Abstract
Purpose
To evaluate the therapeutic potential and safety of intravitreal injections of bone marrow mononuclear fraction (BMMF) containing CD34+ cells in patients with atrophic age-related macular degeneration (AMD).
Methods
Ten patients with atrophic AMD and best-corrected visual acuity (BCVA) in the worse-seeing eye of ≤20/100 were enrolled in this study. The bone marrow from all patients was aspirated and processed for mononuclear cell separation. A 0.1 mL suspension of BMMF CD34+ cells was injected into the vitreous cavity of the worse-seeing eye. Patients were evaluated at Baseline and 1,3,6,9 and 12 months after injection. Ophthalmic evaluation included BCVA measurement, microperimetry, infrared imaging, fundus autofluorescence and SD-optical coherence tomography at all study visits. Fluorescein angiography was performed at Baseline and at 6 and 12 months after intravitreal therapy.
Results
All patients completed the 6-month follow-up, and six completed the 12-month follow-up. Prior to the injection, mean BCVA was 1.18 logMAR (20/320−1), ranging from 20/125 to 20/640−2, and improved significantly at every follow-up visit, including the 12-month one, when BCVA was 1.0 logMAR (20/200) (P<0.05). Mean sensitivity threshold also improved significantly at 6, 9 and 12 months after treatment (P<0.05). Considering the area of atrophy identified by fundus autofluorescence, significant mean BCVA and mean sensitivity threshold improvement were observed in patients with the smallest areas of atrophy. Fluorescein angiography did not identify choroidal new vessels or tumor growth.
Conclusion
The use of intravitreal BMMF injections in patients with AMD is safe and is associated with significant improvement in BCVA and macular sensitivity threshold. Patients with small areas of atrophy have a better response. The paracrine effect of CD34+ cells may explain the functional improvement observed; however, larger series of patients are necessary to confirm these preliminary findings.
Keywords:
Introduction
Age-related macular degeneration (AMD) is a degenerative and disabling ocular disease that requires effective preventive and curative treatment.Citation1 Its main manifestation is progressive and irreversible loss of central vision in over 50-year-old individuals.Citation2–Citation4 Worldwide, it affects 8.7% of the elderly;Citation5 the prevalence is estimated at 196 million in 2020 and 288 million in 2040.Citation6 It is the leading cause of blindness in over 50-year-old patients in developed countries.Citation7 In developing countries, such as Brazil, it is in the third place, but, due to alterations in the epidemiological profile and aging of the population, this is expected to change.Citation8
The pathogenesis of AMD is not fully understood.Citation9,Citation10 There are two forms: exudative and dry. Exudative AMD occurs less frequently (15%) than dry AMD (85%), with the former accounting for two-thirds of the cases of significant visual loss.Citation3,Citation11,Citation12 Dry AMD can progress to geographic atrophy characterized by well-delineated areas of hypopigmentation or depigmentation due to an absence or attenuation of the underlying retinal pigment epithelium.Citation13 Mechanisms for the development of geographic atrophy include ischemia, senescence, oxidative and photo-oxidative damage and inflammation, either directly or through apoptotic mechanisms.Citation11,Citation14
While anti-vascular endothelial growth factor (anti-VEGF) therapy has revolutionized the treatment of exudative AMD, the only approved treatment for dry AMD to date is based on the Age-Related Eye Disease Study (AREDS). This study demonstrated that daily oral supplementation with antioxidant vitamins and minerals reduced the risk of developing an advanced disease in 5 years by 25%.Citation9,Citation10
Treatment of dry AMD poses a challenge as there is no approved therapy available. Knowing the pathology and its limitations, many researchers have looked for therapeutic alternatives, including the use of stem cells.Citation15
Some studies have evaluated the trophic effect of autologous hematopoietic stem cells (HSCs) derived from the bone marrow for retinal diseases.Citation11,Citation16–Citation20
The objective of this study was to evaluate the safety and efficacy of intravitreal injections of bone marrow mononuclear fraction (BMMF) containing CD34+ cells in patients with atrophic AMD.
Methods
A prospective, non-randomized, open study was conducted to assess changes in visual acuity and results of fluorescein angiography, infrared imaging, fundus autofluorescence, optical coherence tomography (OCT) and microperimetry induced by intravitreal injections of BMMF containing CD34+ cells in patients with dry AMD.
The study protocol adhered to the principles of the Declaration of Helsinki, and the study was registered as a clinical trial (NCT01518127). It was approved by local and national institutional review committees (Comitê Nacional de Ética e Pesquisa – CONEP no. 15978), and all the participants signed consent forms.
The patients were evaluated at the Retina and Vitreous Outpatient Clinic in the Hospital das Clínicas of the Medical School of Ribeirao Preto, University of Sao Paulo (HCFMRP-USP), Sao Paulo, between January 2014 and May 2015.
Inclusion and exclusion criteria
The inclusion criteria included the following: male or female; aged >50 years; a diagnosis of geographic atrophy due to AMD in either eye; an Early Treatment Diabetic Retinopathy Study (ETDRS) best-corrected visual acuity (BCVA) of 60 letters or worse in the study eye; no prior or current choroidal neovascularization in either eye; adequate caregiver support and access to medical care in the local community; able to provide written informed consent prior to any study-related procedures; and agree to comply in good faith with all conditions of the study and to attend all required study visits.
The exclusion criteria were the following: retinal disease other than AMD in study eye which, in the investigator’s opinion, may pose a safety risk or interfere with the study; choroidal neovascularization due to a cause other than AMD; media opacity in the study eye that interferes with fundus imaging or is likely to require surgery during the study period; any of the treatments to the study eye within 14 days prior to dosing (ranibizumab [Lucentis], bevacizumab [Avastin], pegaptanib [Macugen], or other VEGF inhibitor), or likely requirement of one of the above treatments within 14 days of LFG316 administration; any of the following within 30 days prior to dosing: photodynamic therapy in the study eye, extrafoveal or juxtafoveal thermal laser photocoagulation in the study eye, systemic or topical (ophthalmic) steroid use in the study eye, bacterial keratitis in the study eye, or intraocular surgery (including cataract surgery) in the fellow eye; any of the following within 90 days prior to dosing: intraocular surgery (including cataract surgery) in the study eye, or intravitreal or periocular corticosteroid injection in the study eye; and participation in another interventional clinical study, or use of any experimental treatment for AMD or any other investigational new drug within 12 weeks prior to the start of study treatment.
Clinical trials solely involving over-the-counter vitamins, supplements or diets were not considered as criteria to exclude the patients from study participation.
The patients underwent blood tests to screen for infections and to analyze blood coagulation.
Ophthalmologic evaluations
The BCVA was measured, according to the standards recommended by the ETDRS (1985), always by the same examiner at every return visit before any other procedure. At baseline, and 1, 3, 6, 9 and 12 months after treatment, patients were subjected to infrared imaging, fundus autofluorescence test, OCT and microperimetry besides an ophthalmologic evaluation by slit lamp biomicroscopy, with and without dilatation, applanation tonometry and retinal mapping with an indirect ophthalmoscope and a 20D lens. Fluorescein angiography was performed at baseline, and 6 and 12 months after the procedure.
Fluorescein angiography, infrared imaging and fundus autofluorescence
The examinations were performed with a Spectralis device (Heidelberg Engineering, Heidelberg, Germany) in all patients at baseline, and 1, 3, 6, 9 and 12 months after treatment except for fluorescein angiography which was performed at baseline, and at 3, 6 and 12 months to evaluate the extent of the AMD lesion and the possible growth of tumors or neovessels.
Optical coherence tomography
Patients were examined at baseline and at all subsequent visits using a Spectralis OCT device (Heidelberg Engineering). A contour with 19 horizontal sections at a distance of 236 μm and centered on the fovea was used. The total field was of 20°×15° (5.7×4.3 mm), with an average of 25 frames in each section.
Microperimetry
Microperimetry was performed using an MAIA apparatus (Centervue, Padova, Italy) with the images being acquired by scanning laser ophthalmoscopy. Sensitivity was measured from 0 to 36 dB, and color was coded; the field of the infrared image was 36°×36°, and perimetry was performed in a field of 30°×30° with a luminance of 4 asb. The Full-Threshold 4–2 test, which assesses the retina in detail, was used.
Collection of bone marrow aspirate
The technique used to obtain bone marrow aspirate is similar to the method employed in oncological and hematological procedures for bone marrow transplantation. The procedure was performed in the Bone Marrow Transplantation Sector of the HCFMRP-USP by a team of hematologists. After antisepsis with iodopovidone and lidocaine anesthesia, a needle was introduced into the posterior iliac crest, and 5–10 mL of bone marrow was aspirated using a syringe containing heparin.
Cell processing
The bone marrow aspirate was processed on the same day using Ficoll-Hypaque gradient centrifugation (Amersham Pharmacia) to isolate mononuclear cells. Then, the cells was resuspended in sterile saline and centrifuged again. These cells are a mixed population of single-nucleus cells including monocytes, lymphocytes and hematopoietic stem and progenitor cells. The fraction of mononuclear cells was immunophenotypically characterized by flow cytometry employing a panel of monoclonal antibodies to determine the presence and percentage of stem cells (CD34+) and mature cells of the hematopoietic and lymphoid cell lines. The number of cells to be infused was, on average, 1.68×104 cells as a 0.1-mL suspension. The total number (approximately 1 million cells) refers to the total number containing these different cells. The percentage of CD34+ cells ranged from 0.6% to 2%.
Intravitreal injection
All patients received a single 0.1-mL intravitreal autologous injection of BMMF with CD34+ cells in the eye with the worst visual acuity in which all the examinations should be performed. The cells were collected from the bone marrow of each patient (autologous cells), and therefore, there was no need to use immunotherapy.
All intravitreal injections were performed by the same researcher. The technique followed established rules including preprocedural periocular and ocular preparation using topical polyvinylpyrrolidone with iodine (PVPI) and topical ophthalmic PVPI, respectively, and the establishment of a surgical field with a sterile blepharostat. The intravitreal injection was applied using a 30-gauge needle in the upper temporal quadrant via pars plana at 4 and 3.5 mm from the limbus in phakic and pseudophakic eyes, respectively. After the procedure, antibiotic eye drops (fourth-generation quinolone) were prescribed four times per day for 7 days.
Statistical analysis
The JMP® statistics program version 10.0.0 was used for all analyses.
Results
Ten patients with AMD were enrolled in this study. All completed 6 months of evaluation, nine completed 9 months and six completed 12 months. Patients 7, 8, 9, and 10 did not attend the 12th-month visit due to difficulty in transportation, since they lived in distant cities.
Visual acuity
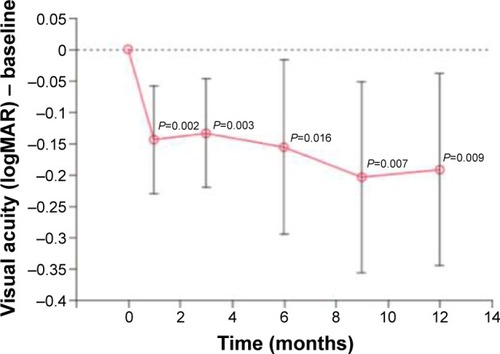
The mean BCVA before the injection of CD34+ cells was 1.18 logMAR (20/320+1). At 3, 6, and 12 months after the intravitreal injection, the mean BCVAs were 1.04 logMAR (20/200−2), 1.02 logMAR (20/200−1), and 1.0 logMAR (20/200), respectively. The BCVAs ranged from 20/125−1 to 20/800 at baseline, increasing to 20/63−1 and 20/500−2 in the first month and to 20/40 and 20/800 in the sixth month. There was a significant difference at each follow-up time point ( and ).
Table 1 Visual acuity measured before the intravitreal injection and 3 months after the procedure
Fluorescein angiography
No changes in perfusion and no tumors or neovessels were found following the intravitreal injection.
Infrared imaging and fundus autofluorescence
There was no significant difference in the size of atrophic areas assessed by infrared imaging and by fundus autofluorescence. Three patients with the largest areas in one of these examinations were selected to compare the data from the visual acuity and microperimetry examinations ().
Figure 2 The size of the atrophic area of patients 1 (left) and 4 (right) measured by infrared examination.
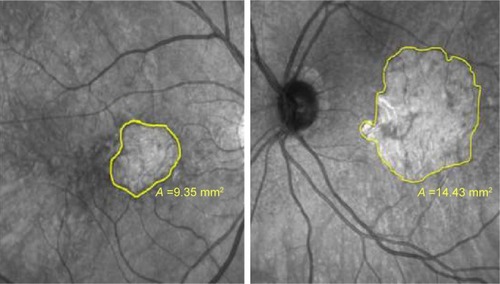
The sixth-month postprocedural analysis of the three patients with the largest atrophic areas showed no statistical significance for the BCVA and microperimetry parameters compared to baseline including for the bivariate contour ellipse area (BCEA) and the mean threshold sensitivity. In the seven patients with the smallest atrophic areas, significant differences were observed for the BCVA (P=0.03) and the mean threshold sensitivity on comparing the baseline data with those of the sixth postprocedural month (P=0.01). Moreover, an analysis of the BCEA showed that there was a trend towards significance (P=0.07).
Optical coherence tomography
No changes were found in the macular thickness or anatomy by OCT at any time point.
Microperimetry
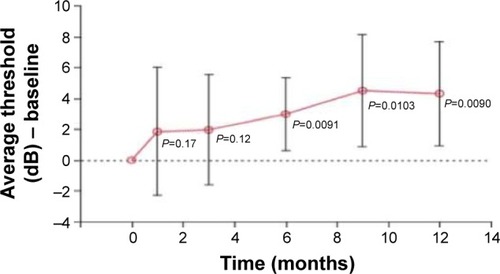
Two parameters were evaluated by microperimetry: the average threshold sensitivity and the BCEA. Despite the improvement in sensitivity, it was not significant in the third month, but the difference was significant at 6, 9, and 12 months ( and ).
Figure 4 Microperimetries at baseline and 6 months after the procedure of the ten eyes that received intravitreal injections. The visual acuity was measured using the Snellen chart and average threshold.
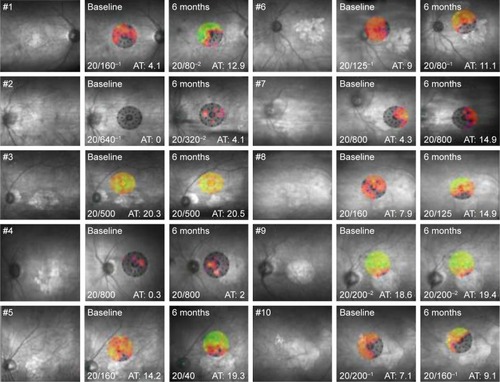
The average threshold sensitivity at baseline was 8.5 dB. In the first, sixth, and twelfth months, the average threshold sensitivities were 10.4, 11.6, and 12.15 dB, respectively.
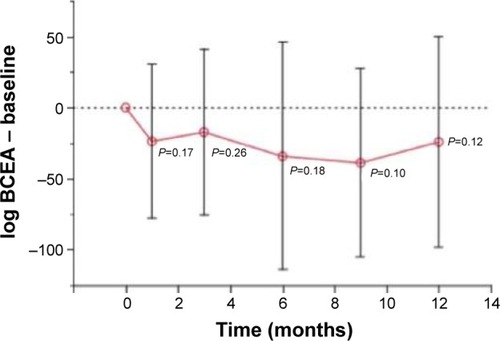
The evaluation of fixation was carried out by an analysis of the BCEA, which used two ellipses formed by various fixation points. Theoretically, the larger the ellipse, the more points the patient presented in the fixation. Probably because it is more difficult to fix at one point, other places of fixation are “looked for.” In the present study, there was a decrease in the BCEA, albeit without statistical significance ().
Discussion
Stem cells have a great potential for treating degenerative diseases of the retina.Citation21,Citation22 In the current study, ten patients with dry AMD received a single intravitreal injection of a 0.1-mL suspension of BMMF containing CD34+ cells. Several studies have already demonstrated that these cells derived from bone marrow can be incorporated after intravitreal injections. Otani et al were one of the first to demonstrate the presence of HSCs in mice up to 6 months after intravitreal injections.Citation23 Caballero et al also demonstrated that bone marrow CD34+ cells have the capacity to engraft in the damaged retina and its vasculature after intravitreal injections.Citation24
Significant effects were observed in the follow-up of patients. The procedure was not associated with clinically significant inflammation, and there was no detectable impairment in visual function, showing that the procedure is safe. This finding corroborates previous research, such as the Phase I study by Siqueira et al, which reported the absence of side effects with the intravitreal use of BMMF in patients with retinitis pigmentosa (RP) (three cases) and cone dystrophy (two cases).Citation12
Jonas et al, in a pilot study of three patients with AMD, glaucoma and diabetic retinopathy separately, also showed that the intravitreal use of these cells is safe.Citation25 Park et al performed a Phase I study with six patients (six eyes) suffering from ischemia or retinal degeneration; these authors found no local or systemic side effects.Citation26
The trophic effect of cell therapy was analyzed in the present study. The main cells used for this type of evaluation are HSCs. These cells are multipotent, but the primary mechanism of action of these cells appears to be a paracrine trophic effect rather than direct cell replacement. These cells secrete neurotrophic and angiogenic trophic factors such as ciliary neurotrophic factor, basic fibroblast growth factor, and VEGF. The biologics secreted by these stem cells have been referred to as secretomes, some of which are packages of extracellular vesicles. Probably, these factors act on cells that are suffering, especially in the area corresponding to hyper-autofluorescence. It is expected that these cells have no action in the atrophic area because they do not have cell replacement capacity.Citation20 According to Park et al,Citation20 acting on the damaged retina may represent a more viable approach to the treatment of retinal dysfunction than direct cell replacement as most retinal disorders are associated with more than one type of damage. Thus, cell therapy would not be disease specific and would be more universally applicable.Citation20
It is well known that the primary mechanism of HSCs is their trophic effect; however, Tomita et al demonstrated in studies of mice with retinal injury that these cells are capable of integrating and differentiating into retinal neurons.Citation27
In the current study, significant improvements in the visual acuity were seen at all time points. Of the ten patients, six improved by more than one line of letters, three of whom improved by two lines or more; after 3 months, two improved by less than one line, and two remained stable. Previous studies with AMD and other diseases have demonstrated the possibility of improving poor vision with mononuclear cells containing CD34+ cells.Citation12,Citation26 In a study by Siqueira et al, improvements in vision were observed in all five patients evaluated even though they had a worse visual acuity of 20/200 and more advanced disease.Citation12,Citation28 Park et al also showed improvement in the BCVA of two or more lines of letters in four of six patients with diagnoses such as Stargardt disease, AMD, central retinal artery and vein occlusion and RP.Citation26 The improvement ranged from 3 to 65 letters; in the present study, the improvement ranged from 3 to 19 letters.
OCT showed no change in anatomy or thickness. Siqueira et al also observed no change in macular thickness.Citation12 Park et al performed tomography with an adaptive optics device and observed that hyperreflective images measuring the same size as CD34+ cells appeared in the retinal layers 1 month after the intravitreal injection in three patients.Citation26
Fluorescein angiography was performed in all patients. One of the effects of HSC therapy is to promote neovascularization and improve retinal perfusion.Citation23 The injection of BMMF in mice rescued endothelial cells.Citation23,Citation29 Even so, no changes in macular perfusion were observed in the ten patients of the current study. On the other hand, this test, by analyzing possible tumor growth and development of choroidal neovascularization during the follow-up, was very important to show the safety of the procedure. Seven patients had improvements in the microperimetry test, while two showed a slight decline in the threshold sensitivity within the first month after the intravitreal injection. At 6 months, nine patients presented greater sensitivity compared to the preprocedural exam, and at 12 months, five of the six patients analyzed showed improvements. Park et al also reported improvements in microperimetry in three out of the five patients evaluated after HSC intravitreal injections.Citation26
On correlating the area of hypoautofluorescence or atrophy identified by the infrared test with improvements in the visual acuity and threshold sensitivity evaluated by microperimetry, it was explicit that patients with greater areas of geographic atrophy responded less to the procedure; there was improvement, albeit nonsignificant, in the parameters. The group with the smallest area of atrophy presented better results. It is believed that BMMF therapy may not only stabilize the process but also decrease the scotoma area by the functional rescue of distressed cells.
Conclusion
Intravitreal injection of BMMF containing CD34+ cells is safe in patients with atrophic AMD. The preliminary results demonstrated a significant improvement in visual acuity, especially in patients with smaller areas of geographic atrophy probably because of the trophic effect of these cells. Studies with larger study samples and the use of technologies such as adaptive optics are important for a better understanding of the behavior of these cells in retinal diseases.
Disclosure
The authors report no conflicts of interest in this work.
References
- CenturionVLacavaACCaballeroJCLente intraocular com cromóforo amarelo – resultados [Intraocular lens with yellow chromophore – results]Rev Bras Oftal2004637–8424431 Portuguese [with English abstract]
- FritscheLGFarissRNStambolianDAbecasisGRCurcioCASwaroopAAge-related macular degeneration: genetics and biology coming togetherAnnu Rev Genomics Hum Genet20141515117124773320
- KhanMAgarwalKLoutfiMKamalAPresent and possible therapies for age-related macular degenerationISRN Ophthalmol2014201460839025097787
- MavijaMAlimanovicEJaksicVKasumovicSSCekicSStamenkovicMTherapeutic modalities of exudative age-related macular degenerationMed Arch201468320420825568535
- CascellaRRagazzoMStrafellaCAge-related macular degeneration: insights into inflammatory genesJ Ophthalmol2014201458284225478207
- WongWLSuXLiXGlobal prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysisLancet Glob Health201422e106e11625104651
- FriedmanDSO’ColmainBJMuñozBPrevalence of age-related macular degeneration in the United StatesArch Ophthalmol200412256457215078675
- ÁvilaMAlvesMRNishiMAs condições de saúde ocular no Brasil [The Conditions of Ocular Health in Brazil]1st edSao PauloConselho Brasileiro de Oftalmologia2015 Portuguese
- Age-Related Eye Disease Study 2 Research Group (AREDS2)Lutein + zeaxanthin and omega-3 fatty acids for age-related macular degeneration: the Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trialJAMA2013309192005201523644932
- Age-Related Eye Disease Study 2 Research Group (AREDS2)ChewEYSanGiovanniJPFerrisFLLutein/zeaxanthin for the treatment of age-related cataract: AREDS2 randomized trial report no 4JAMA Ophthalmol2013131784385023645227
- SiqueiraRCStem cell therapy for retinal diseases: updateStem Cell Res Ther2011265022206617
- SiqueiraRCMessiasAVoltarelliJCScottIUJorgeRIntravitreal injection of autologous bone marrow-derived mononuclear cells for hereditary retinal dystrophy: a phase I trialRetina20113161207121421293313
- MedinaNHEpidemiologia do envelhecimento: estudo oftalmológico populacional de idosos [Epidemiology of Aging: Population-Based Ophthalmologic Study of the Elderly] [PhD thesis]Sao PauloUniversidade Federal de São Paulo1997 Portuguese
- SchwartzSDHubschmanJPHeilwellGEmbryonic stem cell trials for macular degeneration: a preliminary reportLancet2012379981771372022281388
- TaskintunaIElsayedMEAASchatzPUpdate on clinical trials in dry age-related macular degenerationMiddle East Afr J Ophthalmol2016231132626957835
- SiqueiraRCVoltarelliJCMessiasAMJorgeRPossible mechanisms of retinal function recovery with the use of cell therapy with bone marrow-derived stem cellsArq Bras Oftalmol201073547447921225138
- SiqueiraRCélulas tronco para tratamento de doencas da retina – estudos clínicos [Stem cells for treatment of retinal diseases – clinical studies]Rev Univ Vis2015874850 Portuguese [with English abstract]
- SiqueiraRCMessiasAMessiasKQuality of life in patients with retinitis pigmentosa submitted to intravitreal use of bone marrow-derived stem cells (Reticell-clinical trial)Stem Cell Res Ther2015612925890251
- ParkSSCaballeroSBauerGLong-term effects of intravitreal injection of GMP-grade bone marrow derived CD34+ cells in NOD-SCID mice with acute ischemia-reperfusion injuryInvest Ophthalmol Vis Sci201253298699422247454
- ParkSSMoisseievEBauerGAdvances in bone marrow stem cell therapy for retinal dysfunctionProgr Retin Eye Res201756148155
- PellegriniGDe LucaMArsenijevicYTowards therapeutic application of ocular stem cellsSemin Cell Dev Biol200718680581817959397
- TibbettsMDSamuelMAChangTSHoACStem cell therapy for retinal diseaseCurr Opin Ophthalmol201223322623422450217
- OtaniADorrelMIKinderKRescue of retina degeneration by intravitreally injected adult bone marrow-derived lineage-negative hematopoietic stem cellsJ Clin Invest2004114676577415372100
- CaballeroSSenguptaNAfzalAIschemic vascular damage can be repaired by healthy, but not diabetic endothelial progenitor cellsDiabetes20075696096717395742
- JonasJBWitzens-HarigMArsenievLHoADIntravitreal autologous bone marrow-derived mononuclear cell transplantation: a feasibility reportActa Ophthalmol2008862022522617900263
- ParkSSBauerGAbediMPontowSPanorgiasAJonnalRIntravitreal autologous bone marrow CD34+ cell therapy for ischemic and degenerative retinal disorders: preliminary phase 1 clinical trial findingsInvest Ophthalmol Vis Sci2014561818925491299
- TomitaMAdachiYYamadaHBone marrow-derived stem cells can differentiate into retinal cells in injured rat retinaStem Cells200220427928312110696
- SiqueiraRCMessiasAVoltarelliJCMessiasKArcieriRSJorgeRResolution of macular edema associated with retinitis pigmentosa after intravitreal use of autologous BM-derived hematopoietic stem cell transplantationBone Marrow Transplant201348461261323000646
- DorrellMIOtaniAAguilarEMorenoSKFriedlanderMAdult bone marrow-derived stem cells use R-cadherin to target sites of neovascu-larization in the developing retinaBlood200410393420342714726407