Abstract
Purpose
To determine the incidence of interface fluid syndrome (IFS) secondary to steroid-induced elevation of intraocular pressure (IOP) following laser in situ keratomileusis (LASIK) in myopic Egyptian patients.
Methods
This retrospective case series study was conducted at El-Gowhara Private Eye Center. The medical records of 1,807 patients (3,489 eyes), who underwent LASIK to correct myopia from April 2012 to December 2015 were included. The patients were operated on and reviewed by one surgeon (AAG) for IFS after LASIK associated with elevation of IOP (as compared to preoperative values).
Results
This paper reports the incidence of 2.9% (54 patients) (102 eyes) of IFS induced by increased IOP after LASIK in Egyptian patients. The medical records of 1,807 patients (3,489 eyes) with mean age ± standard deviation (SD) 26.4±2.7 years, who presented with mean myopia ± SD −4.50±1.3 D, mean astigmatism ± SD −1.43±0.8, mean IOP ± SD 15.2±1.2 mmHg, and mean central corneal thickness ± SD 549±25.6 μm, were included. The preoperative anterior and posterior segments, corneal topography, and Schirmer’s test were unremarkable.
Conclusion
Limiting topical steroids and routinely measuring the IOP post-LASIK are necessary steps to prevent IFS, especially in case of myopia. A high index of suspicion is required to make a diagnosis. High-resolution optical coherence tomography is helpful to confirm the diagnosis.
Introduction
Laser in situ keratomileusis (LASIK) is the most common surgery to correct refractive errors including myopia, hyperopia, and astigmatism.Citation1 Topical corticosteroids are universally prescribed after LASIK, usually for 7–10 days (depending on the circumstances) to treat inflammatory processes.Citation1
Alternatively, it is reported that a significant increase in intraocular pressure (IOP) occurs in 5%–30% of steroid users.Citation2–Citation4 Also, it typically occurs 2–6 weeks after steroid use, but it has also been recognized to occur within hours of steroid use in some patients.Citation2–Citation4 The risk factors associated with steroid response include glaucoma or a family history of steroid response. Moreover, 88% of patients with myopia >5.00 D have increased pressures with steroid use.Citation5
The elevated IOP can cause aqueous humor to enter the cornea and collect at the interface under the corneal flap. This collection is termed interface fluid syndrome (IFS).Citation6–Citation8
Among interface complications, diffuse lamellar keratitis (DLK) is a well-defined condition in the literature (most common and the most common etiology mistaken for other conditions), and there have been some reports of another entity that is clinically identical to DLK. This entity is called pressure-induced inter-lamellar stromal keratitis (PISK) or IFS. However, unlike DLK, stoppage of the corticosteroid drops resulted in its resolution, suggesting that this is a steroid-induced pressure phenomenon rather than an inflammatory reaction.Citation9
Recently, Tourtas and CursiefenCitation10 created the term “pressure-induced stromal keratopathy (PISK)” which preserves the most common abbreviated term “PISK”, which also precisely and most efficiently conveys the etiology of the condition.
IFS is the most commonly used term to define fluid collection at the interface under the corneal flap.Citation7 It was first described by Lyle and Jin.Citation8 Some authors use the term “pressure induced stromal keratitis” interchangeably.Citation9 Early detection of interface fluid secondary to steroid-induced elevation of IOP, and differentiation from DLK is critical since prolonged use of steroids to treat the presumed DLK diagnosis will worsen the condition.Citation11–Citation13
So, the purpose of this study was to determine the incidence of IFS secondary to steroid-induced elevation of IOP following LASIK in myopic Egyptian patients.
Patients and methods
The medical records of 1,807 patients (3,489 eyes), who underwent LASIK to correct myopia from April 2012 to December 2015 at the El-Gowhar Eye Center in Ismailia, Egypt, who were operated on and reviewed by one surgeon (AAG), were included.
Preoperatively, patients underwent complete eye examinations, including slit-lamp examination, IOP with applanation tonometry, indirect fundoscopy, and corneal topography (Sirius; CSO, Florence, Italy). Snellen chart was used to measure uncorrected visual acuity and best spectacle-corrected visual acuity.
All eyes underwent LASIK using the 500 kHz Amaris excimer laser (Schwind eye-tech-solutions, Kleinostheim, Germany). Flaps were cut using the Moria M2 microkeratome (Moria, Antony, France), and the corrected optical zones ranged from 5.8 to 7.0 mm in diameter. All eyes had routine procedures.
Postoperative treatment included topical application of tobramycin 3 mg/mL and dexamethasone 1 mg/mL five times per day for the first week, tapered to three times per day for the following 21 days, and sodium hyaluronate 0.15 g five times per day for 5 weeks. The patients were evaluated by complete ocular examinations on the first day, first week, fourth week, and sixth week postoperatively.
When patients complained of decreased vision with flap edema, IOP measurement by ocular response analyzer (ORA), anterior optical coherence tomography (OCT), and fundus examination (hardly seen in some patients) were done. In patients with IFS and elevated IOP, topical corticosteroids were discontinued, and topical anti-glaucoma medications were started with timolol maleate 0.5% twice daily. If a further topical medication was required, carbonic anhydrase inhibitors, alpha-2 selective adrenergic agonist, and prostaglandin derivatives were added until the IOP returned to preoperative levels. All the patients with IFS and elevated IOP were followed 6 months post-improvement.
This study adhered to the guidelines of the Declaration of Helsinki and was approved by the research ethics committee of the Faculty of Medicine, Suez Canal University. Informed written consent was obtained from all patients after explanation of LASIK procedures and the surgical technique. Because of the retrospective nature of the study design and the large number of patients, no informed consent was required to review the medical records. The confidentiality of the patients’ data was ensured.
Statistical analysis
Data were imported into Statistical Package for the Social Sciences (SPSS version 20.0; IBM Corporation, Armonk, NY, USA) software for analysis. Baseline characteristics of the study population were presented as frequencies and percentages (%) or mean values and standard deviations (SDs). Differences between frequencies in the groups were compared by Chi-square test or Fisher’s exact probability test of expected values. Differences between means in the groups were compared by Student’s t-test or Mann–Whitney U test according to the normality of data. A P-value of <0.05 was considered significant.
Results
Preoperatively, 1,807 patients (3,489 eyes) aged from 21 to 38 years (mean ± SD 27.4±5 years) presented with myopia ranging from −2.00 to −7.00 D (mean ± SD −4.50±1.3 D), and astigmatism ranging from −1.00 to −4.00 D (mean ± SD −1.43±0.8). IOP ranged between 14 and 18 mmHg (mean ± SD 15.2±1.2 mmHg). Central corneal thickness ranged from 514 to 580 μm (mean ± SD 549±25.6 μm). The anterior and posterior segments, corneal topography, and Schirmer’s test were unremarkable. shows the preoperative characteristics of all patients.
Table 1 Preoperative characteristics of all patients
Postoperatively, 54 patients (102 eyes) (2.9%) developed IFS secondary to IOP elevations between 17 and 36 days after LASIK (mean ± SD 19±4.2 days). This IFS group was aged between 23 and 34 years (mean ± SD 26.2±4.2 years). The best spectacle-corrected visual acuity decreased in all patients by 2–4 lines. All eyes in the IFS group had myopia with spherical equivalent ranging from −0.75 to −2.5 D (mean ± SD −1.5±0.6 D) and astigmatism ranging from −0.5 to −1.5 D (mean ± SD −0.78±0.4). IOP ranged between 28 and 43 mmHg (mean ± SD 35±3.4 mmHg), central corneal thickness ranged from 580 to 689 μm (mean ± SD 665±15.6 μm). shows postoperative characteristics of IFS group ( and ).
Figure 1 Anterior segment optical coherence tomography of the interface fluid as optical space between the corneal flap and the stroma.
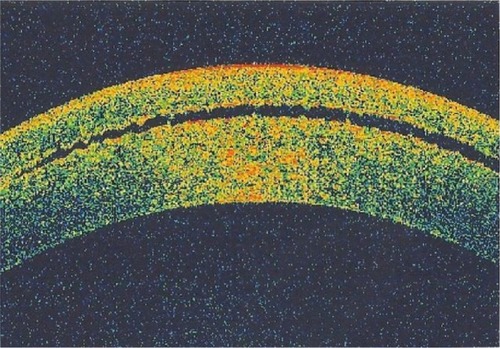
Figure 2 Corneal topography shows thickening of the cornea due to flap edema and interface fluid.
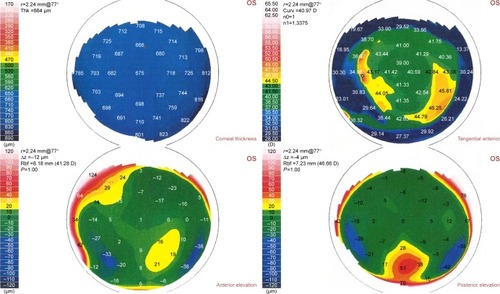
Table 2 Postoperative characteristics of IFS group
There were no significant differences between preoperative characteristics of IFS group and control group (LASIK patients who did not develop IFS) (). No cases with post-LASIK IOP elevations without interface fluid were recorded.
Table 3 Comparison between the preoperative characteristics of IFS group and control group (LASIK patients who did not develop IFS)
After cessation of topical steroids and use of topical anti-glaucoma medications, the IOP and uncorrected visual acuity returned to the expected values within 4–6 weeks of treatment. Flap edema and corneal haze disappeared after the IOP improved to normal levels.
Discussion
The LASIK interface is the potential space between anterior and posterior corneal lamellae, which is a new anatomical region in the cornea and came into existence with LASIK procedures. Within it, some biochemical processes take place after construction of the corneal flap, including limitation of corneal wound healing and intercellular reorganization.Citation14 The anatomy of this interface permits a diversity of unusual complications to arise from different etiologies, often with similar clinical conditions.Citation15
IFS is a relatively rapid steroid response resulting in high IOP with fluid accumulation in the corneal interface between corneal flap and stroma. The amount of this fluid may be relatively small, resulting in diffuse haziness without any obvious fluid,Citation11 or it may be marked, with a visible fluid cleft separating the anterior flap from the stroma.Citation12 IFS typically appears early after LASIK, but also many years after LASIK. Ortega-Usobiaga et alCitation16 reported a case of IFS of the left eye after cataract surgery in a patient who had undergone previous LASIK surgery 10 years ago.
IFS appears to be a more complex condition to recognize than other interface complications due to unclear nomenclature in the literature, and the wide variety of presentations.Citation15 “Keratitis” is a misnomer in this condition, as in vivo confocal microscopic findings of interlamellar stromal keratopathy induced by elevated IOP have demonstrated that no actual keratocyte inflammation is present.Citation17,Citation18 IFS is technically correct.Citation15
This study is the first to report the incidence of 2.9% IFS secondary to steroid-induced elevation of IOP following LASIK in myopic Egyptian patients. This may be due to the topical use of dexamethasone 0.1% post-LASIK, which is a potent steroid for preoperative topical use, or the genetic predisposition of the Egyptian patients (who may be high responders to steroids).
The high incidence of IFS in this series could have been influenced by the duration of the postoperative regimen of dexamethasone (1 month) and the dose (five times daily for the first week). Ortega-Usobiaga et alCitation19 reported a very large series of LASIK treatments (55,255 patients) and postoperative regimen of dexamethasone 1 mg/mL three times a day for only 1 week.
No studies have reported the incidence of IFS following LASIK. There are only clinical manifestations of some recorded cases and of how to differentiate it from DLK.
The degree of the fluid accumulated in the corneal interface masks precise IOP measurements when measured using a Goldmann applanation tonometer (GAT). Real IOP is greater than IOP measured centrally, and peripheral measurements generate a more precise IOP.Citation20 When the interface fluid is a small amount, IOP measurements may be elevated, but still low. However, with a larger amount of interface fluid clefts, IOP will measure very low due to the cushioning effect of this fluid.Citation15
The measurement of IOP in a patient chronically using steroids after LASIK (more than 2 weeks) due to different causes is a critical issue, even in early postoperative cases. IOP measurement is artifactually reduced after routine LASIK;Citation21 so, any increased IOP postoperatively deserves further investigation. Whenever IOP measurements are doubtful, different means should be tried to determine IOP; this includes rebound tonometry, ORA, dynamic contour tonometry or Schiotz.Citation22–Citation24 Previous reports on IFS recommended performing IOP measurement on the periphery of the cornea outside the edge of the corneal flap (no interface fluid), using the rebound tonometerCitation20 to obtain a better estimate of the IOP. However, this may overestimate IOP due to the higher corneal thickness in the peripheral cornea.Citation25
In conclusion, ophthalmologists are likely to face this condition, so limiting topical steroids and routinely measuring the IOP post-LASIK are necessary steps to prevent IFS, especially in myopic patients. A high index of suspicion is required to diagnose it, and it should be suspected when there is LASIK flap edema with low IOP measured by GAT. High-resolution OCT is helpful to confirm the diagnosis.
Acknowledgments
This research was presented at the 21st Winter Meeting of the European Society of Cataract and Refractive Surgeons (ESCRS) held at Maastricht/the Netherlands; February 10–12, 2017 as a poster. The abstract was published online in the meeting program.
Disclosure
The author reports no conflicts of interest in this work.
References
- PallikarisIGSiganosDSExcimer laser in situ keratomileusis and photorefractive keratectomy for correction of high myopiaJ Refract Corneal Surg19941054985107530099
- ClarkAFBasic sciences in clinical glaucoma: steroids, ocular hypertension, and glaucomaJ Glaucoma19954535436919920699
- BeckerBMillsDWCorticosteroids and intraocular pressureArch Ophthalmol19637050050714078872
- ArmalyMFEffect of corticosteroids on intraocular pressure and fluid dynamics, the effect of dexamethasone in the normal eyeArch Ophthalmol19637048249114078870
- TourtasTKopsachilisNMeillerRKruseFECursiefenCPressure-induced interlamellar stromal keratitis after laser in situ keratomileusisCornea201130892092321734483
- KangSJDawsonDGHoppLMSchmackIGrossniklausHEEdelhauserHFInterface fluid syndrome in laser in situ keratomileusis after complicated trabeculectomyJ Cataract Refract Surg20063291560156216931273
- WaringGO3rdAppropriate term for post-LASIK corneal edemaJ Cataract Refract Surg20093581482148319631151
- LyleWAJinGJInterface fluid associated with diffuse lamellar keratitis and epithelial ingrowth after laser in situ keratomileusisJ Cataract Refract Surg19992571009101210404381
- SmithRJMaloneyRKDiffuse lamellar keratitis. A new syndrome in lamellar refractive surgeryOphthalmology19981059172117269754183
- TourtasTCursiefenCPISK-itis” or “PISK-opathy?Cornea201231210722138586
- BelinMWHannushSBYauCWSchultzeRLElevated intraocular pressure-induced interlamellar stromal keratitisOphthalmology2002109101929193312359617
- HamiltonDRMancheEERichLFMaloneyRKSteroid-induced glaucoma after laser in situ keratomileusis associated with interface fluidOphthalmology2002109465966511927421
- ShaikhNMShaikhSSinghKMancheEProgression to end-stage glaucoma after laser in situ keratomileusisJ Cataract Refract Surg200228235635911821221
- DawsonDGKramerTRGrossniklausHEWaringGO3rdEdelhauserHFHistologic, ultrastructural, and immunofluorescent evaluation of human laser-assisted in-situ keratomileusis corneal woundsArch Ophthalmol2005123674175615955975
- RandlemanJBShahRDLASIK interface complications: etiology, management and outcomesJ Refract Surg201228857558622869235
- Ortega-UsobiagaJMartin-ReyesCLlovet-OsunaFDamas-MateacheBBaviera-SabaterJInterface fluid syndrome in routine cataract surgery 10 years after laser in situ keratomileusisCornea201231670670722382593
- ChengACLawRWYoungALLamDSIn vivo confocal microscopic findings in patients with steroid-induced glaucoma after LASIKOphthalmology2004111476877415051211
- KurianMShettyRShettyBKDeviSAIn vivo confocal microscopic findings of interlamellar stromal keratopathy induced by elevated intraocular pressureJ Cataract Refract Surg20063291563156616931274
- Ortega-UsobiagaJLlovet-OsunaFDjodeyreMRLlovet-RausellABeltranJBavieraJIncidence of corneal infections after laser in situ keratomileusis and surface ablation when moxifloxacin and tobramycin are used as postoperative treatmentJ Cataract Refract Surg20154161210121626096523
- GotoSKohSTodaRInterface fluid syndrome after laser in situ keratomileusis following herpetic keratouveitisJ Cataract Refract Surg20133981267127023796622
- ChangDHStultingRDChange in intraocular pressure measurements after LASIK the effect of the refractive correction and the lamellar flapOphthalmology200511261009101615882906
- ParkHJUhmKBHongCReduction in intraocular pressure after laser in situ keratomileusisJ Cataract Refract Surg200127230330911226799
- FoglaRRaoSKPadmanabhanPInterface fluid after laser in situ keratomileusisJ Cataract Refract Surg20012791526152811566545
- PeposeJSFeigenbaumSKQaziMASandersonJPRobertsCJChanges in corneal biomechanics and intraocular pressure following LASIK using static, dynamic, and noncontact tonometryAm J Ophthalmol20071431394717188041
- LimYYamaanariMFukudaSBirefringence measurement of cornea and anterior segment by office-based polarization sensitive optical coherence tomographyBiomed Opt Express2011282392240221833376
