Abstract
Purpose
The purpose of this study was to determine the short-term outcomes for patients who received intravitreal aflibercept (IVA) with or without intravitreal ranibizumab (IVR) for macular edema (ME) due to branch retinal vein occlusion (BRVO).
Patients and methods
Patients received IVA for ME due to BRVO. Patients who initially received IVA were defined as the treatment-naïve group and those who were switched from IVR to IVA after ME recurrence were defined as the switching group. Patient outcomes were examined at 1 week and 1 month postinjection.
Results
Both groups comprised 27 eyes from 27 patients. There was a significant decrease in central macular thickness (CMT) at 1 week and 1 month postinjection in both groups. There was also a significant improvement in best-corrected visual acuity (BCVA) at 1 week and 1 month postinjection in the treatment-naïve group and 1 month in the switching group. Younger age was associated with a good BCVA at 1 month postinjection in the switching group, and the absence of epiretinal membrane was associated with a reduction in CMT at 1 month postinjection in the switching group.
Conclusion
IVA is temporarily effective for treating ME due to BRVO regardless of a history of IVR use.
Introduction
Retinal vein occlusion (RVO), which can be classified as central retinal vein occlusion (CRVO) or branch retinal vein occlusion (BRVO), is the second most common type of retinal vascular disease after diabetic retinopathy.Citation1,Citation2 RVO can induce a loss of visual acuity due to the presence of macular edema (ME),Citation1–Citation3 current treatments including intravitreal dexamethasone implants, laser treatment, and intravitreal injections of anti-vascular endothelial growth factor (anti-VEGF) agents.Citation3–Citation26 Anti-VEGF therapy is widely used for ME due to BRVO, and positive clinical outcomes have been reported,Citation3–Citation11,Citation27–Citation29 with numerous studies reporting on the successful use of the VEGF antibody ranibizumab (Lucentis; Genentech Inc., South San Francisco, CA, USA), because ranibizumab use is first covered by insurance as anti-VEGF agents for vitreous injection. After ranibizumab, there have been large treatment studies involving the VEGF inhibitor aflibercept (Regeneron Pharmaceuticals Inc., Tarrytown, NY, USA)Citation28, but only one clinical report has described treatment outcomes of intravitreal aflibercept (IVA) for BRVO.Citation29 There have also been few reports of switching from ranibizumab to aflibercept for the treatment of ME due to CRVO,Citation24,Citation25 and none for ME due to BRVO. The short-term outcomes of treatments involving aflibercept and switching from ranibizumab to aflibercept for ME due to BRVO were therefore evaluated.
Patients and methods
Ethics
This retrospective study was conducted in accordance with the Declaration of Helsinki. All necessary authorizations were obtained from the Institutional Review Board of the Juntendo University Urayasu Hospital, Urayasu City, Japan. Fully informed written consent was obtained from all study participants.
Patients
Patients were treated with aflibercept for ME due to BRVO between June 2015 and April 2016. The inclusion criteria were as follows: age ≥18 years; symptomatic BRVO with retinal edema involving the foveal center; and foveal thicknesses >300 µm at the initial visit (measured by optical coherence tomography). Exclusion criteria included patients who had received intravitreal bevacizumab (IVB) injection, scatter photocoagulation, or grid laser photocoagulation for ME. The patients were classified into two groups, a treatment-naïve group subsequently treated with IVA and a switching group initially treated with intravitreal ranibizumab (IVR) injection and then switched to IVA because of the recurrence of ME.
Visual acuity and central macular thickness (CMT) were measured at preinjection and at 1 week and 1 month after the IVA. The ratio of patients in the treatment-naïve and switching groups in which the CMT was <300 µm at 1 month after the IVA was compared. The best-corrected visual acuity (BCVA) was measured by using a Landolt chart and converted to the logarithm of the minimum angle of resolution (logMAR).
Preinjection parameters in the switching group were correlated with a CMT <300 µm at 1 month after the IVA and a logMAR ≤0.15 (Snellen chart 20/28) at 1 month after the IVA. The preinjection parameters included age; sex; the duration from onset; the number of IVR injections before switching to IVA; and presence or absence of hypertension, diabetes, cystoid ME, subretinal fluid, and epiretinal membrane (ERM).
Statistical analysis
Data were analyzed by using StatView software for Windows (SAS, Cary, NC, USA). The repeated measures analysis of variance and Dunnett’s test of multiple comparisons were used for comparisons at different time points before and after the injections, as a statistical test for related, not independent groups, was required. Fisher’s exact test was used to compare the ratios between the two groups. The Mann–Whitney U test was used to compare the age, the duration from onset, the number of IVR injections before the IVA injection, and CMT at preswitching. Fisher’s exact test was used for categorical variables such as sex and the presence or absence of hypertension, diabetes, cystoid ME, subretinal fluid, and ERM. Multivariate logistic regression was used to analyze the preswitch parameters. P<0.05 was accepted as statistically significant.
Results
Baseline characteristics
A total of 27 eyes from 27 patients comprised both the treatment-naïve and switching groups. The mean age was 66.4±11.0 years in the treatment-naïve group and 72.0±8.4 years in the switching group. The preinjection characteristics of the patients in both groups are summarized in .
Table 1 Patient demographics and characteristics
Visual acuity and CMT
Changes in the BCVA and CMT from the treatment-naïve and switching groups are shown in and , respectively. In the treatment-naïve group, the BCVA improved from a logMAR value of 0.49 (20/62) at preinjection to 0.34 (20/44) at 1 week post-IVA and 0.27 (20/37) at 1 month post-IVA. Compared with the preinjection, there was a significant improvement in BCVA at both 1 week and 1 month post-IVA. In the switching group, the BCVA improved from a logMAR value of 0.48 (20/60) at preinjection to 0.44 (20/55) at 1 week post-IVA and 0.39 (20/49) at 1 month post-IVA. Compared with the preinjection, there was no significant improvement in BCVA at 1 week post-IVA, but there was a significant improvement in BCVA at 1 month post-IVA.
Figure 1 Improvement of BCVA and CMT from preinjection in the treatment-naïve group. There was a significant improvement at 1 week and 1 month post-IVA for both the BCVA and CMT.
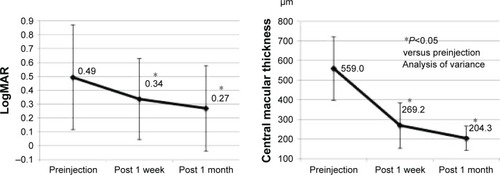
Figure 2 Improvement of BCVA and CMT from preswitch in the switching group. There was a significant improvement in BCVA at 1 month post-IVA and a significant improvement in CMT at 1 week and 1 month post-IVA.
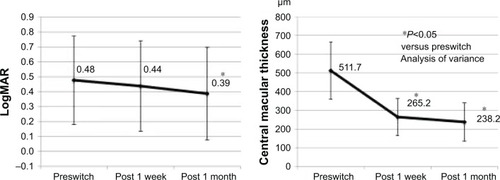
In the treatment-naïve group, the mean CMT decreased from 559.0 µm at preinjection to 269.2 µm at 1 week post-IVA and 204.2 µm at 1 month post-IVA. In the switching group, the mean CMT decreased from 511.7 µm at pre-injection to 265.2 µm at 1 week post-IVA and 238.2 µm at 1 month post-IVA. Compared with the preinjection, there was a significant decrease in the mean CMT at both 1 week and 1 month post-IVA in both the groups.
There were 26 of 27 eyes (96.3%) in the treatment-naïve group and 23 of 27 eyes (85.2%) in the switching group where the CMT was <300 µm at 1 month post-IVA, but there was no significant difference between the two groups (P=0.35).
Preswitch parameter associated with a beneficial response
In the switching group, univariate analysis showed that age and duration from onset were preswitch parameters associated with a decreased logMAR of ≤0.15 (20/28) at 1 month post-IVA (). Here, multivariate logistic regression analysis showed that younger age was an independent pre-switch parameter associated with a logMAR ≤0.15 (20/28). Univariate analysis also showed that the absence of ERM in the switching group was a preswitch parameter associated with a CMT <300 µm at 1 month post-IVA ().
Table 2 Preswitching factors associated with visual acuity at 1 month post-IVA
Table 3 Preswitching parameters associated with CMT at 1 month post-IVA
Discussion
Numerous studies have reported on the use of anti-VEGF therapies to treat ME due to BRVO.Citation3–Citation11,Citation27–Citation29 The BRAVO and HORIZON studies were large-scale studies that evaluated the effectiveness of IVR treatment for ME due to BRVO,Citation3,Citation27 while VIBRANT study investigated the efficacy of IVA treatment.Citation28 There are numerous reports describing switch therapy for age-related macular degeneration (AMD),Citation30–Citation34 but few reports describe the use of switch therapy for RVO.Citation24,Citation25,Citation35 Switching from steroid to anti-VEGF injections for RVO,Citation35 switching from IVR to IVA for CRVO,Citation24 and switching from IVB or IVR to IVA for CRVO have all been previously reported.Citation25 For ischemic CRVO, Lehmann-Clarke et al found that switching from IVR to IVA made the injection interval longer.Citation24 Therefore, this study would benefit from observing longer follow-up periods and consideration of the length of injection intervals.
No studies investigated switching from other anti-VEGF therapies to aflibercept, but switching from IVB to dexamethasone implants for treating ME due to BRVO has been reported.Citation35 By switching from IVB to dexamethasone or from dexamethasone to IVB, both visual acuity and CMT were significantly improved. In this study, switching to IVA from IVR showed both functional and anatomical improvement, as both the visual acuity and CMT significantly improved at 1 month postinjection.
At 1 month post-IVA, there was no significant difference between the treatment-naïve and switching groups for the CMT becoming ≤300 µm. However, the background characteristics of the two groups were different, so a simple comparison was not suitable. The difference in the durations from disease onset between the two groups was large, which can influence the short-term CMT.
In addition, this study showed that the visual acuity of a younger patient increased to a logMAR of ≤0.15 (20/28) at 1 month when switching to post-IVA. It is thought that older patients did not adequately recover their retinal function when the ME disappears. Furthermore, Yasuda et al reported that age is one of the risk factors associated with the onset of RVO,Citation36 so age is also thought to be related to the severity of RVO.
In the switching group, the CMT was rarely <300 µm at 1 month post-IVA in ME patients with ERM. A previous study reported a case of AMD with vitreomacular adhesion that was insensitive to anti-VEGF treatment.Citation37 This report involved a very short timeframe, but it had similar outcomes for BRVO as the present report. It is thought that pro re nata dosing involving IVA is sufficient for switching patients without ERM because of their sensitivity to IVA, but switching of patients with ERM would likely require multiple injections. However, this study only examined patients until 1 month post-IVA and did not examine whether the ME improved after the second IVA, so in these patients it is necessary to follow the progress of the ME after multiple IVA injections. In addition, in patients with ME due to BRVO with ERM, it might be necessary to perform a vitrectomy.
Limitations
The limitations of this study included the small sample size and the short-term follow-up period of 1 month after switching. To overcome these concerns, future studies should determine the effects of IVA over a longer timeframe.
Conclusion
The treatment outcomes at 1 month post-IVA injection for patients with ME due to BRVO were reported. IVA is temporarily effective for treating ME due to BRVO regardless of a history of IVR use.
Disclosure
The authors report no conflicts of interest in this work.
References
- KleinRMossSEMeuerSMKleinBEThe 15-year cumulative incidence of retinal vein occlusion. The Beaver Dam eye studyArch Ophthalmol200812651351818413521
- US Census BureauAnnual estimates of the population by sex and five-year age groups for the United States: April 1, 2000 to July 1, 2007 NC-EST2007-01. Release Date: May 1, 2008. Available from: https://www.census.gov/prod/2008pubs/p70-117.pdfAccessed February 16, 2010
- CampochiaroPAHeierJSFeinerLBRAVO InvestigatorsRanibizumab for macular edema following branch retinal vein occlusion: six-month primary end point results of a phase III studyOphthalmology20101171102111220398941
- SakanishiYLeeAUsui-OuchiAItoREbiharaNTwelve-month outcomes in patients with retinal vein occlusion treated with low-frequency intravitreal ranibizumabClin Ophthalmol2016101161116527382250
- CampochiaroPAWykoffCCSingerMMonthly versus as-needed ranibizumab injections in patients with retinal vein occlusion: the SHORE studyOphthalmology20141212432244225060610
- BrownDMCampochiaroPABhisitkulRBSustained benefits from ranibizumab for macular edema following branch retinal vein occlusion: 12-month outcomes of a phase III studyOphthalmology20111181594160221684606
- CampochiaroPASophieRPearlmanJRETAIN Study GroupLong-term outcomes in patients with retinal vein occlusion treated with ranibizumab: the RETAIN StudyOphthalmology201412120921924112944
- Nghiem-BuffetSFajnkuchenFBuffetMIntravitreal ranibizumab and/or dexamethasone implant for macular edema secondary to retinal vein occlusionOphthalmologica201423221622225413000
- BrynskovTKempHSørensenTLIntravitreal ranibizumab for retinal vein occlusion through 1 year in clinical practiceRetina2014341637164324646663
- TanMHMcAllisterILGilliesMERandomized controlled trial of intravitreal ranibizumab versus standard grid laser for macular edema following branch retinal vein occlusionAm J Ophthalmol201415723724724112635
- MylonasGSacuSDunavoelgyiRMacula Study GroupResponse of retinal sensitivity to ranibizumab treatment of macular edema after acute branch retinal vein occlusionRetina2013331220122623584689
- ScottIUIpMSVanVeldhuisenPCSCORE Study Research GroupA randomized trial comparing the efficacy and safety of intravitreal triamcinolone with standard care to treat vision loss associated with macular Edema secondary to branch retinal vein occlusion: the Standard Care vs Corticosteroid for Retinal Vein Occlusion (SCORE) study report 6Arch Ophthalmol20091271115112819752420
- IpMSScottIUVanVeldhuisenPCSCORE Study Research GroupA randomized trial comparing the efficacy and safety of intravitreal triamcinolone with observation to treat vision loss associated with macular edema secondary to central retinal vein occlusion: the Standard Care vs Corticosteroid for Retinal Vein Occlusion (SCORE) study report 5Arch Ophthalmol20091271101111419752419
- KorobelnikJFKodjikianLDelcourtCTwo-year, prospective, multicenter study of the use of dexamethasone intravitreal implant for treatment of macular edema secondary to retinal vein occlusion in the clinical setting in FranceGraefes Arch Clin Exp Ophthalmol20162542307231827286894
- no authors listedArgon laser photocoagulation for macular edema in branch vein occlusion. Branch Vein Occlusion Study GroupAm J Ophthalmol1984982712826383055
- NomaHFunatsuHMimuraTShimadaKMacular sensitivity and morphology after intravitreal injection of triamcinolone acetonide for macular edema with branch retinal vein occlusionRetina2012321844185222653545
- NomaHMimuraTMacular sensitivity and morphology after intravitreal injection of triamcinolone acetonide for macular edema secondary to central retinal vein occlusionClin Ophthalmol201261901190623204832
- BrownDMCampochiaroPASinghRPCRUISE InvestigatorsRanibizumab for macular edema following central retinal vein occlusion: six-month primary end point results of a phase III studyOphthalmology20101171124113320381871
- CampochiaroPABrownDMAwhCCSustained benefits from ranibizumab for macular edema following central retinal vein occlusion: twelve-month outcomes of a phase III studyOphthalmology20111182041204921715011
- BergerARCruessAFAltomareFOptimal treatment of retinal vein occlusion: Canadian expert consensusOphthalmologica201523462526088287
- BrownDMWykoffCCWongTPRAVE Study GroupRanibizumab in preproliferative (ischemic) central retinal vein occlusion: the rubeosis anti-VEGF (RAVE) trialRetina2014341728173524914476
- OguraYRoiderJKorobelnikJFGALILEO Study GroupIntravitreal aflibercept for macular edema secondary to central retinal vein occlusion: 18-month results of the phase 3 GALILEO studyAm J Ophthalmol20141581032103825068637
- HeierJSClarkWLBoyerDSIntravitreal aflibercept injection for macular edema due to central retinal vein occlusion: two-year results from the COPERNICUS studyOphthalmology20141211414142024679444
- Lehmann-ClarkeLDiraniAMantelIAmbresinAThe effect of switching ranibizumab to aflibercept in refractory cases of macular edema secondary to ischemic central vein occlusionKlin Monbl Augenheilkd201523255255525902119
- PapakostasTDLimLvan ZylTIntravitreal aflibercept for macular oedema secondary to central retinal vein occlusion in patients with prior treatment with bevacizumab or ranibizumabEye (Lond)201630798426449196
- CampochiaroPAHafizGMirTAScatter photocoagulation does not reduce macular edema or treatment burden in patients with retinal vein occlusion: the RELATE trialOphthalmology20151221426143725972260
- HeierJSCampochiaroPAYauLRanibizumab for macular edema due to retinal vein occlusions: long-term follow-up in the HORIZON trialOphthalmology201211980280922301066
- CampochiaroPAClarkWLBoyerDSIntravitreal aflibercept for macular edema following branch retinal vein occlusion: the 24-week results of the VIBRANT studyOphthalmology201512253854425315663
- WangJKHuangTLSuPYChangPYTsengYYIntravitreal aflibercept for macular edema secondary to branch retinal vein occlusion in Chinese patientsEye Sci201530636626902063
- Pinheiro-CostaJCostaJMBeatoJNSwitch to aflibercept in the treatment of neovascular AMD: one-year results in clinical practiceOphthalmologica201523315516125896317
- MichalewskiJNawrockiJTrębińskaMMichalewskaZSwitch to a single dose of aflibercept in bevacizumab nonresponders with AMDCan J Ophthalmol20144943143525284099
- EhlkenCJungmannSBöhringerDAgostiniHTJunkerBPielenASwitch of anti-VEGF agents is an option for nonresponders in the treatment of AMDEye (Lond)20142853854524722504
- EhlersJPSpirnMJShahCPRanibizumab for exudative age-related macular degeneration in eyes previously treated with alternative vascular endothelial growth factor inhibitorsOphthalmic Surg Lasers Imaging20104118218920307035
- MassambaNDiraniAButelNEvaluation of outer retinal tubulations in eyes switched from intravitreal ranibizumab to aflibercept for treatment of exudative age-related macular degenerationGraefes Arch Clin Exp Ophthalmol2017255616727397583
- ChristopheCBronAMStraubMRetinal vein occlusions: therapeutic switch in macular oedema treatment with a 12-month follow-upOphthalmic Res20165515215826756383
- YasudaMKiyoharaYArakawaSPrevalence and systemic risk factors for retinal vein occlusion in a general Japanese population: the Hisayama studyInvest Ophthalmol Vis Sci2010513205320920071683
- LeeSJKohHJEffects of vitreomacular adhesion on anti-vascular endothelial growth factor treatment for exudative age-related macular degenerationOphthalmology201111810111020678805
