Abstract
Purpose
Artificial tear formulations typically contain a water-soluble polymer to enhance residence time, moisture retention, and binding to the mucin coat of the ocular surface, which facilitate corneal healing. This study investigated the potential advantages of combining carboxymethylcellulose (CMC) and hyaluronic acid (HA) polymers in a single formulation.
Materials and methods
Individual CMC and HA solutions were prepared and tested for bulk viscosity in comparison to a solution that combined CMC and HA. Rheometry determined the differences between solutions at increasing shear rates, simulating eye movement and blinking.
Results
The bulk viscosity of the individual 0.5% CMC and 0.1% HA solutions was 2.5 and 5.7 cP, respectively. The viscosity of the combined solution (13.1 cP) was 60% higher than predicted by additive effects. Rheometry revealed shear rates between 10/second (open eye) and 10,000/second (blinking eye). At these rates, viscosity ranged from 2.7 to 3.5 cP for 0.5% CMC, 2.8 to 6.8 cP for 0.1% HA, and 5.2 to 15.3 cP for the 0.5% CMC–0.1% HA combination. Low-shear viscosity of the CMC–HA combination increased 48% over the sum of the individual solutions, but high-shear viscosity remained virtually unchanged. Data from CMC and HA solutions at higher concentrations were consistent with these results.
Conclusion
Combining CMC and HA polymers produced a synergistic increase in low-shear viscosity (which cannot be fully explained by simple molecular entanglement), while the high-shear viscoelasticity of the combined solution remained unaffected. These data suggest that CMC–HA combinations have properties that may be used to formulate artificial tears that optimize ocular retention (through higher low-shear viscosity), while minimizing blur and stickiness during blinking (through lower high-shear viscosity).
Introduction
Dry eye disease is a common ocular condition that has a high impact on the quality of life of affected patients, owing to discomfort and/or visual disability.Citation1 It is a multifactorial disease associated with tear film hyperosmolarity and inflammation of the ocular surface, which can cause mild to incapacitating symptoms, such as itching, burning, blurred vision, mucous discharge, and photophobia.Citation1–Citation3
Artificial tear solutions are the mainstay of care for patients with mild dry eye symptoms, providing relief that can minimize corneal damage. Unpreserved formulations (which reduce the risk of developing preservative-associated side effects) are also often used concomitantly with prescribed therapies in patients with moderate to severe disease.Citation3–Citation5 Artificial tear formulations typically contain a water-soluble polymerCitation6 to provide enhanced residence time, retention of moisture, and binding to the mucin coat of the ocular surface, which facilitate corneal healing. Carboxymethylcellulose (CMC; also known as carmellose)Citation7 is a polymer () that has been used as an active ingredient in artificial tear solutions for many years.Citation8 Its clinical efficacy, binding capacity to ocular surface cells, and enhancement of corneal wound healing have been demonstrated in various model systems.Citation7,Citation9–Citation17 Hyaluronic acid (HA), also a polymer (), is used as an artificial tear ingredient because of its favorable hydrating, viscoelastic, and wound-healing properties.Citation5,Citation18–Citation27 These polymers, available in a range of molecular weights representing varying chain lengths, are generally dissolved in a dilute aqueous solution for use as a lubricant eyedrop, along with appropriate excipients, such as buffers, tonicity agents, and preservatives.
Figure 1 Structure of CMC and HA.
Abbreviations: CMC, carboxymethylcellulose; HA, hyaluronic acid.
Given the distinct biochemical, biophysical, and therapeutic properties of CMC and HA, we investigated combining them in a single artificial tear formulation. We compared the properties of a formulation containing both CMC and HA with those of each polymer individually, focusing on viscosity because it can increase retention time, hence improving moisture retention and clinical outcomes.Citation13 Multiple combinations were assessed based on concentrations of the polymers, with the goal of selecting formulas for subsequent clinical development.
Materials and methods
Solutions containing 0.5% or 1.0% CMC (low-viscosity type, approximately 90 kDa; Ashland Specialty Ingredients, Wilmington, DE, USA), 0.1%, 0.15%, or 0.25% HA (high molecular weight, >1,000 kDa; HTL Biotechnology, Javené, France), plus the combinations 0.5% CMC + 0.1% HA, 0.5% CMC + 0.15% HA, or 1.0% CMC + 0.25% HA were prepared in phosphate-buffered saline or a buffered solution (pH 7.2) containing glycerin, sodium borate, boric acid, sodium citrate, erythritol, L-carnitine, KCl, MgCl2, CaCl2, and stabilized oxychloro complex (Purite) as a preservative (similar to marketed artificial tear formulations). The solubilization process involved standard pharmaceutical methods. Briefly, buffers and tonicity agents were dissolved at moderate temperatures (40°C–50°C), and the polymers were added sequentially with rapid and sustained mixing to ensure complete dissolution. A combination of heat and filter sterilization techniques were then utilized to prepare final solutions for testing or clinical use.
The viscoelastic properties of each solution were assessed at low (10/second) and high (10,000/second) shear rates (to simulate eye movement and blinking, respectively) using a programmable rheometer (AR2000; TA Instruments, New Castle, DE, USA). Measurements were taken every 60 seconds using plate and cone geometry (5° angle, 60 mm steel cone). CMC and HA solutions were also tested for bulk viscosity at a fixed shear rate (60 rpm, or approximately 79.2/second) using a Brookfield viscometer and SC4-18 spindle (Brookfield Engineering, Middleboro, MA, USA), and compared with formulations combining both CMC and HA. All measurements were performed at 25°C in ≥3 replicates.
Results
CMC and HA polymers both exhibited shear thinning (ie, viscoelastic behavior) under shear strain when analyzed individually on the rheometer. Although each polymer exhibited a characteristic curve owing to its specific molecular properties, viscosity at a given shear rate was affected by polymer concentration in both cases (). For CMC 0.5% and 1.0%, viscosity varied from 3.5 cP (at 10/second) to 2.7 cP (at 10,000/second) and 5.8 cP (at 10/second) to 4.7 cP (at 10,000/second), respectively. For HA 0.1%, 0.15%, and 0.25%, viscosity at the same two shear rates were 6.8 and 2.8 cP, 14.4 and 3.4 cP, and 47.6 and 5.2 cP, respectively.
Figure 2 Rheological analysis of combinations of low (A), medium (B), and high (C) concentrations of CMC and HA polymers.
Abbreviations: CMC, carboxymethylcellulose; HA, hyaluronic acid.
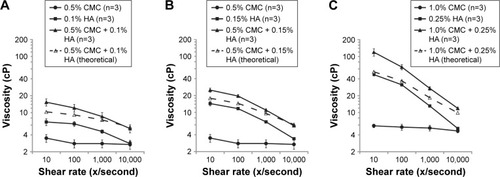
In a rheological analysis of combinations of CMC and HA polymers at 0.5% + 0.1%, 0.5% + 0.15%, and 1.0% + 0.25%, viscosity varied from 15.3 cP (at 10/second) to 5.2 cP (at 10,000/second), 25.0 cP (at 10/second) to 5.8 cP (at 10,000/second), and 127.5 cP (at 10/second) to 11.9 cP (at 10,000/second), respectively. Low (), medium (), and high () concentrations of CMC and HA consistently demonstrated higher viscosity (observed values) than predicted by additive effects (theoretical values) in the 10–10,000/second shear rate range. The effect was greatest at lower levels of shear force, and decreased at higher levels. For example, the combination of 0.5% CMC and 0.1% HA had observed viscosity of 15.3 cP at 10/second, which is 48% higher than the sum of the individual polymer viscosities.
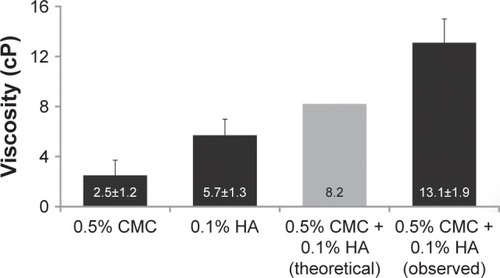
Bulk viscosity measurements on the Brookfield viscometer confirmed that the viscosity of the combined solution (observed) was substantially higher than predicted (theoretical) by the sum of the individual solution viscosities. The measured value (SD) of the 0.5% CMC–0.1% HA combination was 13.1 (1.9) cP, compared with the predicted value of 8.2 cP, representing a 60% increase ().
Figure 3 Bulk viscosity of the combined solution of 0.5% CMC and 0.1% HA was higher than predicted by additive effects.
Abbreviations: CMC, carboxymethylcellulose; HA, hyaluronic acid.
Additional results obtained at the approximate temperature of the tear film (ie, 35°C) revealed similar properties of the combined solution at 35°C and 25°C, despite a slight reduction in absolute viscosity values.
Discussion
Consistent with published reports, rheological testing showed that, individually, CMC and HA (in their native form, not cross-linked or chemically modified) exhibit viscoelastic properties that are affected by total polymer concentration and relative shear force.Citation28,Citation29 In comparison, observed results were higher than expected from additive effects when testing solutions combining CMC and HA. Indeed, rheological findings indicated that this combination of polymers exhibited higher viscosity than predicted from the sum of their individual formulation values, except at the highest shear rate tested (10,000/second), suggesting a synergistic effect, or cooperative interactions, between CMC and HA. This synergy at low shear force could potentially be explained (at least in part) by the fact that the relationship between viscosity and concentration is typically logarithmic, rather than additive,Citation30 but cannot be fully explained by simple molecule entanglement because the increase in low-shear viscosity occurs immediately upon mixing CMC and HA polymers and is readily reversible (data not shown). Both CMC and HA are anionic at physiological pH, exposing multiple hydroxyl and carboxyl water-binding sites that create a net negative charge and generate intra/interchain repulsion, thereby reducing chain–chain binding. It is this electrorepulsion that limits interactions between HA and CMC molecules to weaker, nonionic, fully reversible forces.
While the high molecular weight HA polymer (>1,000 kDa) is predicted to form globular random coiled structuresCitation31 (referred to as “time-average spheres”)Citation32 that include ions and small molecules but exclude macromolecules such as proteins,Citation33 short/low molecular weight HA chains cannot coil and remain somewhat extended and flexible in aqueous solution.Citation31 Similarly, the CMC polymer is not known to coil and is mostly linear/extended in solution.Citation34 In both cases, extension occurs because electrorepulsion at adjacent anionic sites increases as the backbone bends, causing chain rigidity. It is also worth noting that the CMC polymer used in this study was significantly shorter/smaller (approximately 30 times) than the HA polymer. With a CMC:HA weight ratio of 5:1, the CMC:HA molar ratio was >100:1. Nonetheless, CMC is largely excluded from HA spheres (due to its relatively large size compared with water and ions), which likely contributes to the macromolecular interactions between the two anionic polymers and forms the basis for the observed rheological synergy.
Solutes cannot occupy the same space at the same time, and their associated hydration shells may not only control a larger volume than the size of the solute itself but also behave differently than the bulk solution.Citation35 In this “bridged matrix” model, spheres composed of individual HA molecules occupy a greater volume because of their shape and total hydration space, explaining why (at least in part) HA is responsible for the majority of the solution viscosity in the CMC–HA combination, despite the greater amount of CMC by weight and total number of molecules. These HA spheres allow relatively free movement of water, ions, and small molecules, yet exclude CMC, owing to its size and anionic repulsion forces.Citation33,Citation34,Citation36 The partitioning of HA and CMC molecules, occurring to a greater degree than predicted under conditions of low shear (ie, “at rest”), may thus explain the unexpected rheological properties of the CMC–HA combination. High molecular weight HA will swell and occupy nearly all available volume at 0.1%, the concentration at which HA–HA entanglement has been reported.Citation37 If CMC is excluded from the HA spheres, its concentration in the available space will be increased,Citation35 and given that HA and CMC are both negatively charged, any entanglement that occurs will be limited and reversible, despite the proximity of the molecules. In this model, the excluded CMC molecules residing around the HA spheres (more so at rest) provide a stiffening component to the mixture by bridging physical forces between adjacent HA molecules.Citation38 Under conditions of high shear force, compression and elongation of HA are expected to occur, and CMC–HA entanglement/weak binding would be broken as a consequence.
The effectiveness of the CMC–HA combination at 0.5%/0.1% concentrations (Optive Fusion; Allergan plc, Dublin, Ireland) has been reported recently in two multicenter, randomized clinical trials involving patients with mild to severe dry eye.Citation39,Citation40 The effects of this combination on dry eye symptoms that develop after cataract surgery have also been investigated in a randomized study.Citation41 Moreover, preservative-free and multidose preserved formulations with this combination of polymers have now become available. In these formulations, the salt content is significantly lower than in the majority of HA-based products. Osmotic balance is achieved by using organic osmolytes or other compatible solutes, without addition of sodium chloride.Citation29,Citation42 The reduction/absence of Na+ ions in solution increases the availability of anionic binding sites that attract and hold water, further increasing the hydration sphere around the CMC and HA polymer chains and, consequently, increasing viscosity and delivering water to the ocular surface.Citation29,Citation42 Increased viscosity at low shear, such as exists between blinks, should improve retention of each eyedrop instilled in the eye and thus optimize hydration and protection of the ocular surface. On the other hand, reduced viscosity at high shear, such as occurs during blinking, should improve ocular comfort and reduce symptoms of stickiness and blur associated with some eyedrop formulations. Whether formulations such as CMC 1.0% + HA 0.25% may be too concentrated for therapeutic use or better suited for nighttime treatment (when blurred vision is not a concern) remains to be determined. Overall, however, the CMC–HA combination of polymers does produce a synergistic effect that enhances its low-shear viscosity above what would be expected, allowing for greater retention on the ocular surface without increasing the total concentration of the polymers. Above a certain point, the increase in low-shear viscosity does lead to less desirable effects, as previously shown,Citation39 and the more viscous product does not perform as well for a broad range of dry eye patients. Therefore, an optimum formulation of the combination of CMC and HA should consider the resultant viscosity and the target patient population.
Acknowledgments
We would like to posthumously recognize Bereth J Beard, BSc for her efforts and contributions to the conduct of this study and development of the first draft of this manuscript. This study was sponsored by Allergan plc, Irvine, CA, USA. Writing and editorial assistance was provided to the authors by Michele Jacob, PhD, CMPP of Evidence Scientific, Philadelphia, PA, USA, and funded by Allergan plc. All authors met the ICMJE authorship criteria.
Disclosure
PAS and JGV are employees of Allergan plc and report no other conflicts of interest in this work.
References
- American Academy of OphthalmologyPreferred Practice Pattern: Dry Eye SyndromeSan FranciscoAAO2013
- No authors listedThe epidemiology of dry eye disease: report of the Epidemiology Subcommittee of the International Dry Eye WorkShop (2007)Ocul Surf2007529310717508117
- No authors listedExpert consensus in the treatment of dry eye inflammationOphthalmol Times2007327112
- BehrensADoyleJJSternLDysfunctional tear syndrome: a Delphi approach to treatment recommendationsCornea200625890090717102664
- AragonaPPapaVMicaliASantoconoMMilazzoGLong term treatment with sodium hyaluronate-containing artificial tears reduces ocular surface damage in patients with dry eyeBr J Ophthalmol200286218118411815344
- FoodUSAdministrationDrugOphthalmic DemulcentsWashingtonUS Government Printing Office2000
- LentonLMAlbietzJMEffect of carmellose-based artificial tears on the ocular surface in eyes after laser in situ keratomileusisJ Refract Surg1999152 SupplS227S23110202728
- GreneRBLankstonPMordauntJHarroldMGwonAJonesRUnpreserved carboxymethylcellulose artificial tears evaluated in patients with keratoconjunctivitis siccaCornea19921142943011424648
- BaudouinCCochenerBPisellaPJRandomized, phase III study comparing osmoprotective carboxymethylcellulose with sodium hyaluronate in dry eye diseaseEur J Ophthalmol201222575176122287172
- MonacoGCacioppoVConsonniDTroianoPEffects of osmoprotection on symptoms, ocular surface damage, and tear film modifications caused by glaucoma therapyEur J Ophthalmol201121324325020872359
- GarrettQXuSSimmonsPACarboxymethyl cellulose stimulates rabbit corneal epithelial wound healingCurr Eye Res200833756757318600489
- GarrettQSimmonsPAXuSCarboxymethylcellulose binds to human corneal epithelial cells and is a modulator of corneal epithelial wound healingInvest Ophthalmol Vis Sci20074841559156717389485
- SimmonsPAVehigeJGClinical performance of a mid-viscosity artificial tear for dry eye treatmentCornea200726329430217413956
- NoeckerRJComparison of initial treatment response to two enhanced-viscosity artificial tearsEye Contact Lens200632314815216702870
- NilforoushanMRLatkanyRASpeakerMGEffect of artificial tears on visual acuityAm J Ophthalmol2005140583083516310460
- AlbietzJMLentonLMMcLennanSGEarlMLA comparison of the effect of Refresh Plus and Bion tears on dry eye symptoms and ocular surface health in myopic LASIK patientsCLAO J20022829610012054380
- DieboldYHerrerasJMCallejoSArguesoPCalongeMCarbomer-versus cellulose-based artificial-tear formulations: morphologic and toxicologic effects on a corneal cell lineCornea19981744334409676917
- BaeyensVBronABaudouinCEfficacy of 0.18% hypotonic sodium hyaluronate ophthalmic solution in the treatment of signs and symptoms of dry eye diseaseJ Fr Ophtalmol201235641241922483761
- LeeJHAhnHSKimEKKimTIEfficacy of sodium hyaluronate and carboxymethylcellulose in treating mild to moderate dry eye diseaseCornea201130217517921045674
- VogelRCrockettRSOdenNLaliberteTWMolinaLDemonstration of efficacy in the treatment of dry eye disease with 0.18% sodium hyaluronate ophthalmic solution (Vismed, Rejena)Am J Ophthalmol2010149459460120346777
- TroianoPMonacoGEffect of hypotonic 0.4% hyaluronic acid drops in dry eye patients: a cross-over studyCornea200827101126113019034126
- JohnsonMEMurphyPJBoultonMEffectiveness of sodium hyaluronate eyedrops in the treatment of dry eyeGraefes Arch Clin Exp Ophthalmol2006244110911215983814
- GomesJAAmankwahRPowell-RichardsADuaHSSodium hyaluronate (hyaluronic acid) promotes migration of human corneal epithelial cells in vitroBr J Ophthalmol200488682182515148219
- CondonPIMcEwenCGWrightMMackintoshGPrescottRJMcDonaldCDouble blind, randomised, placebo controlled, crossover, multicentre study to determine the efficacy of a 0.1% (w/v) sodium hyaluronate solution (Fermavisc) in the treatment of dry eye syndromeBr J Ophthalmol199983101121112410502570
- YokoiNKomuroANishidaKKinoshitaSEffectiveness of hyaluronan on corneal epithelial barrier function in dry eyeBr J Ophthalmol19978175335369290362
- SnibsonGRGreavesJLSoperNDPrydalJIWilsonCGBronAJPrecorneal residence times of sodium hyaluronate solutions studied by quantitative gamma scintigraphyEye (Lond)19904Pt 45946022226990
- DeLuiseVPPetersonWSThe use of topical Healon tears in the management of refractory dry-eye syndromeAnn Ophthalmol19841698238246508097
- BenchabaneABekkourKRheological properties of carboxymethyl cellulose (CMC) solutionsColloid Polym Sci20082861011731180
- KobayashiYOkamotoANishinariKViscoelasticity of hyaluronic acid with different molecular weightsBiorheology19943132352448729484
- ThomasDKThomasTAJViscosity–concentration relationships in solutions of high polymersJ Appl Polym Sci196038129131
- CowmanMKMatsuokaSExperimental approaches to hyaluronan structureCarbohydr Res2005340579180915780246
- CowmanMKSchmidtTARaghavanPSteccoAViscoelastic properties of hyaluronan in physiological conditionsF1000Res2015462226594344
- FraserJRLaurentTCLaurentUBHyaluronan: its nature, distribution, functions and turnoverJ Intern Med1997242127339260563
- ChytilMLiškováKJanečekJThe influence of counterions of different valency on carboxymethylcellulose viscoelastic behaviorMitchellGRheology: Theory, Properties and Practical Applications1st edHauppauge (NY)Nova Science Publishers2014
- MurphyLRMatubayasiNPayneVALevyRMProtein hydration and unfolding – insights from experimental partial specific volumes and unfolded protein modelsFold Des1998321051189565755
- GuillaumieFFurrerPFelt-BaeyensOComparative studies of various hyaluronic acids produced by microbial fermentation for potential topical ophthalmic applicationsJ Biomed Mater Res A201092A414211430
- BeckerLCBergfeldWFBelsitoDVFinal report of the safety assessment of hyaluronic acid, potassium hyaluronate, and sodium hyaluronateInt J Toxicol2009284 Suppl567
- EllisRJMacromolecular crowding: obvious but underappreciatedTrends Biochem Sci2001261059760411590012
- SimmonsPALiuHCarlisle-WilcoxCVehigeJGEfficacy and safety of two new formulations of artificial tears in subjects with dry eye disease: a 3-month, multicenter, active-controlled, randomized trialClin Ophthalmol2015966567525931807
- LabetoulleMChiambarettaFShirlawALeabackRBaudouinCOsmoprotectants, carboxymethylcellulose and hyaluronic acid multi-ingredient eye drop: a randomised controlled trial in moderate to severe dry eyeEye (Lond) Epub2017428
- MencucciRBoccaliniCCaputoRFavuzzaEEffect of a hyaluronic acid and carboxymethylcellulose ophthalmic solution on ocular comfort and tear-film instability after cataract surgeryJ Cataract Refract Surg20154181699170426432128
- GribbonPHengBCHardinghamTEThe molecular basis of the solution properties of hyaluronan investigated by confocal fluorescence recovery after photobleachingBiophys J19997742210221610512840
