Abstract
Purpose
The purpose of this study was to evaluate the potential benefits of intravitreal aflibercept injections for the treatment of choroidal neovascularization (CNV) secondary to chorioretinitis.
Methods
In this uncontrolled, prospective cohort study, 15 eyes of 14 consecutive patients affected by CNV associated with ocular toxoplasmosis were treated with intravitreal aflibercept (2 mg) pro re nata and observed over a 12-month follow-up period. The primary outcome was the change in best-corrected visual acuity (BCVA) from baseline to month 12. Secondary outcomes included change in central retinal thickness (CRT) in the foveal area on optical coherence tomography (OCT) from baseline to month 12, the number of intravitreal aflibercept injections administered, and safety.
Results
Mean (standard deviation [SD]) BCVA improved significantly from 0.36 (0.23) at baseline to 0.64 (0.3) at month 12 (P=0.0002). Mean (SD) CRT on OCT showed a reduction from 317 (74) µm at baseline to 254 (43) µm (P=0.0002) at month 12. A mean (SD) of 1.7 (0.5) injections (range, 1–2 injections) were performed during the study period. No cases of endophthalmitis, uveitis, stroke, or retinal detachment were noted. No patient demonstrated an intraocular pressure >20 mmHg at any study visit.
Conclusion
Intravitreal aflibercept showed a positive clinical effect and was well tolerated for the treatment of CNV associated with chorioretinitis. The results could be helpful for selecting a treatment for CNV secondary to chorioretinitis.
Introduction
In addition to the well-known causes of choroidal neovascularization (CNV) such as age-related macular degeneration (AMD), pathologic myopia, and angioid streaks, CNV may develop as a complication of chorioretinitis, with an incidence of 2%. The prevalence of CNV secondary to chorioretinitis varies among different entities, and commonly occurs in presumed ocular histoplasmosis (3.8%), toxoplasmosis (TX, 0.3%–19%), punctate inner choroidopathy (17%–40%), idiopathic multifocal choroiditis (33%), and serpiginous choroiditis (SC, 4.7%). CNV has also been reported in up to 9% of patients with Vogt–Koyanagi–Harada disease.Citation1
There is no uniform consensus on the management of CNV secondary to chorioretinitis because of the lack of randomized or controlled clinical trials, which have not been performed due to the rarity of this condition.
There are different treatment strategies for chorioretinitis-associated CNV, including photodynamic therapy (PDT),Citation2–Citation4 anti-vascular endothelial growth factor (VEGF) agents such as bevacizumab and ranibizumab,Citation5–Citation7 surgery,Citation8,Citation9 local and systemic corticosteroids, and combinations of these methods.Citation10
A review article published in 2014 describes the three main therapies available for CNV secondary to chorioretinitis in the last decade as PDT, intravitreal bevacizumab, and intravitreal ranibizumab.Citation1 Aflibercept (EYLEA®, Regeneron Pharmaceutical Inc, Tarrytown, NY, USA), initially named VEGF Trap-Eye, is the most recent anti-VEGF agent to be granted approval by the US Food and Drug Administration (2011) for the treatment of neovascular AMD. The ocular formulation of aflibercept has been specifically purified and buffered to minimize the risk of eye toxicity when injected intravitreally.Citation11 To the authors’ knowledge, there are no published articles describing the use of intravitreal aflibercept in this pathology. However, in 2017, a short-term study showed that off-label intravitreal ziv-aflibercept is safe and effective in a small series of patients with CNV secondary to choroiditis.Citation12
The purpose of this report was to evaluate the potential benefits of intravitreal aflibercept injections for the treatment of CNV secondary to chorioretinitis.
Materials and methods
Protocol
This study was a prospective, noncontrolled case series. Fourteen consecutive patients (15 eyes) presented to the Department of Inflammatory Pathology of the Eye of the state institution “The Filatov Institute of Eye Diseases and Tissue Therapy of the National Academy of Medical Sciences of Ukraine” with signs and symptoms consistent with CNV associated with chorioretinitis, and were examined and screened for study entry. The diagnosis of active CNV at baseline was based on slit lamp biomicroscopy and was confirmed with spectral domain optical coherence tomography (OCT) and fluorescein angiography (FA). Inclusion criteria were naïve CNV secondary to TX and inactive TX. Ocular exclusion criteria included acute TX, any other possible cause of CNV, known glaucoma or clinical suspicion of glaucoma on presentation, ocular hypertension (intraocular pressure [IOP] >24 mmHg), and fundus observation complicated by media opacity. Non-ocular exclusion criteria included pregnancy, lactation, and the inability of patients to sign informed consent. Eligible patients were given an extensive explanation concerning their disease and the proposed study and treatment. Written informed consent was obtained from all individual participants included in the study.
This study was approved by the state institution “The Filatov Institute of Eye Diseases and Tissue Therapy of the National Academy of Medical Sciences of Ukraine” Research Ethics Board before commencement. All procedures were performed in accordance with the 1964 Declaration of Helsinki.
Intervention
An obligatory loading injection of intravitreal aflibercept was administered at baseline. Subsequent injections were given on an as-needed (pro re nata) schedule. Aflibercept (2 mg) was injected intravitreally. Sterile propracaine drops were instilled in the affected eye. The eye and surrounding periocular skin were then prepped with 5% betadine solution. A lid speculum was placed in the eye and the anesthetized area was cleaned with 5% betadine. A variable caliper was utilized to mark the pars plana, 3.5 mm from the limbus for phakic patients and 3 mm for pseudophakic patients. A 27-gauge needle was then introduced through this mark into the vitreous cavity and the aflibercept was injected. The needle was retracted and the injection site was covered with a sterile, cotton-tipped applicator. A drop of topical ofloxacin was placed in the eye and the speculum was removed. The patients were instructed to continue the ofloxacin drops four times daily for 5 days.
Re-treatment at a 4-week interval was required after the first injection of aflibercept. Re-treatment criteria included persistence of CNV and/or recurrence of increased retinal thickness in the foveal area >50 µm, subretinal or intraretinal fluid, and/or intraretinal cysts on OCT, or subretinal hemorrhage observed during dilated fundus examination.
Study visits
All the enrolled patients underwent baseline ophthalmologic examination and investigations, including measurement of the decimal best-corrected visual acuity (BCVA), IOP measurements, biomicroscopy, and dilated fundus examination, as well as spectral domain OCT (Spectralis, Heidelberg Engineering, Heidelberg, Germany) of the macular area, color fundus photography, and FA (TRC 50 – EX; Topcon, Tokyo, Japan). FA was administered in all cases for confirmation of the absence of posterior uveitis activity.
Subsequently, all patients were reexamined monthly for 6 months, and then once every 3 months up to month 12. Follow-up examinations involved ophthalmologic examination, including BCVA measurement, IOP measurement, and dilated fundus examination. OCT and color fundus photography were also repeated at each of these visits. FA was performed in the case of decrease in BCVA and/or increase in macular edema.
The following adverse events were monitored: chori-oretinitis relapse, elevated IOP, endophthalmitis, retinal tear, retinal detachment, and vitreous hemorrhage. All other complications encountered were also noted.
Outcome measures
The primary outcome was the change in BCVA from baseline to month 12. Secondary outcomes included change in central retinal thickness (CRT) in the foveal area on OCT from baseline to month 12, the number of intravitreal aflibercept injections administered, and safety.
Analysis
Differences between final and initial BCVA and CRT were compared using paired Student’s t-test, and a P-value of <0.05 was considered significant. All analyses were performed with Statistica for Windows version 10.0 (Dell Software, Round Rock, TX, USA).
Results
A total of 15 eyes of 14 patients with CNV secondary to ocular TX were examined at baseline and followed up for 12 months. There were eleven females and three males, and the mean age was 37 years (range, 16–56 years). Ocular TX was inactive in all eyes during the follow-up period. All patients presented with predominantly classic CNV in the macular area. CNV was subfoveal in seven eyes and juxtafoveal in eight eyes. The mean (SD) baseline decimal BCVA was 0.36 (0.23) (range, 0.06–0.8). The mean (SD) baseline retinal thickness in the foveal area was 317 (74) µm (range, 239–528 µm).
The mean (SD) BCVA improved to 0.64 (0.3) (range, 0.15–1.0) at month 12 (P=0.0002). At 12 months, visual acuity improved by ≥3 lines in three eyes (20%), by ≥2 to <3 lines in eight eyes (53%), and by ≥1 to <2 lines in three eyes (20%). Vision was unchanged in one eye (7%). None of the treated eyes lost ≥1 line.
The mean (SD) CRT decreased to 254 (43) µm (range, 192–362 µm) (P=0.0002) at month 12. The mean (SD) number of injections during the study period was 1.7 (0.5) (range, 1–2 injections). No cases of persistent CNV were noted during the 12 months of follow-up.
One-year outcomes are summarized in .
Table 1 One-year follow-up of intravitreal aflibercept for choroidal neovascularization associated with chorioretinitis
Complications
Adverse events observed after intravitreal injections included eye pain (four of 25 injections, 16%), subconjunctival hemorrhage (three of 25 injections, 23%), and vitreous reflux (seven of 25 injections, 28%). No cases of chorioretinitis relapse, endophthalmitis or uveitis, stroke, or retinal detachment were noted. No patient demonstrated an IOP >20 mmHg in any study visit.
Report of case
The patient whose case is reported provided informed written consent for publication of their case details, including any images.
Case
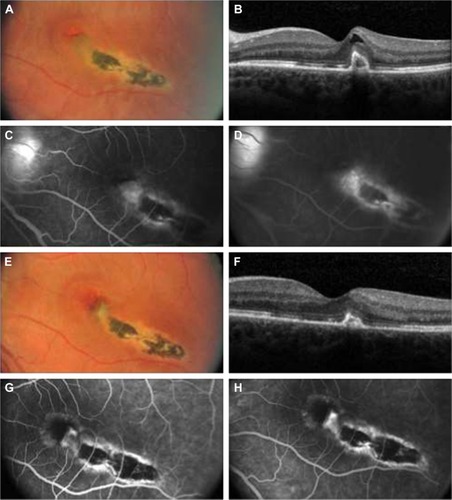
A 28-year-old woman with blurred vision in the left eye was referred to us. Her diagnosis was inactive chorioretinitis with CNV of the left eye. She had been treated in the clinic with acute chorioretinitis and papilledema 4 months before. Decimal BCVA was 1.0 in the right eye and 0.3 in the left eye. IOP was normal in both eyes. Biomicroscopic examination was normal. There was no cellular reaction in the anterior chamber or vitreous cavity. The right eye fundus examination was normal. The left eye fundus examination showed a chorioretinal scar with macular edema and hemorrhage (). FA and OCT confirmed the presence of CNV in the left eye (). After obtaining the written informed consent, the intravitreal aflibercept injection was performed. One month later, BCVA increased to 0.6, but CNV was still active and the patient received a second injection. There was no chorioretinitis, and no CNV relapses, at the subsequent follow-up visit (). This was confirmed by FA and OCT (). BCVA increased to 1.0 and remained stable throughout the follow-up period.
Figure 1 (A) Baseline fundus photographs (OS) show a macular scar with fibrosis, subretinal fluid, and hemorrhage. (B) Baseline optical coherence tomographic (OCT) scan shows increased retinal thickening and a highly reflective subretinal complex. (C and D) Baseline early- and late-phase fluorescein angiographic images show leakage from choroidal neovascularization. (E) Posttreatment fundus photographs taken at 4 months show a macular scar and no evidence of subretinal fluid or hemorrhage. (F) Posttreatment OCT scan taken at 4 months shows decreased retinal thickness and a persistent highly reflective subretinal complex. (G and H) Posttreatment early- and late-phase fluorescein angiographic images taken at 4 months show no evidence of leakage.
Discussion
There are limitations to this study due to its small number of patients, and no statistical comparison with other studies of CNV secondary to chorioretinitis is provided, due to great differences in protocols and a diverse etiology of chorioretinitis in different studies.
In this study, the etiology of chorioretinitis in all patients was TX. As such, the data are compared with other studies in which patients had this same etiology.Citation4,Citation6,Citation13,Citation14 It is well known that the natural history of CNV secondary to toxoplasmic chorioretinitis has a poor visual prognosis.Citation15 The different treatment modalities used for CNV secondary to TX include laser photocoagulation, submacular surgery, PDT, and anti-VEGF agents.Citation13
The majority of studies regarding CNV secondary to TX report on treatment with PDT.Citation2,Citation3,Citation13,Citation14
Neri et alCitation3 evaluated the efficacy and safety of PDT in the long-term control of subfoveal CNV associated with toxoplasmic retinochoroiditis. The records of 13 patients with classic subfoveal CNV associated with toxoplasmic retinochoroiditis treated with PDT were reviewed, and nine patients were enrolled in the study. The authors evaluated BCVA and CNV diameter. At the 48-month follow-up, BCVA and major CNV diameter were stable or improved in all patients. Mean (SD) BCVA improved from 0.29 (0.19) at baseline to 0.54 (0.16) at month 48 (P<0.0001) and mean (SD) major CNV diameter improved from 1,463.67 (431.47) µm at baseline to 846 (326.5) µm at month 48.Citation3
Nessi et alCitation14 described three patients treated with PDT. In the first case (one PDT session), visual acuity decreased during the follow-up, in the second case (three PDT sessions) BCVA was stabilized at 0.3, and in the third case (four PDT sessions) BCVA was stabilized at 0.2.
Mauget-Faÿsse et alCitation2 evaluated the efficacy and safety of full-dose PDT verteporfin for young adults and children with subfoveal CNV associated with toxoplasmic retinochoroiditis. Eight patients were treated. Mean visual acuity increased from 20/225 (range, 20/400–20/50) to 20/123 (range, 20/200–20/25) during a mean follow-up period of 25 months (range, 5–49 months). Persistent closure of CNV was achieved in all eight patients (mean number of treatments, 1.75). Vascular anastomosis developed in the treated area in two patients, but there was no additional visual loss. No significant adverse effects of PDT were observed.Citation2
Rishi et alCitation13 described the case of combination treatment (PDT and intravitreal bevacizumab) in a pediatric patient. The patient underwent PDT. This was followed by an intravitreal injection of bevacizumab after 2 days. CNV regressed after 8 weeks of follow-up and remained stable at 8 months of follow-up. The initial visual acuity improved from 20/120 to 20/30.
There was one case of surgery for subfoveal CNV with toxoplasmic retinochoroiditisCitation8 and there were a few reports of intravitreal injections for CNV associated with toxoplasmic chorioretinitis.Citation16,Citation17
Shah and ShahCitation16 reported a case of CNV secondary to ocular TX in an 18-year-old female patient with an initial BCVA of 20/80. She was treated with a single intravitreal injection of ranibizumab. Visual acuity improved to 20/30 at the 4-week follow-up visit after injection and was maintained through the last follow-up visit at 2 years.Citation16
Kianersi et alCitation18 described a small series of four patients with TX. Leakage diminished in all patients over 6–25 months of follow-up. A reduction in CRT was recorded in all patients. Visual acuity increased to normal levels and was preserved until the end of follow-up. In this study, only one injection was required to achieve good visual outcomes. This study does have some limitations, including the limited number of patients.
Ben Yahia et alCitation17 reported two young patients with CNV secondary to ocular TX who each received a single intravitreal injection of bevacizumab as a primary or rescue therapy. After a follow-up of 12 and 10 months, respectively, visual acuity improved, and features of active neovascularization resolved with no recurrence.
In this study, almost one-third of the patients achieved vision stabilization after a single intravitreal aflibercept injection, and almost two-thirds had stable vision after two injections. This is a lower number of injections than is required in eyes with CNV secondary to other diseases, such as AMD.
There are suggestions in the literature that the good response of CNV secondary to chorioretinitis to anti-VEGF treatment may be related to the fact that VEGF plays an important role as a pro-inflammatory mediator. In cases of inflammatory CNV, the blockade of VEGF therefore not only impedes the process of angiogenesis, but also attenuates the underlying process for CNV development.Citation19
There were no cases of chorioretinitis reactivation during the follow-up period. There were no systemic or local adverse events, and treatment was well tolerated.
In conclusion, intravitreal aflibercept showed a positive clinical effect and was well tolerated for the treatment of CNV associated with chorioretinitis. The results of this study may be helpful in selecting treatment for CNV secondary to chorioretinitis.
Acknowledgments
The authors take full responsibility for the scope, direction, and content of the manuscript, and have approved the submitted manuscript. Medical writing support was provided by Corey Eagan, MPH, of PAREXEL, and this study was funded by Bayer Pharmaceuticals.
Disclosure
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or nonfinancial interest (such as personal or professional relationships, affiliations, knowledge, or beliefs) in the subject matter or materials discussed in this manuscript. The authors report no conflicts of interest in this work.
References
- D’AmbrosioETortorellaPIannettiLManagement of uveitis-related choroidal neovascularization: from the pathogenesis to the therapyJ Ophthalmol2014201445042824868454
- Mauget-FaÿsseMMimounGRuiz-MorenoJMVerteporfin photodynamic therapy for choroidal neovascularization associated with toxoplasmic retinochoroiditisRetina200626439640316603957
- NeriPMercantiLMariottiCSalvoliniSGiovanniniALong-term control of choroidal neovascularization in quiescent congenital toxoplasma retinochoroiditis with photodynamic therapy: 4-year resultsInt Ophthalmol2010301515619183856
- OliveiraLBReisPAPhotodynamic therapy-treated choroidal neovascular membrane secondary to toxoplasmic retinochoroiditisGraefes Arch Clin Exp Ophthalmol2004242121028103015064953
- ArevaloJFAdanABerrocalMHIntravitreal bevacizumab for inflammatory choroidal neovascularization: results from the Pan-American Collaborative Retina Study Group at 24 monthsRetina201131235336320890239
- MathurGGeorgeAESenPPaediatric choroidal neovascular membrane secondary to toxoplasmosis treated successfully with anti-vascular endothelial growth factorOman J Ophthalmol20147314114325378880
- KramerMAxer-SiegelRJaouniTBevacizumab for choroidal neovascularization related to inflammatory diseasesRetina201030693894420168273
- AdanAMateoCWolley-DodCSurgery for subfoveal choroidal neovascularization in toxoplasmic retinochoroiditisAm J Ophthalmol2003135338638712614761
- UemuraAThomasMAVisual outcome after surgical removal of choroidal neovascularization in pediatric patientsArch Ophthalmol2000118101373137811030819
- DhingraNKellySMajidMABaileyCBDickADInflammatory choroidal neovascular membrane in posterior uveitis-pathogenesis and treatmentIndian J Ophthalmol201058131020029141
- SemeraroFMorescalchiFDuseSParmeggianiFGambicortiECostagliolaCAflibercept in wet AMD: specific role and optimal useDrug Des Devel Ther20137711722
- BraimahIZStewartMVidekarCDedhiaCJChhablaniJIntravitreal ziv-aflibercept for the treatment of choroidal neovascularisation associated with conditions other than age-related macular degenerationBr J Ophthalmol2017 Epub ahead of print
- RishiPVenkataramanARishiECombination photodynamic therapy and bevacizumab for choroidal neovascularization associated with toxoplasmosisIndian J Ophthalmol2011591626421157079
- NessiFGuex-CrosierYAmbresinAZografosLPhotodynamic therapy with verteporfin for subfoveal choroidal neovascularization secondary to toxoplasmic chorioretinal scarKlin Monbl Augenheilkd2004221537137315162283
- AtmacaLSSimsekTBatiogluFClinical features and prognosis in ocular toxoplasmosisJpn J Ophthalmol200448438639115295668
- ShahNJShahUNIntravitreal ranibizumab for the treatment of choroidal neovascularization secondary to ocular toxoplasmosisIndian J Ophthalmol201159431831921666322
- Ben YahiaSHerbortCPJenzeriSIntravitreal bevacizumab (Avastin) as primary and rescue treatment for choroidal neovascularization secondary to ocular toxoplasmosisInt Ophthalmol200828431131618401552
- KianersiFNaderiBANaderiBZGhanbariHIntravitreal bevacizumab for treatment of choroidal neovascularization secondary to toxoplasmic retinochoroiditis: a case seriesSemin Ophthalmol201530318118724175641
- WolfAThurauSKookDIntravitreal bevacizumab for choroidal neovascularization secondary to inflammationActa Ophthalmol2010887e295e29619860782
