in previous nepafenac studies.Citation20,Citation21,Citation31 Overall, no new safety signals were identified with the extended use of nepafenac 0.1% for up to 90 days following cataract surgery in either study.Citation17,Citation18
Conclusion
These results support the clinical benefit with the prophylactic use of nepafenac suspension 0.1%, beginning preoperatively, in reducing the risk of postoperative ME and for early and sustained improvement in BCVA outcomes following an uneventful cataract surgery in patients with diabetes and NPDR.
Acknowledgments
The study was funded by Alcon Research Ltd (now a Novartis company), Fort Worth, TX, USA. Medical writing support for this manuscript was provided by Shivani Vadapalli (Novartis Healthcare Pvt Ltd, Hyderabad, India).
Disclosure
Rishi P Singh is a consultant for Alcon, Regeneron, Genentech, Zeiss, and Shire and conducts sponsored research for Genentech/Roche, Regeneron, and Apellis. Giovanni Staurenghi reports personal fees from Novartis, personal fees from Alcon, personal fees from Bayer, personal fees from Allergan, personal fees from Boehringer Ingelheim, personal fees from Genentech, personal fees from Roche, grants and personal fees from Zeiss Meditec, grants and personal fees from Heidelberg Engineering, grants and personal fees from Optos, and grants and personal fees from Centervue outside the submitted work. Ayala Pollack has received research grant from Alcon, Bayer, Neovista, Novartis, Ophthotech, Regeneron, Quark, and Santen. Robert Lehmann is a consultant for Alcon and conducts sponsored research for Baush & Lomb, AMO, WaveTech, Omerous, and AVS. Adeniyi Adewale, Thomas Walker, and Dana Sager are employees of Alcon Research Ltd, Fort Worth, TX. The authors report no other conflicts of interest in this work.
References
- WieldersLHLambermontVASchoutenJSPrevention of cystoid macular edema after cataract surgery in non-diabetic and diabetic patients: a systematic review and meta-analysisAm J Ophthalmol2015160596898126232601
- KimSJSchoenbergerSDThorneJEEhlersJPYehSBakriSJTopical nonsteroidal anti-inflammatory drugs and cataract surgery: a report by the American Academy of OphthalmologyOphthalmology2015122112159216826123091
- HendersonBAKimJYAmentCSFerrufino-PonceZKGrabowskaACremersSLClinical pseudophakic cystoid macular edema. Risk factors for development and duration after treatmentJ Cataract Refract Surg20073391550155817720069
- BenitahNRArroyoJGPseudophakic cystoid macular edemaInt Ophtalmol Clin2010501139153
- ErikssonUAlmABjarnhallGGranstamEMatssonAWMacular edema and visual outcome following cataract surgery in patients with diabetic retinopathy and controlsGraefes Arch Clin Exp Ophthalmol2011249334935920827486
- KreplerKBiowskiRSchreySJandrasitsKWedrichACataract surgery in patients with diabetic retinopathy: visual outcome, progres sion of diabetic retinopathy, and incidence of diabetic macular oedemaGraefes Arch Clin Exp Ophthalmol2002240973573812271370
- BakerCWAlmukhtarTBresslerNMDiabetic Retinopathy Clinical Research Network Authors/Writing CommitteeMacular edema after cataract surgery in eyes without preoperative central involved diabetic macular edemaJAMA Ophthalmol2013131787087923599174
- DowlerJGFSehmiKSHykinPGHamiltonAMThe natural history of macular edema after cataract surgery in diabetesOphthalmology1999106466366810201584
- ChuCJJohnstonRLBuscombeCSallamABMohamedQYangYCUnited Kingdom Pseudophakic Macular Edema Study Group OphthalmologyRisk factors and incidence of macular edema after cataract surgery: a database study of 81984 eyesOphthalmology2016123231632326681390
- RossettiLChaudhuriJDickersinKMedical prophylaxis and treatment of cystoid macular edema after cataract surgery. The results of a meta-analysisOphthalmology199810533974059499767
- KesselLTendalBJørgensenKJPost-cataract prevention of inflammation and macular edema by steroid and nonsteroidal anti-inflammatory eye drops: a systematic reviewOphthalmology2014121101915192424935281
- European Medical Agency Assessment Report: Nevenac EMA/CHMP/167525/2016 Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Assessment_Report_-_Variation/human/000818/WC500212717.pdfAccessed October 5, 2016
- ILEVRO™ (nepafenac ophthalmic suspension 0.3%) [package insert]Fort Worth, TXAlcon Laboratories, Inc2014 Available from: http://ecatalog.alcon.com/PI/Ilevro_us_en.pdfAccessed April 27, 2016
- KeTLGraffGSpellmanJMYanniJMNepafenac, a unique nonsteroidal prodrug with potential utility in the treatment of trauma-induced ocular inflammation: II. In vitro bioactivation and permeation of external ocular barriersInflammation200024437138410850858
- WaltersTRaizmanMErnestPGaytonJLehmannRIn vivo pharmacokinetics and in vitro pharmacodynamics of nepafenac, amfenac, ketorolac, and bromfenacJ Cataract Refract Surg20073391539154517720067
- ChastainJESandersMECurtisMADistribution of topical ocular nepafenac and its active metabolite amfenac to the posterior segment of the eyeExp Eye Res2016145586726474497
- SinghRAlpernLJaffeGJEvaluation of nepafenac in prevention of macular edema following cataract surgery in patients with diabetic retinopathyClin Ophthalmol201261259126922927737
- PollackAStaurenghiGSagerDMukeshBReiserHSinghRProspective randomized clinical trial to evaluate the safety and efficacy of nepafenac treatment for the prevention of macular edema associated with cataract surgery in patients with diabetic retinopathyBr J Ophthalmol20161527378485
- SinghRLehmannRMartelJNepafenac 0.3% following cataract surgery in patients with diabetic retinopathy: results of two randomized Phase 3 studiesOphthalmology Epub201734
- MiyakeKOtaIMiyakeGNumagaJNepafenac 0.1% versus fluorometholone 0.1% for preventing cystoid macular edema after cataract surgeryJ Cataract Refract Surg20113791581158821855758
- HariprasadSMCallananDGaineySHeYGWarrenKCystoid and diabetic macular edema treated with nepafenac 0.1%J Ocul Pharmacol Ther200723658559018001248
- American Medical Association2016Registry Individual Measure Flow-Measure #191 (NQF 0565): Cataracts: 20/40 or Better Visual Acuity within 90 Days Following Cataract Surgery – National Quality Strategy Domain: Effective Clinical Care Available from: http://www.mdinteractive.com/files/uploaded/file/CMS2016/2016_PQRS_Measure_191_11_17_2015.pdfAccessed on May 6, 2016
- MittraRABorrilloJLDevSMielerWFKoenigSBRetinopathy progression and visual outcomes after phacoemulsification in patients with diabetes mellitusArch Ophthalmol2000118791291710900103
- BakerCWAlmukhtarTBresslerNMMacular edema after cataract surgery in eyes without preoperative central-involved diabetic macular edemaJAMA Ophthalmol2013131787087923599174
- LundströmMManningSBarryPSteneviUHenryYRosenPVisual outcome of cataract surgery; study from the European registry of quality outcomes for cataract and refractive surgeryJ Cataract Refract Surg201339567367923499065
- MorrisDFraserSDGrayCCataract surgery and quality of life implicationsClin Interv Aging20072110510818044082
- FergusonVMGSpaltonDJContinued breakdown of the blood aqueous barrier following cataract surgeryBr J Ophthalmol19927684534561390524
- PollackALeibaHBukelmanAOliverMCystoid macular oedema following cataract extraction in patients with diabetesBr J Ophthalmol19927642212241390489
- HenricssonMHeijlAJanzonLDiabetic retinopathy before and after cataract surgeryBr J Ophthalmol19968097897938942374
- ZaczekAOlivestedtGZetterstromCVisual outcome after phacoemulsification and IOL implantation in diabetic patientsBr J Ophthalmol19998391036104110460771
- WolfEJBraunsteinAShihCBraunsteinREIncidence of visually significant pseudophakic macular edema after uneventful phacoemulsification in patients treated with nepafenacJ Cataract Refract Surg20073391546154917720068
- MathysKCCohenKLImpact of nepafenac 0.1% on macular thickness and postoperative visual acuity after cataract surgery in patients at low risk for cystoid macular oedemaEye (Lond)2010241909619229275
- AlmeidaDRPKhanZXingLProphylactic nepafenac and ketorolac versus placebo in preventing postoperative macular edema after uneventful phacoemulsificationJ Cataract Refract Surg20123891537154322795976
- TzelikisPFVieiraMHidaWtComparison of ketorolac 0.4% and nepafenac 0.1% for the prevention of cystoid macular oedema after phacoemulsification: prospective placebo-controlled randomised studyBr J Ophthalmol201599565465825385061
- AlmeidaDRPJohnsonDHollandsHEffect of prophylactic nonsteroidal anti-inflammatory drugs on cystoid macular edema assessed using optical coherence tomography quantification of total macular volume after cataract surgeryJ Cataract Refract Surg2008341646918165083
- AsanoSMiyakeKOtaIReducing angiographic cystoid macular edema and blood-aqueous barrier disruption after small-incision phacoemulsification and foldable intraocular lens implantation: multicenter prospective randomized comparison of topical diclofenac 0.1% and betamethasone 0.1%J Cataract Refract Surg2008341576318165082
- HirneissCNeubauerASKampikASchonfeldCLComparison of prednisolone 1%, rimexolone 1% and ketorolac tromethamine 0.5% after cataract extraction: a prospective, randomized, double-masked studyGraefes Arch Clin Exp Ophthalmol2005243876877315756571
- HolzerMPSolomonKDSandovalHPVromanDTComparison of ketorolac tromethamine 0.5% and loteprednol etabonate 0.5% for inflammation after phacoemulsification: prospective randomized double-masked studyJ Cataract Refract Surg2002281939911777716
- WangQWYaoKXuWBromfenac sodium 0.1%, fluorometholone 0.1% and dexamethasone 0.1% for control of ocular inflammation and prevention of cystoid macular edema after phacoemulsificationOphthalmologica2013229418719423429038
- DonnenfeldEDHollandEJSolomonKDA multicenter randomized controlled fellow eye trial of pulse-dosed difluprednate 0.05% versus prednisolone acetate 1% in cataract surgeryAm J Ophthalmol2011152460961721704965
- DonnenfeldEDPerryHDWittpennJRSolomonRNattisAChouTPreoperative ketorolac tromethamine 0.4% in phacoemulsification outcomes: pharmacokinetic response curveJ Cataract Refract Surg20063291474148216931258