Abstract
Background
The primary objective of this study was to investigate the level of agreement between IOL-Master and OB-820 ocular biometers.
Materials and methods
In this prospective randomized case series, we measured the anterior chamber depth (ACD), the axial length (AL), the corneal radii (R1, R2), the ratio of mean corneal radius and spherical equivalent and the corneal astigmatism (cylinder [Cyl]) before and after cataract extraction surgery.
Results
Significant differences between pre- and postoperative data were observed for ACD for both biometric devices (P<0.01) and Cyl parameter in IOL-Master. Range and 95% limits of agreement (LoA) were clinically significantly different for AL parameter pre- and postoperatively and for R2 and radius and spherical equivalent postoperatively (P<0.001). The rest of the parameters presented sufficient 95% LoA, which imply good agreement.
Conclusion
In clinical practice, the IOL-master and OB-820 should not be used interchangeably due to discrepancy in the important AL parameter. Both biometers may provide consistent results regarding Cyl, ACD and R1.
Introduction
Ocular biometry is an essential procedure in ophthalmology as it is used to calculate the optical power of the intraocular lens (IOL) that replaces the cataract lens in pseudophakic cataract corrections.Citation1,Citation2 An integrated component of optical biometry is accurate measurement of the axial length (AL) of the eye, the most influencing parameter in modern formulas.Citation3,Citation4 AL measurement is based on the principles of transmission, reflection and absorption of light and sound through various media.
The following methods depend on echos in order to get information about the structures inside the eye.Citation5 In A-scan biometry (amplitude modulation), the reflection of a thin parallel sound beam is translated by the biometer into spikes arising from the baseline.Citation6 The height of the spikes is relevant to the strength of the reflecting echo that strikes each interface in the eye, and the distance between the spikes is relevant to the time needed in order for the echo to travel between each interface. Taking into account the different velocities of the echo transmission in the different ocular media through clear lens 1,640 m/s, cataract lens 1,629 m/s and aqueous 1,532 m/s, it is possible to differentiate between the different intraocular tissues.
In B-scan biometry, the reflected echoes are represented as a multitude of dots in a gray scale depending on the density of the tissue and are being imaged as a “slice” of the depicted structure. B-scan is primarily used to reconstruct ocular topography.Citation7–Citation9
However, A-scan ocular biometry suffers from high variability of measurements, which can partly be explained by the fact that the probe has to be in contact with the corneal epithelium. Therefore, a series of ocular biometry technologies have been developed to address the limitations of A-scan biometry: 1) the optical biometry with ZEISS IOL-Master (Carl Zeiss Meditec, Jena, Germany). This is a noncontact biometry device that can determine the AL of the eye by partial coherence interferometry (PCI). It can also measure the anterior chamber depth (ACD) using visual pachymetry and at the same time provide keratometric data. The AL is measured with an accuracy of ±0.01 mm in a measurement scale of 14–39 mm.Citation10 2) WaveLight OB-820 (WaveLight, Erlangen, Germany), which is also a noncontact PCI biometry device and the first to use low coherence reflectometry. It provides measurements of the AL, central corneal thickness, ACD and crystalloid lens thickness. It has the ability to analyze 16 different measurements alongside the optical axis and four different keratometric ones with a single scan of the eye.Citation11
The function of both these devices is based on Mickelson’s interferometer. It is a device that produces interference fringes by splitting a beam of monochromatic light so that one beam strikes a fixed mirror and the other a movable mirror. When the reflected beams are brought back together, an interference pattern results.Citation12 This pattern is detected by an appropriate detector. The spatial frequency of the wave crest depends on the difference of the optical route of the two beams until they cohere. In this way, if their difference is an integer multiple of the wavelength λ, then constructive interference occurs. In case the difference is an integer multiple of λ/2, there is destructive interference. Therefore, by altering the distance between the two split beams, the spatial frequency of the waves is also modified. This is accomplished using a reflector that can move forward and backward from the beam splitter.Citation13
It becomes obvious that both aforementioned devices utilize cutting-edge technology to calculate certain indexes that are essential in modern cataract surgery. However, a thorough search of the published literature returned no information of the level of agreement between these devices.
Within this context, the primary objective of this study was to assess the level of agreement of a series of biometric parameters between IOL-Master and WaveLight OB-820 in a sample of cataract patients.
Materials and methods
Setting
This was a prospective study. The protocol adhered to the tenets of the Declaration of Helsinki. The institutional review board of the Democritus University of Thrace approved the protocol, and written informed consent was obtained from all participants.
Participants
Participants were recruited from the Cataract Service on a consecutive-if-eligible basis and underwent full biometric examination using the IOL-Master and OB-820. They were between the ages of 60 and 80 years with senile grade II and grade III cataracts. Exclusion criteria for all study participants included diabetic macular edema, age-related macular degeneration, neurologic disorders that affect the neuroretina and the optic nerve, fundus photocoagulation or other intraocular procedures, edema or scarring of the cornea. Patients with intraoperative complications such as posterior capsule rupture, malposition of IOL, hemorrhage or use of stitches at the corneal incision were also excluded.
Data collection
The variables for this survey were obtained with IOL-Master and OB-820.
Data collection was performed in a consistent way by the same experienced operator. Regarding IOL-Master, five measurements were obtained for AL calculation with signal-to-noise ratio (SNR) at least >100. Preoperative data collection was obtained with the appropriate “phakic” setting on the software of the device. On the other hand, postoperative data were collected with the “pseudophakic” setting, which also offers further parameters to determine the type of the IOL, such as silicone, memory and acrylic.
Regarding OB-820, for corneal thickness, 16 point measurements in each eye were arranged in two rings. The outer ring diameter is 2.30 mm, and the inner ring diameter is 1.65 mm. The white-to-white distance is determined by photographing the iris and combining it with the keratometric values. These measurements represent the diameter of an ideal circle. The AL is measured by means of the patient’s visual optical line. An integrated conversion factor uses the established IOL calculation formulas that are originally derived from ultrasound biometry. The pupil diameter is obtained after at least five valid measurements. Those are possible even after refractive or other surgical procedures, as there are appropriate settings as well (phakic, aphakic, pseudophakic and silicon-filled eye).
The following parameters were calculated and evaluated both preoperatively and postoperatively: 1) ACD, 2) AL, 3) horizontal corneal diameter (R1), 4) vertical corneal diameter (R2), 5) radius/spherical equivalent (RSE) and 6) cylinder (Cyl).
Statistical analysis
The normality of measured data was evaluated by Kolmogorov–Smirnov test. Normal distribution data were assessed by Student’s t-test. Nonparametric data were assessed with Mann–Whitney U-test. Level of agreement between the two devices was assessed by Bland–Altman analysis. P-values <0.05 were considered as statistically significant. All statistical analyses were performed using SPSS version 16 (SPSS Inc., Chicago, IL, USA).
Results
Fifty-six patients (28 men and 28 women, 65.2±11.5 years) were recruited and populated the study group. Detailed demographic parameters are presented in . No difficulty in accurate measurements was encountered for our patients’ sample. Regarding preoperative and postoperative comparisons for both modalities, nonsignificant differences could be detected for the majority of measured parameters (). As it was expected, ACD was postoperatively increased both in IOL-Master (3.17±0.44 mm, 4.53±0.54 mm, P=0.03) and in OB-820 (3.23±0.41 mm, 4.62±0.34 mm, P=0.04). However, Cyl demonstrated significant postoperative difference only in IOL-Master (P=0.02). Another important outcome of the study was that, despite the fact that the AL did not significantly change prior to surgery and postoperatively when the parameter was statistically evaluated for each instrument, the actual difference of AL between IOL-Master and OB-820 was significant (P=0.03; ). This finding suggested a probable discrepancy in the limits of agreement (LoA) for the AL parameter, which was confirmed by the Bland–Altman analysis that was performed for all studied parameters. As presented in Bland–Altman plots ( and ), AL presented significant different range and 95% LoA (P<0.001) for both preoperative and postoperative measurements. In fact, OB-820 presented significant higher values for all measurements, with the observed discrepancy to be more pronounced for longer eyes (ie, with higher ALs). Moreover, the postoperative values of R2 and RSE parameters demonstrated significant discrepancy as well (P<0.001) ( and ).
Figure 1 Bland–Altman plot of AL (preoperative).
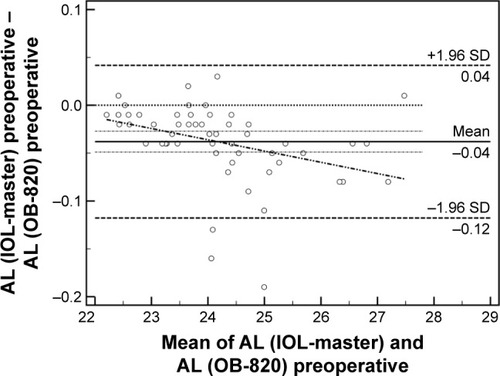
Figure 2 Bland–Altman plot of AL (postoperative).
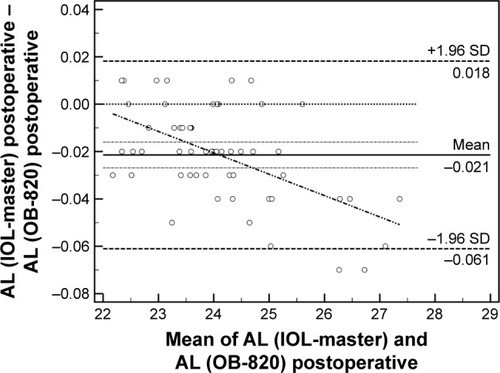
Figure 3 Bland–Altman plot of R2 (postoperative).
Abbreviation: SD, standard deviation.
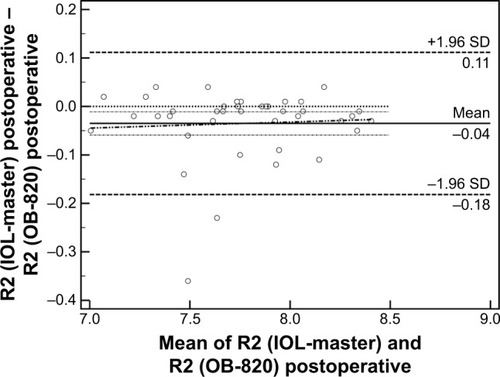
Figure 4 Bland–Altman plot of RSE (postoperative).
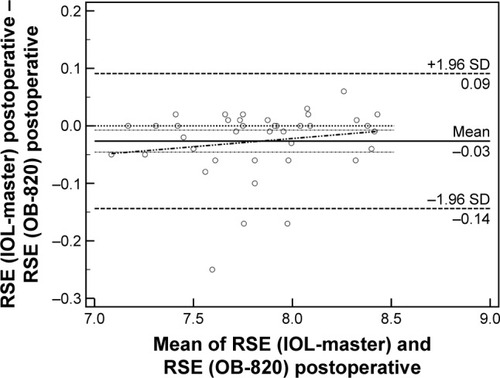
Table 1 Demographic characteristics of study participants
Table 2 Preoperative and postoperative values of measured parameters
Table 3 Comparisons of preoperative and postoperative differences
Discussion
Cataract is still the primary cause of reversible blindness in the world, and phacoemulsification is the most commonly performed operation in medicine.Citation14 Cutting-edge technology in cataract extraction surgery in terms of premium lenses, viscoelastics and emulsification strategies attempts to deliver optimal visual outcomes to the patient.Citation15–Citation17 However, among the most essential prerequisites is the accurate calculation of the dioptric power of the IOL that will substitute the cataract lens.Citation18,Citation19 A series of mathematical formulas have been developed that predict the necessary dioptric power of the artificial lens taking into account anatomical landmarks of the eye.Citation20–Citation24 However, modern ocular biometry presents variability before and after the cataract extraction surgery; moreover, significant discrepancies might be encountered between two different devices.Citation25 Moreover, there were several studies that presented the high validity and repeatability of IOL-Master-derived measurements.Citation26,Citation27 Nevertheless, none of them presented the validity or repeatability of OB-820 and the comparison of two modalities.
Within this context, this study attempted to evaluate the level of agreement between two prevalent biometric devices (ie, the IOL-Master and OB-820) prior to and following the operation. For this purpose, we recruited a sample of patients who were candidates for cataract extraction surgery, and we measured a series of parameters necessary for the calculation of the IOL power. Our outcomes suggested the following: 1) Further to the expected change in the ACD, both devices demonstrated nonsignificant differences between preoperative and postoperative values for the majority of studied parameters. Only the Cyl parameter presented significant change when measured by the IOL-Master. 2) Significant discrepancy in the level of agreement was detected for the AL both preoperatively and postoperatively, and 3) the postoperative values of R2 and RSE parameters demonstrated significant discrepancy as well. Since this is the first study to evaluate the level of agreement between IOL-Master and OB-820, we conducted a thorough literature regarding potential discrepancies in prevalent biometric devices. The majority of published reports are comparative trials regarding IOL-Master and LENSTAR LS 900 (Haag Streit AG).Citation28–Citation30 These reports suggest minor differences in measured parameters and adequate level of agreement between the two prevalent instruments.Citation31,Citation32
Conclusion
Our outcomes suggest that OB-820 presents significant difference with IOL-Master in AL measurements, suggesting that these biometry devices should not have been used interchangeably, since it is well known that AL is an integral component of modern formulas that estimate the dioptric power of the IOL. However, larger cohorts of cataractic patients and stratification according to their cataract level are necessary to confirm our results and contribute to the body of knowledge on the important issue of ocular biometry.
Acknowledgments
No financial support was received for this study.
Disclosure
None of the authors have received reimbursements, fees, funding, or salary in the past 5 years from an organization that may in any way gain or lose financially from the publication of this manuscript, either now or in the future. None of the authors hold any stocks or shares in an organization that may in any way gain or lose financially from the publication of this manuscript, either now or in the future. None of the authors have received reimbursements, fees, funding, or salary from an organization that holds or has applied for patents relating to the content of the manuscript. None of the authors of this manuscript have a financial interest in any material or method related to this work. No conflicting relationship exists for the authors. None of the authors has any proprietary interests or conflicts of interest related to this submission. It is not simultaneously being considered for publication in any other journal. The authors report no other conflicts of interest in this work.
References
- FedorovSNKolinkoAIKolinkoAIEstimation of optical power of the intraocular lensVestn Oftalmol1967802731
- HowesFWPatient workup for cataract surgeryYanoffMDukerJOphthalmology4th ed Chap. 5.3St Louis, MOMosby Elsevier2009410420
- LeeACQaziMAPeposeJSBiometry and intraocular lens power calculationCurr Opin Ophthalmol2008191131718090891
- WangLShirayamaMMaXJKohnenTKochDDOptimizing intraocular lens power calculations in eyes with axial lengths above 25.0 mmJ Cataract Refract Surg201137112018202722018365
- ByrneSFGreenRLUltrasound of the Eye and Orbit2nd edSt. Louis, MOMosby, Inc2002
- ByrneSFA-Scan Axial Eye Length Measurements; a Handbook for IOL CalculationsMars Hill, NCGrove Park Publishers19956264
- AgarwalAFanSInvernizziACharacterization of retinal structure and diagnosis of peripheral acquired retinoschisis using high-resolution ultrasound B-scanGraefes Arch Clin Exp Ophthalmol20162541697525904297
- SinghRInvernizziAAgarwalAKumariNGuptaAEnhanced depth imaging spectral domain optical coherence tomography versus ultrasonography B-Scan for measuring retinochoroidal thickness in normal eyesRetina201535225025625105311
- MüllerHRThe diagnosis of internal carotid artery occlusion by directional Doppler sonography of the ophthalmic arteryNeurology19722288164673409
- FreemanGPesudovsKThe impact of cataract severity on measurement acquisition with the IOLMasterActa Ophthalmol Scand200583443944216029267
- BuckhurstPJWolffsohnJSShahSNarooSADaviesLNBerrowEJA new optical low coherence reflectometry device for ocular biometry in cataract patientsBr J Ophthalmol200993794995319380310
- HariharanPBasics of InterferometrySan Diego, CAAcademic Press2010
- KanellopoulosAJAsimellisGCorrelation between central corneal thickness, anterior chamber depth, and corneal keratometry as measured by Oculyzer II and WaveLight OB820 in preoperative cataract surgery patientsJ Refract Surg2012281289590023092131
- WHOglobal Data on Visual Impairments 2010GenevaWHO20126
- LabirisGGiarmoukakisAPatsiamanidiMPapadopoulosZKozobolisVPMini-monovision versus multifocal intraocular lens implantationJ Cataract Refract Surg2015411535724962144
- LabirisGSideroudiHRousopoulosKKozobolisVPCohesive versus dispersive-cohesive ophthalmic viscosurgical device in torsional intelligent phacoJ Cataract Refract Surg201541368168225804586
- RobertsTVLawlessMSuttonGHodgeCUpdate and clinical utility of the LenSx femtosecond laser in cataract surgeryClin Ophthalmol201610202127799728
- BollingerKELangstonRHSWhat can patients expect from cataract surgery?Cleve Clin J Med200875319318383928
- ScheinODKatzJBassEBThe value of routine preoperative medical testing before cataract surgeryN Engl J Med2000342316817510639542
- HolladayJTStandardizing constants for ultrasonic biometry, keratometry, and intraocular lens power calculationsJ Cataract Refract Surg1997239135613709423908
- NgDTRoweNAFrancisICIntraoperative complications of 1000 phacoemulsification procedures: a prospective studyJ Cataract Refract Surg19982410139013959795858
- KurimotoYParkMSakaueHKondoTChanges in the anterior chamber configuration after small-incision cataract surgery with posterior chamber intraocular lens implantationAm J Ophthalmol199712467757809402823
- PereiraFACronembergerSUltrasound biomicroscopic study of anterior segment changes after phacoemulsification and foldable intraocular lens implantationOphthalmology200311091799180613129880
- LongDAMonicaMLA prospective evaluation of corneal curvature changes with 3.0- to 3.5-mm corneal tunnel phacoemulsificationOphthalmology199610322262328594506
- RozemaJJWoutersKMathysenDGTassignonMJOverview of the repeatability, reproducibility, and agreement of the biometry values provided by various ophthalmic devicesAm J Ophthalmol201415861111112025128596
- Lopez de la FuenteCSanchez-CanoASeguraFPinillaIComparison of anterior segment measurements obtained by three different devices in healthy eyesBiomed Res Int2014201449808024987687
- DincUAGorgunEOncelBYenerelMNAlimgilLAssessment of anterior chamber depth using Visante optical coherence tomography, slitlamp optical coherence tomography, IOL Master, Pentacam and Orbscan IIzOphthalmologica2010224634134620453540
- RabsilberTMJepsenCAuffarthGUHolzerMPIntraocular lens power calculation: clinical comparison of 2 optical biometry devicesJ Cataract Refract Surg201036223023420152602
- ChenY-AHirnschallNFindlOEvaluation of 2 new optical biometry devices and comparison with the current gold standard biometerJ Cataract Refract Surg201137351351721244866
- HuiSYiLComparison of two optical biometers in intraocular lens power calculationIndian J Ophthalmol201462993125370395
- MylonasGSacuSBuehlWRitterMGeorgopoulosMSchmidt-ErfurthUPerformance of three biometry devices in patients with different grades of age-related cataractActa Ophthalmol2011893e237e24121310011
- ShinJWSeongMKangMHLeeYGComparison of ocular biometry and postoperative refraction in cataract patients between Lenstar® and IOL Master®J Korean Ophthalmol Soc2012536833838
